Abstract
Objective
To examine the effect of acute exhaustive exercise versus rest on short‐term zinc kinetics in men.
Design
Crossover design, wherein all subjects were their own control.
Setting
University setting, where subjects were free living.
Participants
12 healthy, sedentary men, 25–35 years of age.
Interventions
70Zn was infused 10 min after exercise or at rest. Plasma zinc concentrations were measured at baseline and 2, 5, 10, 15, 30, 45, 60, 75, 90 and 120 min after exercise or rest. Haematocrit was measured before and after exercise to assess changes in plasma volume.
Main outcome measurements
Plasma zinc (primary), serum creatine kinase and serum cortisol concentrations (secondary).
Results
Plasma zinc concentrations decreased (p<0.05) after exercise, with a mean (SD) nadir of 13.9% (4.1%) observed at 70 min after exercise. There were increases in the size of the rapidly exchangeable plasma zinc pool (Qa; from 3.1 (0.2) to 3.6 (0.2) mg; p<0.05) and the liver zinc pool (Qb; from 10.2 (0.6) to 11.4 (0.8) mg; p = 0.12).
Conclusion
Exercise seems to cause a shift of plasma zinc into the interstitial fluid and liver after exercise, which may reflect the acute stress response of strenuous exercise.
Plasma zinc concentrations have been shown to decline with acute stress. This decline is thought to be related to an increased uptake of zinc by the liver and bone marrow for synthesis of acute‐phase proteins. Strenuous exercise induces an acute‐phase response; previous investigators have measured both declines and increases in plasma zinc levels after strenuous exercise.1,2,3,4,5,6,7
Anderson et al1 observed no changes in serum zinc concentration immediately after a 6‐mile run in male runners, but reported a significant decrease in serum zinc values 3 h after exercise. They attributed the decrease in serum zinc concentration to a redistribution of zinc from the serum to the tissues. Singh et al6 reported a 33% decrease in plasma zinc levels after 5 days of continuous physical and psychological stress in 119 Navy Sea, Air and Land trainees, possibly in response “to tissue damage and the inflammatory effect of physical activity”. Decreases in plasma zinc levels immediately after exercise have also been attributed to an acute‐phase response.2 In addition, plasma zinc concentrations have been shown to decrease after exercise in zinc‐deficient subjects, but to increase after exercise with zinc repletion.3 Zinc leakage from the muscle due to muscle damage from exercise might have been the cause of significant increases in total zinc concentration after aerobic and resistance exercise.4,5
The disparity in results in the aforementioned studies might be due to variations in the timing of blood draws, fitness status of subjects, exercise intensity, type and duration, as well as zinc status. To date, stable isotopes of zinc have not been used to assess the kinetics of zinc metabolism after an exhaustive exercise bout. The use of a kinetic model of zinc metabolism in exercise studies will provide a new insight into the changes observed in plasma zinc concentrations.
The purpose of the present investigation was to measure short‐term changes in zinc kinetics, using the stable isotope 70Zn, to define the kinetics of zinc metabolism after an acute, strenuous bout of exercise in sedentary men. Changes in plasma zinc, serum creatine kinase and serum cortisol concentrations with maximal, acute exercise were also assessed to provide an indication of the overall stressor of the exercise.
Materials and methods
Subjects
This study was approved by the Committee for Protection of Human Subjects at the University of California, Berkeley, California, USA. An electrocardiogram, a medical history, physical examination and a physical activity questionnaire were completed on each subject as part of the screening process. All subjects read and signed an informed consent form.
Criteria used for subject selection required participants to (a) be men between 25 and 35 years of age, (b) be sedentary (exercised for <1 h on ⩽2 days/week), (c) be non‐smokers, (d) have body mass index (kg/m2) ⩽25, (e) have no history of any type of chronic disease, (f) have no recent acute illness or trauma within the past 6 months (which can affect zinc status) and (g) have no recent major body weight changes. A total of 12 male subjects were recruited for participation in this study.
Study design
Each subject was studied twice at exercise to exhaustion on a bicycle and at rest; the studies were approximately 1 month apart. The order of the two treatments was randomised. Two subjects were measured on the same day: one would be placed through the exercise regimen while the other rested. The evening before each study day, subjects were given a standardised meal and instructed to fast after 20:00 h (table 1). Each subject was required to consume the meal between 18:00 h and 20:00 h the evening before each study day. The standardised meal was provided to control the amount of energy and zinc consumed the evening before each testing day. Furthermore, the standardised meal and specific time of consumption controlled the effect of the previous meal on plasma zinc concentrations.8
Box: Composition of standardised meal provided the night before each testing session
Frozen cheese ravioli and spiral spaghetti with marinara sauce and garlic bread (Marie Callender's Retail Foods, Los Angeles, California, USA)
Two 5.5 Oz cans of apple juice (Tree Top, Selah, Washington, DC, USA)
One 4.5 Oz can of mixed fruit in heavy syrup (Del Monte Foods, San Francisco, California, USA); Four Marie Lu cookies (MC Cookie, Oakland, California, USA)
Protocol for studies of zinc kinetics during rest
Each subject arrived at the metabolic unit at the University of California at Berkeley at 07:00 h, after an overnight fast. Subjects then reclined supine on a bed. After a short period of rest, a nurse placed a catheter into the antecubital vein and obtained a fasting blood sample (10 ml) for plasma zinc, serum creatine kinase, and serum cortisol concentrations, and haematocrit. Blood was collected into Monovette mineral‐free, ammonium‐heparin blood collection tubes (Sarstedt, Nümbrecht, Germany). After the baseline blood sample was collected, the nurse gave an intravenous stable isotope of 0.1 mg 70Zn through a butterfly catheter into the opposite arm of the blood draws. Blood was sampled at intervals of 2, 5, 10, 15, 30, 45, 60, 75, 90 and 120 min after isotope infusion. Approximately 10 ml of blood was obtained at each interval.
After the final blood draw, body weight, height, skinfold measures and circumferences were determined and hydrostatic weighing performed to assess body composition. Body weight was measured to the nearest 0.25 kg while subjects were wearing swim trunks, and height was measured to the nearest 0.5 cm using a stadiometer. Body mass index (weight (kg)/height (m2)) was then calculated.
Waist and hip circumferences were measured in centimeters with a flexible measuring tape, and the waist‐to‐hip ratio calculated. Skinfolds were measured at six sites: triceps, subscapula, suprailiac, abdomen, thigh and calf (Lange Skinfold Calipers, Cambridge Scientific Industries, Cambridge, Maryland, USA). All measures were performed on the right side of the body, three times per site, with the average of each site used to calculate the total sum of skinfolds (mm).
Hydrostatic weighing was performed in a similar way as that described by Katch and McArdle.9 Before entering the tank, subjects were weighed in their swimsuits and measured for residual lung volume via the oxygen dilution technique.10 Each subject was then weighed in a stainless steel, square tank while sitting on a canvas strap attached to a scale (9 kg capacity × 10 g; Acme Scale, Oakland, California, USA). Each subject performed 10 trials, with the three highest trials averaged to compute body density. Percentage body fat was calculated using the Siri equation.11 After all measurements were completed, subjects were provided lunch and discharged.
Protocol for studies of zinc kinetics during strenuous exercise
At the exercise time point, each subject arrived at the metabolic unit at 07:00 h after an overnight fast. The same procedures for catheter placement and blood draws were performed as during the resting time point; however, before infusion of the stable isotope of 70Zn, the subjects exercised on a bicycle ergometer to exhaustion. Subjects were connected to a one‐lead electrocardiogram and to a finger heart rate analyser (SensorMedics Corporation, Yorba Linda, California, USA) to assess heart rate. Heart rate and blood pressure were monitored before, during every minute of each stage of exercise testing and for 2–3 min after exercise. During exercise testing, each subject wore a mouthpiece that was connected to a metabolic cart (SensorMedics Corporation, Yorba Linda, California, USA). Resting oxygen consumption (VO2) was measured, followed by a warm‐up of approximately 2 min without any resistance. Subjects then cycled on a bicycle ergometer (Ergo‐metrics 800S, SensorMedics Corporation, Yorba Linda, California, USA) until maximal oxygen consumption (VO2max) was achieved. The exercise test began at 50 W and increased 25 W every 2 min.
The criteria used for achieving VO2max were (1) attaining the predicted maximal heart rate using the Karvonen method (220−age = predicted maximum heart rate)12; (2) a levelling off or a decrease in VO2 with increases in workload; and (3) achieving a respiratory exchange ratio (VCO2/VO2) of ⩾1. Borg's Rating of Perceived Exertion Scale (20‐point scale)13 was used at each stage of the exercise test to assess the subjects' perception of the exercise intensity. After exercise testing, the subjects cooled down for approximately 2–3 min. Subjects were then escorted to the blood drawing area, where they reclined supine on a bed. The nurse infused a stable isotope of 70Zn (in the opposite arm of the catheter) about 10 min after completion of the exercise bout (this time included the 2–3 min post‐exercise cool‐down period). Blood was drawn at the same time points, after infusion, as during the resting protocol. When all blood collections were completed, subjects were provided lunch and discharged.
Blood preparation and analyses
Haematocrit levels were analysed manually in duplicate before and at 12 min after exercise; the values were used to calculate percent plasma volume changes with exercise using the following equation14:
where Hct denotes haematocrit, pre denotes pre‐exercise and post denotes postexercise.
The blood was then centrifuged for 10 min at 1500 g and plasma aliquotted into mineral‐free, polyethylene, microcentrifuge tubes (Out Patient Services, Petaluma, California, USA). Samples were frozen at −20°C until analyses, except those used to evaluate serum creatine kinase concentrations, which were analysed within 7 days using a colorimetric reaction (Sigma Diagnostics, St Louis, Missouri, USA).
Serum cortisol concentrations were analysed by radioimmunoassay (Diagnostic Products, Los Angeles, California, USA). As serum cortisol concentrations remain virtually unchanged at rest, the baseline (pre‐exercise) values were compared with serum cortisol concentrations observed during the 2‐h period after exercise.
Plasma zinc concentrations were analysed on an Atomic Absorption Spectrophotometer (Thermo Jarrell Ash Smith‐Heiftje 22, Thermo Jarrell Ash, Franklin, Massachusetts, USA) after 1:8 dilution with 0.125 N ultrapure hydrochloric acid (Seastar Chemicals, Seattle, Washington, DC, USA). Plasma internal standards (bovine liver standard, National Institute of Standards and Technology, Gaithersburg, Maryland, USA) that were analysed had an average coefficient of variation of 3.7%.
The enrichment of the plasma with the isotope 70Zn was analysed on a VG Plasma Quad Inductively Coupled Plasma‐Mass Spectrometer ThermoElectron Corporation, West Palm Beach, Florida, USA). Sample preparation for Inductively Coupled Plasma‐Mass Spectrometer was a slightly modified version of that described by Turnlund and Keyes.15 In brief, all plasma samples were ashed and purified via ion exchange chromatography using only ultrapure acids (Seastar hydrochloric acid, Seastar Chemicals, Seattle, Washington, DC, USA; Optima Nitric Acid, Fisher Scientific, Pittsburgh, Pennsylvania, USA). The frozen plasma samples were thawed and transferred into acid‐washed Pyrex petri dishes and 4 ml of concentrated nitric acid was added to each sample. The sample/acid‐containing petri dishes were then placed on a hot plate under a fume hood and were slowly evaporated to dryness. Once the samples had cooled, they were covered with acid‐washed Pyrex lids and placed into a low‐temperature asher (Bronson/IPC, Hayward, California, USA) overnight for elimination of any organic compounds. The remaining mineral ash was dissolved in 10 ml of 2.5 M hydrochloric acid. Ion exchange chromatography was used to isolate zinc from the mineral ash. Detailed methods are described elsewhere.16
Kinetic analysis
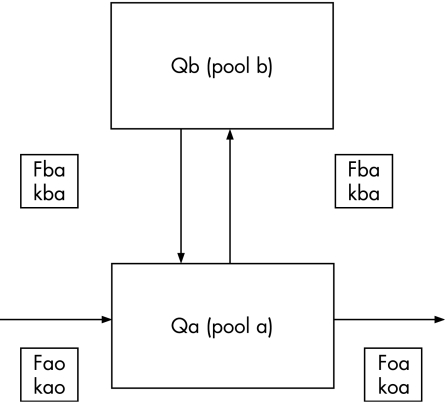
The regression of the ratio of 70Zn/67Zn in the plasma over time was analysed using a computer regression analysis programme (Regression, 1990–2, Blackwell Scientific Software, Oxford, UK). The minimum number of exponential terms needed to characterise each decay curve was defined, and subsequently, a compartmental assessment of the data was conducted using techniques described by Shipley and Clark17 and used previously by the authors.18,19,20 The computer regression programme and the dose of 70Zn infused into each subject were used to compute the sizes of pools a and b (Q), the turnover rates of the pools (k values) and the flux of zinc between the pools (F values). Isotope ratios were converted to tracer:tracee ratios using the method described in detail by Lowe et al.16 This method accounts for all the naturally occurring isotopes of zinc.16 Figure 1 shows the two‐compartment model of zinc kinetics.
Figure 1 Two‐compartment model of plasma zinc kinetics obtained from healthy men who were assessed at rest and after exhaustive exercise. Qa, plasma zinc pool; Qb, liver zinc pool; Kba, fractional turnover rate of Qa into Qb; Kab, fractional turnover rate of Qb into Qa; Koa, fractional turnover rate of Qa to pools outside the system; Fab, zinc transfer rate to Qa from Qb; Fba, zinc transfer rate to Qb from Qa; Fao, zinc transfer rate to Qa from pools outside the system; Foa, zinc transfer rate to pools outside the system from Qa. Zinc transfer rate is measured as mg/min. Adapted from Lowe et al.21
Dietary records
Between each testing period, subjects were required to weigh and record dietary intakes for 3 days (two weekdays and one weekend day). The intake of total kilojoules (kJ), carbohydrate, fat, protein and zinc intake was conducted using Nutritionist III, V.8.5, 1993, computer program (N‐squared, Salem, Oregon, USA).
Statistical analyses
Anthropometric data, physical fitness measures and dietary intakes were averaged to provide subject characteristics. Changes in haematocrit levels were used to calculate plasma volume changes before exercise with those after exercise.14 Serum cortisol concentrations were analysed using one‐way repeated measures analysis of variance. As serum cortisol concentrations were not measured during the resting time point, the pre‐exercise values were assumed to be equivalent to resting values (Statview SE+Graphics, Abacus Concepts, Berkeley, California, USA). Serum creatine kinase and plasma zinc concentrations were analysed using repeated measures analysis of variance with two trial factors of condition (exercise v rest) and time (SPSS‐X V.3.1). Tukey's post hoc test was used if significant interactions occurred. The 70Zn kinetic parameters for the exercise and resting time points were analysed using paired t tests (Microsoft Excel 2000, Microsoft Corporation, Redmond, Washington, USA). The level of significance was set a priori at 0.05. We did not conduct formal sample size estimation for this study because: (1) we used the same number of subjects who participated in many other studies of zinc kinetics by the same researchers and (2) this was a crossover design, which increased the statistical power of the study.
Results
Tables 2 and 3 summarise subjects' physical attributes and physical fitness characteristics, respectively. The men were of normal weight‐for‐height and of average physical fitness.
Table 2 Mean subject characteristics.
| Age (years) | 28.8 (2.9) |
| Body weight (kg) | 74.6 (12.9) |
| Height (cm) | 182.2 (10.4) |
| Body mass index (kg/m2) | 22.4 (3.4) |
| Waist‐to‐hip ratio | 0.85 (0.04) |
| Sum of skinfolds (mm; six sites)* | 83.8 (41.8) |
| Percent body fat (hydrostatic weighing) | 16.9 (7) |
| Lean body mass (kg; hydrostatic weighing) | 61.2 (7.1) |
Values represent means (SD).
*The six sites were triceps, subscapula, suprailiac, abdomen, thigh and calf; all skinfold measurements were taken on the right side of the body.
Table 3 Mean physical fitness characteristics.
| Maximum watts achieved | 220.8 (56.2) |
| Maximal oxygen consumption (ml/kg/min) | 41.5 (10.4) |
| Respiratory exchange ratio (VCO2/VO2) | 1.1 (0.05) |
| Energy expenditure during exercise (kilocalories/min) | 16.1 (4.4) |
| Maximum heart rate (beats/min) | 179.9 (15) |
| Maximum systolic blood pressure (mm Hg) | 174 (23.6) |
| Maximum diastolic blood pressure (mm Hg) | 57.8 (4.4) |
| Borg's ating of perceived exertion (1 to 20 scale) | 18.1 (1.5) |
Values represent means (SD).
Dietary zinc intakes averaged 114% of the Dietary Reference Intake of 11 mg/day for adult men (n = 5).22 Subjects consumed an average of 11 127 (3217) kJ/day, 101 (33) g/day of protein, 373 (132) g/day of carbohydrate and 88 (27) g/day of total fat.
The mean (standard error of the mean (SEM) value of haematocrit increased from 45.1% (0.65%) before exercise to 47.5% (0.83%) after exercise. Using van Beaumont et al's quotient,14 this is consistent with a 9.2 (1.6%) reduction in plasma volume after the acute bout of maximal cycle ergometry exercise.
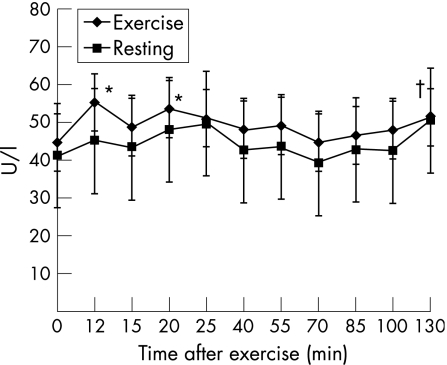
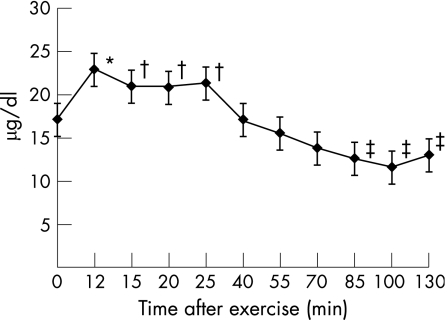
The changes in serum creatine kinase and cortisol concentrations after exercise suggest that an acute‐phase response was induced by the exercise bout. Serum creatine kinase concentrations increased by 36.7%(10.7%) (p<0.05) at 12 min after exercise (2 min after isotope infusion) and remained higher than resting values throughout the 130‐min post‐exercise period (120 min after isotope infusion; fig 2). Serum cortisol levels increased 44% (15.2%) (p<0.05) at 12 min after exercise (2 min after isotope infusion); they remained raised until 25 min after exercise and then declined to a nadir of 28.8% (6.6%) (p<0.05) below baseline values at approximately 100 min after exercise (fig 3).
Figure 2 Changes in serum creatine kinase concentrations at rest and after exercise. Values represent means (SEM). *Significantly greater than pre‐exercise values (p = 0.012 (12 min) and p = 0.04 (20 min)). †Trend to be greater than pre‐exercise values (p = 0.09 (130 min)).
Figure 3 Changes in serum cortisol concentrations after exercise. Values represent means (SEM). As serum cortisol concentrations remain virtually unchanged at rest, the baseline (pre‐exercise) values were compared with serum cortisol concentrations observed during the 2‐h period after exercise. *Significantly greater than pre‐exercise values (p = 0.014). †Trend to be greater than pre‐exercise values (p = 0.10 (15 min); p = 0.06 (20 min); p = 0.07 (25 min)). ‡Significantly lower than pre‐exercise values (p = 0.03 (85 min); p = 0.003 (100 min); p = 0.02 (130 min)).
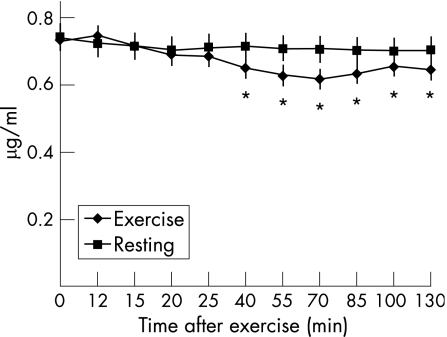
Plasma zinc concentrations decreased (p<0.05) with exercise compared with baseline values (fig 4). The nadir, observed at 70 min after exercise (60 min after isotope infusion), was 13.9% (4.1%) lower than baseline values. No differences were seen at 12 min after exercise when a 9% reduction in plasma volume was taken into account. Haemoconcentration, associated with the decline in plasma volume, might have obviated a fall in plasma zinc earlier in the post‐exercise period.
Figure 4 Changes in plasma zinc concentrations (µg/ml) at rest and after exercise. Values represent means (SEM). *Significantly lower than pre‐exercise values (p = 0.04 (40 min); p = 0.02 (55 min); p = 0.012 (70 min); p = 0.004 (85 min); p = 0.04 (100 min); p = 0.011 (130 min)).
The zinc kinetic data best fit a two‐compartment model (fig 1). The size of pool a (Qa) significantly increased (p = 0.05) about 15% during the 130 min after the exercise bout (table 3). The size of pool b (Qb) also tended to increase after exercise, but the 12% increase did not reach significance (p = 0.12; table 4).
Table 4 Calculated values of pool size, turnover rate and zinc flux for the resting and exercise trials.
| Resting | Exercise | |
|---|---|---|
| Qa (mg) | 3.12 (0.2) | 3.59 (0.24)* |
| Qb (mg) | 10.21 (0.62) | 11.40 (0.83)† |
| Koa (×10−2/min) | 0.90 (0.16) | 0.79 (0.18) |
| Kba (×10−2/min) | 8.03 (0.44) | 8.13 (0.73) |
| Kaa (×10−2/min) | 9.11 (0.53) | 8.72 (0.88) |
| Kbb and Kab (×10−2/min) | 2.49 (0.17) | 2.54 (0.25) |
| Foa and Fao (mg/min) | 0.03 (0.003) | 0.03 (0.005) |
| Fab and Fba (mg/min) | 0.24 (0.009) | 0.28 (0.02) |
Values represent means (SEM).
Qa, plasma pool; Qb, liver pool; Koa, fractional turnover rate of Qa to the outside; Kba, fractional turnover rate of Qa into Qb; Kaa, total fractional turnover rate within Qa; Kbb, total fractional turnover rate within Qb; Kab (Kbb), fractional turnover rate of Qb into Qa; Foa, flux of Qa to the outside; Fao (Foa), flux of the outside to Qa; Fab, flux of Qb into Qa; Fba (Fab), flux of Qa into Qb.
*Significantly greater than resting value (p = 0.05).
†Trend to be greater than resting value (p = 0.12).
The fractional turnover rate of zinc in the pools (Koa, Kba and Kab), the flux or amount of zinc between the pools (Fab and Fba) with exercise and the flux of zinc out of the system to other pools (Foa) did not differ between resting and exercising conditions (table 4). The relationship between pool size and flux is pool size (Q) × turnover rate (K) = flux (sum of F's exiting pool). Although there was a slight increase in flux between a and b during and after exercise, these changes were not significant.
Discussion
The main purpose of our investigation was to measure short‐term changes in zinc kinetics using the stable isotope 70Zn, to define the kinetics of zinc metabolism after an acute, strenuous bout of exercise in sedentary men. A simple two‐compartment model was used to analyse the kinetic data. The increases in serum creatine kinase and cortisol levels, combined with the haemoconcentration of zinc, confirm that an acute stress response was induced among our subjects who were required to exercise on a cycle ergometer until exhaustion. We observed a 37% increase in serum creatine kinase about 12 min after exercise, validating that the stressor of the bicycle ergometry exercise was great enough to elicit the release of this acute phase protein. Our results are similar to those of Singh et al,6 who reported a 266% increase in serum creatine kinase concentrations after 5 days of intense physical activity.
In our study, a 14% decrease in plasma zinc concentration at 70 min after exercise occurred despite a 9% reduction in plasma volume, which would have resulted in a haemoconcentration of zinc remaining in the circulation. Our results are similar to those of Singh et al,6 who reported a 33% reduction in plasma zinc concentration after 5 days of highly intensive physical and psychological stress. The greater degrees of percentage change observed by Singh et al6 were probably due to the greater duration and intensity of the exercise.
Our subjects appeared to be in good zinc status before the study, with an average plasma zinc concentration of 0.73 (0.04) g/ml. A possible explanation of the reduction of plasma zinc level could be that, immediately after exercise, there is an increased uptake of zinc by the muscles followed by an increased muscle catabolism, with zinc subsequently released back into the circulation. This could account for the immediate reduction of plasma zinc concentrations after exercise, and the subsequent increase after recovery from exercise. The acute‐phase proteins may also be responsible for the reduction of plasma zinc levels immediately after exercise. Interleukin 1 and/or interleukin 6 may sequester zinc, via increased hepatic metallothionein production, into the liver as a result of the acute stress of exercise.3,6
In contrast with our results, Hetland et al23 reported a maximal increase of 53% in serum zinc concentration, in all but two subjects, immediately after a 50‐km cross‐country skiing competition. Perhaps the plasma zinc levels increased due to red blood cells lysing as a result of the effect of the exercise, which caused an initial release of zinc from the red blood cells. Another explanation for the rise in plasma zinc concentration after exercise may be the leakage of zinc from the muscle into the extracellular fluid,4,24 because there is an increased rate of protein and amino acid catabolism after a strenuous bout of exercise. Furthermore, lactate dehydrogenase, a zinc metalloenzyme, is one among the cellular proteins that have been reported to leak into the circulation after an intensive bout of exercise.24 These could be two reasons why Ohno et al5 observed increases in plasma zinc concentrations immediately after exercise in their subjects, despite the use of a bicycle ergometry protocol. Lukaski et al3 found increases in plasma zinc concentrations immediately after cycle ergometry in five healthy men who had recently been repleted with zinc after a previous depletion of tissue zinc. The zinc repletion could have been the main reason for the increased post‐exercise concentrations of zinc.
Although we reported a decline in plasma zinc levels, Qa was larger when subjects were recovering from exercise than when they were at rest. Qa is thought to represent the plasma and part of the interstitial fluid.18 The increase in Qa suggests that zinc moved from the plasma into the interstitial space. Furthermore, Qb tended to increase, which is thought to be primarily in the liver.18 Lowe et al18 reported increases in both Qa and Qb after injection of Escherichia coli (a stressor) endotoxin in rats. Exercise, like E coli, is a stressor; therefore, the response of a decline in plasma zinc levels with a concomitant increase in Qa and Qb seems to be a normal response. As we do not have an appropriate exercise model with which to compare our data, it could also be speculated that Qa is increasing at the expense of another, more slowly exchanging compartment.
Changes in oncotic pressure with exercise could also account for the fall in plasma zinc concentrations after exercise, and the subsequent increase in Qa and Qb. Changes in muscle metabolism with exercise can alter the oncotic pressure as a result of an accumulation of muscle metabolites in the cell, which would then cause an increased movement of fluid into the cell.25 Movement of water into the cells will cause a rise in oncotic pressure, favouring a shift of fluid from the vascular space into the interstitial fluid.25 Furthermore, >95% of zinc in the serum is bound to albumin and α2‐macroglobulin.21 Proteins shifting from the vascular space into the interstitial space may result in an increased oncotic pressure.25 The rate of albumin exiting the capillaries rises during exercise25 and may be expedited by such hormones as serum cortisol, which may increase capillary permeability. We observed an increase in serum cortisol concentrations up to about 25 min after exercise.
Similar findings of a reduction in plasma zinc concentrations were reported by others23 who measured short‐term changes in zinc kinetics after a meal. Meal consumption, infection and exercise are all stressors to the physiological system, and generally result in decreases in plasma zinc concentrations. The increased serum cortisol levels reported in our study further support this explanation, as possible actions of increased serum cortisol concentrations observed during and after exercise may be related to its roles in gluconeogenesis and modifications in cellular permeability.25
The significant reduction in plasma zinc concentration after exercise and the subsequent increases in Qa and Qb suggest that plasma zinc shifted into the interstitial fluid and the liver. The possible mechanisms stated above support our findings. Nonetheless, the change in plasma zinc could have occurred as a result of a decreased movement of zinc from the other rapidly exchanging compartments to the plasma. However, we can only speculate on this.
The implications of this transient reduction in plasma zinc concentrations after exercise, if repeated often, may result in chronically low plasma zinc concentrations. Upper respiratory tract infections are often observed in many athletes who undergo intense training,26 which could possibly be a result of a reduction in the synthesis of immunological proteins (of which zinc is required; ie, thymulin)27 and/or a decrease in zinc–copper superoxide dismutase, an enzyme that plays a major part in the body's antioxidant defence mechanism.
Furthermore, there are many zinc‐dependent enzymes required for energy metabolism, such as succinate dehydrogenase, malate dehydrogenase, carnitine palmityl transferase, creatine phosphokinase, phosphofructose kinase and lactate dehydrogenase.21 Chronically low plasma zinc concentrations could result in a reduction in the synthesis of these enzymes, which could ultimately lead to impaired exercise performance28 and/or energy metabolism.
In conclusion, plasma zinc concentration significantly decreased after exercise, with the nadir occurring at 70 min after exercise. Short‐term zinc kinetics showed a significant increase in Qa with a trend towards an increase in Qb. This may be explained by a shift of plasma zinc into the interstitial fluid and uptake of zinc by the liver. Possible mechanisms include increased cytokine activity and sequestering of zinc into the liver and/or changes in oncotic pressure with exercise. Owing to the fact that there are no steady‐state conditions after exercise, we are aware that our results could have been affected. Nonetheless, we used previous models that have been published under non‐steady‐state conditions to calculate the changes after exercise. We hope that this research will spur further investigations in the use of stable isotopes after exercise, so that we can fully elucidate what occurs with mineral metabolism in non‐steady‐state conditions.
What is already known on this topic
Plasma zinc concentrations have been shown to decline with acute stress (eg, acute infection, trauma and acute stress response).
This decline is thought to be related to an increased uptake of zinc by the liver and bone marrow for synthesis of acute‐phase proteins.
Strenuous exercise induces an acute‐phase response; however, previous investigators have measured both declines and increases in plasma zinc levels after strenuous exercise.
Nonetheless, previous research differed in the timing of blood draws, training status of subjects (ie, sedentary v trained), and exercise intensity, type and duration.
What this study adds
None of the previous investigations used stable isotopes of zinc to assess the kinetics of zinc metabolism after an exhaustive exercise bout.
The use of a kinetic model of zinc metabolism in exercise studies will provide a new insight into the changes observed in plasma zinc concentrations.
This study adds a new line of research in the area of zinc kinetics and exercise metabolism.
It has allowed us to better understand the changes that occur to zinc immediately after exercise.
We hope that this research will stimulate more investigations in the area of zinc (and other mineral) kinetics and exercise.
Thus, we can gain more knowledge of the changes to zinc during exercise, and will be better able to provide athletes and individuals who are physically active with more definitive information on the requirement of zinc (mineral).
Acknowledgements
We thank Dr Alan Volpe of Lawrence Livermore Laboratories for allowing us to use his inductively coupled plasma‐mass spectrometer. We also thank all the dedicated subjects who participated in this study.
Footnotes
Funding: This project was supported by National Institutes of Health Nutrition Training Grant # T32.HD07266‐10.
Competing interests: None.
Each of the authors contributed to the entire process of this paper—from data collection and analyses to the final product of this manuscript.
References
- 1.Anderson R A, Polansky M M, Bryden N A. Acute effects of chromium, copper, zinc, and selected clinical variables in urine and serum of male runners. Biol Trace Elem Res 19846327–336. [DOI] [PubMed] [Google Scholar]
- 2.Aruoma O I, Reilly T, MacLaren D.et al Iron, copper, and zinc concentrations in human sweat and plasma; the effect of exercise. Clin Chim Acta 198817781–88. [DOI] [PubMed] [Google Scholar]
- 3.Lukaski H C, Bolonchuk W W, Klevay L M.et al Changes in plasma zinc content after exercise in men fed a low‐zinc diet. Am J Physiol 1984247E88–E93. [DOI] [PubMed] [Google Scholar]
- 4.Mundie T G, Hare B. Effects of resistance exercise on plasma, erythrocyte, and urine Zn. Biol Trace Elem Res 20017923–28. [DOI] [PubMed] [Google Scholar]
- 5.Ohno H, Yamashita K, Doi R.et al Exercise‐induced changes in blood zinc and related proteins in humans. J Appl Physiol 1985581453–1458. [DOI] [PubMed] [Google Scholar]
- 6.Singh A, Smoak B L, Patterson K Y.et al Biochemical indices of selected trace minerals in men: effect of stress. Am J Clin Nutr 199153126–131. [DOI] [PubMed] [Google Scholar]
- 7.Keen C L. The effect of exercise and heat on mineral metabolism and requirements. In: Marriott BM, ed. Nutritional needs in hot environments: applications for military personnel in field operations. Washington, DC: National Academy Press, 1993117–135. [PubMed]
- 8.Hambidge K M, King J C, Kern D L.et al Pre‐breakfast plasma zinc concentrations: the effect of previous meals. J Trace Elem Electrolytes Health Disease 19904229–231. [PubMed] [Google Scholar]
- 9.Katch F I, McArdle W D.Nutrition, weight control, and exercise, 3rd edn. Philadelphia, PA: Lea & Febiger 1988124–127.
- 10.Wilmore J H, Vodak P A, Parr R B.et al Further simplification of a method for determination of residual lung volume. Med Sci Sports Exerc 198012216–218. [PubMed] [Google Scholar]
- 11.Siri W E. Gross composition of the body. In: Lawrence JH, Tobias CA, eds. Advances in biological and medical physics. Vol 4. New York, NY: Academic Press, 1956 [DOI] [PubMed]
- 12.Pollock M L, Wilmore J L, Fox S M.Exercise in health and diseases: evaluation and prescription for prevention and rehabilitation. Philadelphia, PA: WB Saunders, 1984
- 13.Borg G A V. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 198214377–381. [PubMed] [Google Scholar]
- 14.van Beaumont W, Strand J C, Petrofsky J S.et al Changes in total plasma content of electrolytes and proteins with maximal exercise. J Appl Physiol 197334102–106. [DOI] [PubMed] [Google Scholar]
- 15.Turnlund J R, Keyes W R. Automated analysis of stable isotopes of zinc, copper, iron, calcium and magnesium by thermal ionization mass spectrometry using double isotope dilution for tracer studies in humans. J Micronutr Anal 19907117–145. [Google Scholar]
- 16.Lowe N M, Woodhouse L R, Matel J S.et al A compartmental model of zinc metabolism in healthy women, using oral and intravenous stable isotopes. Am J Clin Nutr 1997651810–1819. [DOI] [PubMed] [Google Scholar]
- 17.Shipley R A, Clark R E.Tracer methods for in vivo kinetics. New York, NY: Academic Press, 1972
- 18.Lowe N M, Bremmer I, Jackson M J. Plasma 65Zn kinetics in the rat. Br J Nutr 199165645–655. [DOI] [PubMed] [Google Scholar]
- 19.Lowe N M, Green A, Rhodes J M.et al Studies of human zinc kinetics using the stable isotopes 70Zn. Clin Sci 199384113–117. [DOI] [PubMed] [Google Scholar]
- 20.Pinna K, Woodhouse L R, Sutherland B S.et al Exchangeable zinc pool masses and turnover are maintained in healthy men with low zinc intakes. J Nutr 1312288–2294. [DOI] [PubMed] [Google Scholar]
- 21.Lowe N M, Woodhouse L R, King J C. A comparison of the short‐term kinetics of zinc metabolism in women during fasting and following a breakfast meal. Br J Nutr 199880363–370. [DOI] [PubMed] [Google Scholar]
- 22.Food and Nutrition Board, Institute of Medicine Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, DC: National Academy Press, 2001 [PubMed]
- 23.Hetland O, Brubak A, Refsum H E.et al Serum and erythrocyte zinc concentrations after prolonged heavy exercise. In: Howald H, Poortmans JR, eds. Metabolic adaptation to prolonged physical exercise. Proceedings of the 2nd International Symposium on Biochemistry of Exercise. Basel, Switzerland: Birkhauser Verlag, 1973367–370.
- 24.Wade C E, Freund B J. Hormonal control of blood volume during and following exercise. In: Gisolfi CV, Lamb DR, eds. Perspectives in exercise science and sports medicine. Vol 3 Fluid homeostasis during exercise Carmel, IN: Cooper Publishing Group, 1990207–245.
- 25.Solomons N W. Zinc and copper. In: Shils ME, Young VR, eds. Modern nutrition in health and disease. 7th edn. Philadelphia, PA: Lea & Febiger, 1988238–262.
- 26.Nieman D C. Exercise, infection, and immunity. Int J Sports Med 199415(Suppl 3)S131–S141. [DOI] [PubMed] [Google Scholar]
- 27.Cunningham‐Rundles S, Bockman R S, Lin A.et al Physiological and pharmacological effects of zinc on immune response. In: Bendich A, Chandra RK, eds. Micronutrients and immune function. New York, NY: New York Academy of Sciences, 1990113–122. [DOI] [PubMed]
- 28.Van Loan M D, Sutherland B, Lowe N M.et al The effects of zinc depletion on peak force and total work of knee and shoulder extensor and flexor muscles. Int J Sport Nutr 19999125–135. [DOI] [PubMed] [Google Scholar]