Abstract
Background
Anabolic androgenic steroids (AAS) are sometimes used by power athletes to improve performance by increasing muscle mass and strength. Recent bioptical data have shown that in athletes under the pharmacological effects of AAS, a focal increase in myocardial collagen content might occur as a repair mechanism against myocardial damage.
Objective
To investigate the potential underlying left ventricular myocardial dysfunction after chronic misuse of AAS in athletes by use of Doppler myocardial imaging (DMI) and strain rate imaging (SRI).
Methods
Standard Doppler echocardiography, DMI, SRI and ECG treadmill test were undertaken by 45 bodybuilders, including 20 athletes misusing AAS for at least 5 years (users), by 25 anabolic‐free bodybuilders (non‐users) and by 25 age‐matched healthy sedentary controls, all men. The mean (SD) number of weeks of AAS use per year was 31.3 (6.4) in users, compared with 8.9 (3.8) years in non‐users, and the mean weekly dosage of AAS was 525.4 (90.7) mg.
Results
The groups were matched for age. Systolic blood pressure was higher in athletes (145 (9) vs 130 (5) mm Hg) than in controls. Left ventricular mass index did not significantly differ between the two groups of athletes. In particular, both users and non‐users showed increased wall thickness and relative wall thickness compared with controls, whereas left ventricular ejection fraction, left ventricular end‐diastolic diameter and transmitral Doppler indexes were comparable for the three groups. Colour DMI analysis showed significantly lower myocardial early: myocardial atrial diastolic wave ratios in users at the level of the basal interventricular septum (IVS) and left ventricular lateral wall (p<0.01), in comparison with both non‐users and controls. In addition, in users, peak systolic left ventricular strain rate and strain were both reduced in the middle IVS (both p<0.001) and in the left ventricular lateral free wall (both p<0.01). By stepwise forward multivariate analyses, the sum of the left ventricular wall thickness (β coefficient = −0.32, p<0.01), the number of weeks of AAS use per year (β = −0.42, p<0.001) and the weekly dosage of AAS (β = −0.48, p<0.001) were the only independent determinants of middle IVS strain rate. In addition, impaired left ventricular strain in users was associated with a reduced performance during physical effort (p<0.001).
Conclusions
Several years after chronic misuse of AAS, power athletes show a subclinical impairment of both systolic and diastolic myocardial function, strongly associated with mean dosage and duration of AAS use. The combined use of DMI and SRI may therefore be useful for the early identification of patients with more diffused cardiac involvement, and eventually for investigation of the reversibility of such myocardial effects after discontinuation of the drug.
Haemodynamic overload due to long‐term training usually involves both left and right ventricles, inducing changes in cardiac structure such as increases in internal cavity diameters, wall thickness and mass, usually described as “athlete's heart”.1,2,3,4,5,6
In competitive athletes, left ventricular hypertrophy often mimics pathological conditions, and the distinction may have important implications, particularly in adulthood when practising regular physical activity.
Anabolic androgenic steroids (AAS) are sometimes used by power athletes to improve performance by increasing muscle mass and strength.7,8,9,10,11 AAS stimulate cellular protein synthesis through androgenic receptors and promote the growth of all organs that have receptors similar to those of androgens.12,13,14,15,16 The effects of the chronic assumption of AAS on human performance and on cardiovascular structures are subjects of intense debate. Recent bioptical data have shown that in athletes under the pharmacological effects of AAS, a focal increase in myocardial collagen content might occur as a repair mechanism against myocardial damage.10
Although standard Doppler echocardiography has been widely used to distinguish athlete's heart from pathological left ventricular hypertrophy, few echocardiographic reports have defined changes in left ventricular morphology and function determined by chronic misuse of AAS.17,18,19,20,21,22,23,24
Doppler myocardial imaging (DMI) and strain rate imaging (SRI) extend Doppler applications beyond the analysis of cardiac blood flows into the measurement of regional myocardial function.25,26,27,28 Our previous reports have documented the usefulness of such techniques in identifying training influence on left ventricular myocardial longitudinal function, and in detecting differences of myocardial function in different kinds of pathological left ventricular hypertrophy.29,30,31,32 However, no data are presently available about the possible effects of AAS misuse on left ventricular regional myocardial function in power athletes.
The aim of this study was to investigate the potential underlying left ventricular myocardial dysfunction after chronic misuse of AAS in athletes by DMI and SRI.
Methods
Study population
Power athletes
After the approval of the institutional ethics committee, we selected a population of 45 top‐level competitive bodybuilders, including 20 athletes misusing AAS for at least 5 years (users), 25 anabolic‐free bodybuilders (non‐users) and 25 age‐matched healthy sedentary controls, all men. Exclusion criteria were coronary artery disease, valvular and congenital heart disease, congestive heart failure, cardiomyopathies, diabetes mellitus, sinus tachycardia and echocardiograms of inadequate quality.
Training protocols
All the participants had trained intensively for 15–20 h/week for >5 years. All the athletes underwent anaerobic isometric static exercise at incremental workloads of 40–60% of maximal heart rate. In particular, their training protocol included 3 h/day of weightlifting at high workload. Maximum self‐reported one‐repetition squat results were 169 (36) kg in non‐users, and 203 (26) kg in users, (p<0.01). In addition to the strength training, non‐users and users performed approximately 2 h of low‐intensity endurance training (“fat burning”).
AAS misuse
The AAS reported to be given by intramuscular injections were metenolone, nandrolone and esters of testosterone. The substances taken orally included fluoxymesterone, mesterolone, metenolone, metandienone, oxandrolone and oxymetholone. All except two participants used combinations of both oral and injectable substances. Three athletes who had sporadic experiences (<1 year; 2–16 IU daily) with growth hormone were excluded from the protocol. Two users had taken cocaine once in the past. The classification into users and anabolic‐free athletes was strengthened by additional measurements of luteinising and follicle stimulating hormones, showing clearly depressed blood concentrations in all users, whereas all non‐users had normal values. The mean (SD) number of weeks of AAS use per year was 31.3 (6.4) in users, compared with 8.9 (3.8) years in non‐users, and the mean (SD) weekly dosage of AAS was 525.4 (90.7) mg.
Sedentary healthy controls
We also studied 25 age‐matched sedentary controls without detectable cardiovascular risk factors. All volunteer controls were recruited in Naples (Italy), selected from participants investigated for work eligibility at our cardiology department and examined in a single centre (Monaldi Hospital, Naples, Italy). None of the controls had structural or functional cardiovascular abnormalities or had received any drugs. We ensured comparability of the groups (athletes and controls) by using frequency matching. Baseline characteristics such as familial history of atherosclerotic disease, smoking behaviour, eating habits and work activities were examined, and for these parameters no significant differences were observed between the groups of patients.
Echocardiographic procedures
Standard Doppler echocardiography, DMI and SRI were performed by digital ultrasound machine (Vivid 7, GE Medical Systems, Milwaukee, Wisconsin, USA), equipped with DMI and SRI capabilities. A variable frequency phased‐array transducer (2.5–3.5–4 MHz) was used for echo‐Doppler and DMI imaging. Cuff blood pressure (mean of three measurements) was estimated by a physician blinded to the examination. All the measurements were analysed by two experienced readers, using the average of 3–5 cardiac cycles.
Standard Doppler echo
M‐mode measurements were performed in a parasternal long‐axis view, by using the criteria of the American Society of Echocardiography. Left ventricular mass was calculated by the Penn convention, and indexed for height2.7 (Cornell adjustment).33 The relative diastolic wall thickness was determined as the ratio between the sum of septal and posterior wall thickness and left ventricular end‐diastolic diameter. Left ventricular ejection fraction was measured using a commercially available software program that applied Simpson's rule on the two‐chamber and four‐chamber views. Circumferential end‐systolic stress was calculated as a measurement of left ventricular afterload using a cylindrical model according to the following formula:
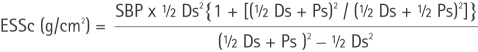 |
where SBP is the systolic blood pressure, Ds the end‐systolic diameter and Ps the posterior wall thickness in systole.34 Stroke volume was obtained by left ventricular outflow Doppler imaging as the product between outflow tract area and left ventricular output velocity integral.35
Standard Doppler‐derived left ventricular diastolic inflow was recorded in an apical four‐chamber view by placing the sample volume at the tips level. The following left ventricular diastolic measurements were measured: E and A peak velocities (m/sec) and their ratio, E‐wave deceleration time (min), and isovolumic relaxation time (min; the time interval between the end of systolic output flow and the transmitral E‐wave onset, by placing pulsed‐Doppler sample volume between outflow tract and mitral valve).36
Colour DMI
By colour reconstructed pulsed‐wave DMI, simultaneous interrogation of multiple myocardial segments within the same cardiac cycle was obtained “off‐line”, using high frame rate colour two‐dimensional DMI cine‐loops to generate time–velocity plots.
In apical four‐chamber view, a Doppler sample volume was placed to correspond with both the IVS and left ventricular lateral wall, at the level of the mitral annulus. The apical view was chosen to obtain a quantitative assessment of regional wall motion almost simultaneous to Doppler left ventricular inflow and outflow, and to minimise the incidence angle between the Doppler beam and the left ventricular and right ventricular longitudinal wall motion. Colour reconstructed pulsed‐wave DMI is characterised by a myocardial systolic wave (Sm) and two diastolic waves‐early (Em) and atrial (Am). Myocardial peak velocity of Sm (m/s), myocardial pre‐contraction time (from the onset of ECG QRS complex to the beginning of Sm) and contraction time (from the beginning to the end of Sm wave; all in ms) were calculated as systolic indexes. Myocardial early diastolic (Em) and atrial diastolic (Am) peak velocities (m/s), Em:Am ratio and regional relaxation time (min; the time interval between the end of Sm and the onset of Em) were determined as diastolic measurements.25,26
Strain rate imaging
Strain rate digital data were acquired in real time, with a frame rate >160 frames/s, and transferred to magneto‐optical discs for off‐line analysis, applying the software incorporated in the ultrasound system. This allowed determination of the strain rate, and the strain of a selected sample volume for each instant during three cardiac cycles. In the apical four‐chamber view, in accordance with previous reports, variations in longitudinal strain rate (1/s) and integrated strain (%) of two segments (basal and middle) of both interventricular septum and left ventricular lateral wall were analysed simultaneously by off‐line analysis of DMI data, and displayed as spectral tracings. To analyse strain rate variables, the strain rate calculation length was set to 10 mm. The strain rate corresponded to the local spatial velocity gradient, and the regional strain was obtained from the integration of the strain rate curves.27,28
Statistical methods
All the analyses were performed by SPSS for Windows release V.11.0. Variables are presented as mean (SD). Analyses of variance by Newman–Keuls post hoc test for multiple comparisons, and t test for unpaired data were performed to estimate differences between groups. Reproducibility of measuring the DMI parameters was determined in 20 participants (10 athletes and 10 controls) by using both Pearson's bivariate two‐tailed correlations and Bland–Altman analysis. Relation coefficients, 95% confidence limits and percentage errors were reported.
Stepwise‐forward, multiple regression analyses were performed to weigh the independent effects of potential determinants not obviously related to each other on a dependent variable. Differences were significant at p<0.05.
Results
Clinical characteristics of the study population
Table 1 shows the clinical characteristics of the study population. The three groups were matched for age. In accordance with the effects of the training, in athletes, systolic blood pressure and body surface area were higher, and heart rate was lower than in controls.
Table 1 Characteristics of the study population.
| Variable | Controls (n = 25) | Users (n = 20) | Non‐users (n = 25) |
|---|---|---|---|
| Age (years) | 33.42.2 | 34.83.2 | 34.23.4 |
| BSA (m2) | 1.880.4 | 2.180.6* | 2.120.5** |
| HR (beats/min) | 78.97.9 | 699.9* | 66.14.4** |
| Systolic BP (mm Hg) | 125.96.1 | 139.97.1* | 130.89.1 |
| Diastolic BP (mm Hg) | 84.73 | 83.94 | 80.74.9 |
BP, blood pressure; BSA, body surface area; HR, heart rate.
Values are mean (SD).
* p<0.01: users versus controls.
**p<0.01: non‐users versus controls.
Standard Doppler echocardiographic analysis
Table 2 shows the details of the standard Doppler echocardiographic analysis. Left ventricular mass index did not significantly differ between the two groups of athletes. In particular, both users and non‐users showed increased wall thickness and stroke volume compared with controls, whereas left ventricular ejection fraction, left ventricular end‐diastolic diameter and transmitral Doppler indexes were comparable between the three groups. Of note, circumferential end‐systolic stress was comparable between users and non‐users.
Table 2 Standard Doppler echocardiographic quantitative analysis.
| Variable | Controls (n = 25) | Users (n = 20) | Non‐users (n = 25) |
|---|---|---|---|
| M‐mode echocardiography | |||
| Septal wall thickness (mm) | 9.31.1 | 12.31.3* | 11.22.1** |
| Posterior wall thickness (mm) | 8.42.1 | 11.81.4* | 10.42.1** |
| LV end‐diastolic diameter (mm) | 47.44.7 | 48.23.5 | 47.44.7 |
| LV end‐systolic diameter (mm) | 28.22.9 | 26.84.1 | 29.32.9 |
| Relative diastolic wall thickness | 0.430.04 | 0.450.06 | 0.460.04 |
| Endocardial fractional shortening (%) | 36.73.7 | 38.12.8 | 38.74.7 |
| LV mass index (g/m2.7) | 48.45.9 | 68.28.7* | 63.49.9** |
| LV ESSc (g/cm2) | 96.110.2 | 118.616.5* | 113.115.2* |
| RV end‐diastolic diameter (mm) | 21.22.8 | 21.42.2 | 20.23.8 |
| Standard Doppler analysis | |||
| LV stroke volume (ml) | 71.43.2 | 80.44.3* | 83.16.2** |
| Mitral peak E velocity m/s) | 0.740.17 | 0.770.17 | 0.780.12 |
| Mitral peak A velocity (m/s) | 0.530.14 | 0.490.14 | 0.530.12 |
| Mitral peak E:A ratio | 1.50.5 | 1.50.8 | 1.60.5 |
| Mitral E deceleration time (ms) | 151.317.7 | 159.317.7 | 160.616.3 |
| Mitral isovolumic relaxation time (ms) | 82.313.4 | 78.316.6 | 85.820.4 |
ESSc, circumferential end‐systolic stress; LV, Left ventricular; RV, right ventricular.
Values are mean (SD).
*p<0.001: users versus controls.
**p<0.01: non‐users versus controls.
†p<0.01 users versus non‐users.
DMI and SRI analyses
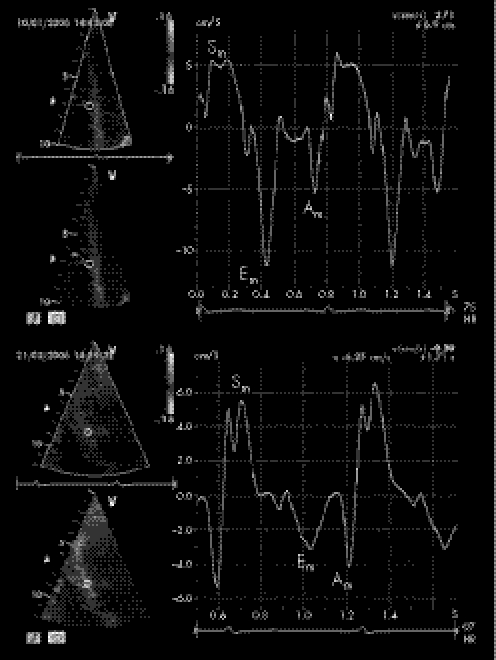
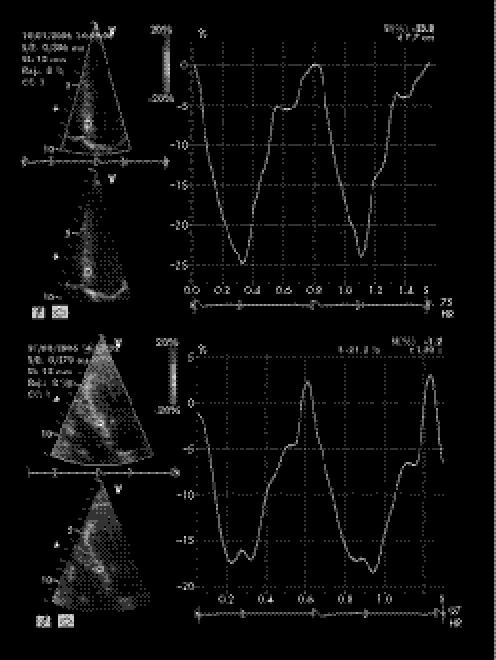
Colour DMI analysis showed significantly lower Em:Am ratios in users at the level of both the basal IVS and left ventricular lateral wall (both p<0.01), in comparison with non‐users (tables 3 and 4). In addition, in users, peak systolic left ventricular strain rate and strain were both reduced in the middle IVS and in left ventricular lateral free wall. Figures 1 and 2 show colour DMI pattern and peak systolic strain of IVS in users (top) and non‐users (bottom).
Table 3 Doppler myocardial imaging assessment of left ventricular lateral wall and interventricular septum.
| Variable | Controls (n = 25) | Users (n = 20) | Non‐users (n = 25) | |||
|---|---|---|---|---|---|---|
| LV lateral wall | ||||||
| Sm peak (m/s) | 0.09 (0.04) | 0.10 (0.04) | 0.11 (0.03) | |||
| PCTm (ms) | 108.6 (13.4) | 100.6 (18.4) | 105.2 (19.7) | |||
| CTm (ms) | 253.8 (21.2) | 254.8 (37.2) | 268.7 (13.4) | |||
| Em peak (m/s) | 0.09 (0.02) | 0.07 (0.02) | 0.14 (0.04)† | |||
| Am peak (m/s) | 0.08 (0.02) | 0.11 (0.02) | 0.12 (0.02) | |||
| Em/Am ratio | 1.1 (0.4) | 0.9 (0.4)** | 1.3 (0.3) | |||
| RTm (ms) | 78.2 (21.2) | 77.2 (21.2) | 78.3 (12.2) | |||
| Interventricular septal wall | ||||||
| Sm peak (m/s) | 0.11 (0.02) | 0.11 (0.03) | 0.12 (0.02) | |||
| PCTm (ms) | 102.5 (13.1) | 101.5 (22.1) | 105.6 (18.2) | |||
| CTm (ms) | 269.2 (10.4) | 275.3 (15.4) | 278.4 (16.1) | |||
| Em peak (m/s) | 0.11 (0.3) | 0.09 (0.3) | 0.15 (0.05)† | |||
| Am peak (m/s) | 0.08 (0.02) | 0.10 (0.02) | 0.10 (0.02) | |||
| Em:Am ratio | 1.3 (0.7) | 0.9 (0.8)* | 1.6 (0.9) | |||
| RTm (ms) | 74.7 (15.1) | 75.7 (15.1) | 76.2 (13.1) | |||
Am, myocardial atrial diastolic wave; CTm, myocardial contraction time; Em, myocardial early diastolic wave; PCTm, myocardial pre‐contraction time; RTm, myocardial relaxation time; Sm, myocardial systolic peak velocity.
†p<0.001: non‐users versus controls and users.
*P<0.001: users versus controls and non‐users.
**P<0.01: users versus controls and non‐users.
Table 4 Strain rate and strain analysis of left ventricular lateral free wall and interventricular septal wall.
| Segment | SR (1/s) | Strain (%) | ||||
|---|---|---|---|---|---|---|
| Controls (n = 25) | Users (n = 20) | Non‐users (n = 25) | Controls | Users | Non‐users | |
| Left ventricular lateral free wall | ||||||
| Basal | −1.8 (0.4) | −1.6 (−0.3)* | −2 (0.4) | −19 (6) | −15 (5)* | −20 (6) |
| Middle | −1.8 (0.2) | −1.1 (0.3)* | −1.9 (0.2) | −24 (4) | −16 (4)* | −26 (4) |
| Interventricular septal wall | ||||||
| Basal | −1.9 (0.4) | −0.76 (0.3)** | −2 (0.4) | −18 (4) | −8 (5)** | −19 (4) |
| Middle | −2 (0.3) | −0.74 (0.3)** | −2.1 (0.3) | −22 (5) | −15 (4)** | −25 (5) |
SR, strain rate.
Values are mean (SD).
*p<0.01: users versus controls and non‐users.
**p<0.001: users versus controls and non‐users.
Figure 1 Colour reconstructed pulsed‐wave Doppler myocardial imaging pattern with the sample volume placed at the level of basal segments of the interventricular septum in power athletes (top, anabolic androgenic steroid users and bottom, non‐users). Sm, myocardial systolic wave; Em, myocardial early diastolic wave; Am, myocardial atrial diastolic wave.
Figure 2 Peak systolic strain of middle interventricular septum in power athletes (top, anabolic androgenic steroid users and bottom, non‐users.
Reproducibility of DMI and SRI measurements
Interobserver variability
Pearson's correlation showed: DMI IVS Em peak: r = 0.98; p<0.001; middle IVS systolic strain: r = 0.92; p<0.001. Bland–Altman analysis showed: Em (95% CI −1.2 to +1.2; percentage error 3.1%), middle IVS systolic strain (95% CI −3.5 to +3.5; percentage error 4.6%).
Intraobserver variability
Pearson's correlation showed: DMI IVS Em: r = 0.98; p<0.001; middle IVS systolic strain: r = 0.93; p<0.001.
Bland–Altman analysis showed: Em (95% CI−1 to +1; percentage error 2.3%), middle IVS systolic strain (95% CI−3.1 to +3.1; percentage error 4.3%).
Bicycle ergometric test
During maximal physical effort, athletes showed a better functional capacity, with greater maximal workload achieved with lower maximal heart rate and systolic blood pressure (table 5).
Table 5 Functional parameters of athletes during physical effort.
| Variable | Users | Non‐users | p Value |
|---|---|---|---|
| Bicycle ergometer | |||
| Maximal HR (beats/min) | 177.9 (12.3) | 178.9 (13.3) | NS |
| Maximal SBP (mm Hg) | 179.44 (21.2) | 168.15 (24.2) | <0.01 |
| Rate–pressure product (beats/min×mm Hg×10−3) | 30.3 (2.3) | 29.5 (3.3) | <0.01 |
| Maximal workload achieved (W) | 168.21 (34.7) | 200.13 (36.4) | <0.001 |
HR, heart rate; SBP, systolic blood pressure.
Values are mean SD.
Relationships of DMI indexes
Separate multiple linear regression analyses were carried out after adjusting for potential confounders such as age, body surface area, heart rate, systolic blood pressure, circumferential end‐systolic stress, Doppler indexes and left ventricular mass index. By stepwise forward multivariate analyses, the sum of left ventricular wall thickness (β coefficient = −0.32, p<0.01), the number of weeks of AAS use per year (β = −0.42, p<0.001) and the weekly dosage of AAS (β = −0.48, p<0.001) were the only independent determinants of middle IVS strain rate. In addition, impaired left ventricular strain in users was associated with reduced performance during physical effort (p<0.001).
Discussion
Long‐term power training is a potent cardiac hypertrophic stimulus. In previous reports, top‐level strength‐trained athletes, mainly involved in static isometric anaerobic exercise, showed increased left ventricular wall thickness and relative wall thickness, with a pattern of left ventricular concentric geometry caused by pressure overload typical of this kind of effort.1,2,3,4,5,6
However, power athletes are increasingly exposing themselves to supraphysiological doses of AAS. These are known to increase skeletal muscle mass and strength—effects that form the basis for their administration to enhance athletic performance. A variety of AAS are often taken simultaneously (so‐called “stacking”), and in doses that result in 10–100‐fold increases in androgen concentrations.10,11
This study shows for the first time the usefulness of DMI and SRI, two non‐invasive techniques, to detect underlying subclinical left ventricular myocardial dysfunction after chronic misuse of AAS in power athletes. The main findings are that: (1) despite normal standard echo parameters, lower myocardial early diastolic peak velocities and impaired systolic deformation indexes were observed at the levels of both left ventricular lateral wall and IVS in AAS users; (2) the number of weeks of AAS use per year and the weekly dosage of AAS were independent determinants of impaired IVS strain rate; and (3) impaired left ventricular strain in users was associated with a reduced performance during physical effort.
Previous reports on the cardiac effects of AAS
Some experimental in vitro studies indicated an adverse effect of AAS on the cardiovascular system. The myocardial cellular destruction of primary myocardial cells in culture treated with AAS was associated with depressed contractile activity, increased lysosomal fragility, and depressed mitochondrial activity.15,16
Additionally, in vivo studies on animal models, performed to show the effects of AAS on the cardiovascular system, detected (1) myocardial structural changes, and (2) cardiac hypertrophy, with both a reduction of response to an inotropic load and an impairment of left ventricular compliance.12,13,14
However, the effects of chronic misuse of AAS on the athlete's ventricular performance are still under investigation. In fact, some authors have not found any left ventricular functional change in athletes under pharmacological efects of AAS, whereas others have found an initial impairment of the left ventricular diastolic function with an enlargement of the left ventricular chamber and an increase in the thickness of the left ventricular wall.17,18,19,20,21,22,23,24
As for athletes' myocardial function, Di Bello et al21 were the first authors to analyse regional function in AAS users by ultrasonic myocardial backscatter. They showed that the cyclic variation index of the myocardial echo amplitude of the septum and of the left ventricular posterior wall in weightlifters clear of drugs was comparable with that of normal subjects, whereas weightlifters who had taken drugs showed a considerably lower cycle variation index at both the septum and left ventricular posterior wall levels.21
Effects of AAS on an athlete's myocardium
DMI analysis
This study emphasises early left ventricular myocardial diastolic dysfunction in AAS users, as significantly lower early diastolic peak velocities were observed at both the IVS and lateral wall levels, despite normal Doppler measurements. As for regional systolic function, AAS users showed normal left ventricular systolic peak velocities by pulsed DMI.
A previous report showed that early diastolic regional velocities evaluated by DMI are directly dependent on myocardial structure, characterised by the percentage of interstitial fibrosis and the myocardial β‐adrenoreceptor density assessed by endomyocardial biopsy.37 In particular, in our experience, we have already detected by DMI a reduction in myocardial early diastolic peak velocities in several abnormal conditions involving the left ventricle, in relation to either increasing load or left ventricular intrinsic myocardial dysfunction.25,29
Such impairment of left ventricular diastolic myocardial function is in strict accordance with the hypothetical models of AAS‐induced adverse cardiovascular effects previously proposed according to basic research: the atherogenic, the thrombothic, the coronary vasospastic and the direct injury models. In fact, there is evidence that an advanced degree of left ventricular hypertrophy in users is accompanied by a disproportionate increase in the connective tissue content as a repair process of the myocardial cellular injury caused by AAS.10 AAS are likely to share such influences on the left ventricular hypertrophic response through actions on the androgen receptor, an almost ubiquitously expressed transcriptional regulator, found in skeletal muscle cells and on cardiac myocytes.16 In addition, being structurally similar to aldosterone, AAS can also cause sodium and water retention, with a consequent increase in blood volume and pressure, and can constitute a potent stimulus on myocardial fibroblastic growth and collagen production.8,9
SRI analysis
Using SRI analysis, a spatial map of longitudinal deformation within the myocardium can be obtained, which can be used for a rapid visual evaluation of local myocardial function and to obtain quantitative information. In addition, this technique overcomes several limitations inherent in measuring regional velocity profiles, because is not influenced by global cardiac displacement and the tethering effects of adjacent segments.27,28
What is already known on this topic
The effects of chronic consumption of anabolic androgenic steroids on human performance and on cardiovascular structures are still a controversial issue.
On the one hand, some authors have found no functional changes of the left ventricle, but others have found an initial impairment of the left ventricular diastolic function with an enlargement of the left ventricular chamber and an increase in the thickness of the ventricular walls.
What this study adds
This study shows for the first time the usefulness of Doppler myocardial imaging and strain rate imaging, two non‐invasive techniques, to detect underlying sub‐clinical left ventricular myocardial dysfunction after chronic misuse of anabolic androgenic steroid in power athletes.
-
The main findings are that:
-
-
Despite normal standard echo parameters, lower myocardial early diastolic peak velocities and impaired systolic deformation indexes were observed at the level of both left ventricular lateral wall and interventricular septum in AAS users;
-
-
The number of weeks of AAS use per year and the weekly dosage of AAS were independent determinants of impaired strain rate.
-
-
In our population of AAS users, despite normal left ventricular ejection fraction and DMI systolic peak velocities, a significant reduction in strain and strain rate indexes at the level of both IVS and left ventricular lateral free walls was detected. Even if the reduction of peak systolic strain is the consequence of change in ventricular afterload or preload, the concomitant reduction of peak strain rate also points out early impairment of left ventricular myocardial contractile function in users, at a time when other global and regional systolic parameters remain normal. Therefore, our findings confirm that SRI is a feasible technique that allows evaluation of ventricular regional wall motion with higher sensitivity than DMI and conventional ultrasound.27,28
Limitations
Our study has several methodological limitations. Firstly, it was based on cross‐sectional rather than longitudinal data. It does not seem reasonable to suppose that power athletes choosing to take anabolic drugs have initial cardiac measurement results different from AAS‐free athletes. A longitudinal study using humans would also give rise to ethical problems.
Secondly, no histological determination of cardiac structure was available, but the use of this invasive technique was not ethically acceptable. No urine or serum measurements of drugs were available, but all athletes were in a wash‐out period, to avoid the acute pharmacological effects induced by AAS on the cardiocirculatory function.
Thirdly, information about the intake of steroids was self‐reported, but it is difficult to assess this in an objective manner. It seems unlikely that the small differences in AAS intake could explain our results.
Finally, training‐related influences are also improbable as an explanation for the differences between the AAS users and non‐users in our study, as the training protocol was the same for all the athletes.
Conclusions
Several years after chronic misuse of AAS, power athletes show a subclinical impairment of both systolic and diastolic myocardial function, strongly associated with mean dosage and duration of AAS use.
To date, the extent to which increased left ventricular muscle mass caused by AAS misuse represents a long‐term risk for cardiac complications is controversial. Previous authors reported the 12‐year mortality to be 12.9% in a group of 62 male powerlifters suspected of AAS use, compared with 3.1% in a control population.38
In light of the results presented in this study, DMI and SRI may be considered effective, non‐invasive and easy repeatable methods for detection of some early myocardial textural changes in AAS users, which could represent the onset of a specific cardiomyopathy. The combined use of DMI and SRI may therefore be useful for early identification of AAS users with more diffused cardiac involvement, and eventually for investigation of the reversibility of such myocardial effects after discontinuation of the drug.
Abbreviations
AAS - anabolic androgenic steroids
DMI - Doppler myocardial imaging
IVS - interventricular septum
SRI - strain rate imaging
Footnotes
Competing interests: None declared.
References
- 1.Huston T P, Puffer J C, Rodney W M. The athletic heart syndrome. N Engl J Med 1985424–32. [DOI] [PubMed] [Google Scholar]
- 2.Maron B J. Structural features of the athlete heart as defined by echocardiography. J Am Coll Cardiol 19867190–203. [DOI] [PubMed] [Google Scholar]
- 3.D'Andrea A, Limongelli G, Caso P.et al Association between left ventricular structure and cardiac performance during effort in two morphological forms of athlete's heart. Int J Cardiol 200286177–184. [DOI] [PubMed] [Google Scholar]
- 4.Pelliccia A, Culasso F, Di Paolo F.et al Physiologic left ventricular cavity dilation in elite athletes. Ann Intern Med 199913023–31. [DOI] [PubMed] [Google Scholar]
- 5.Di Bello V, Lattanzi F, Picano E.et al Left ventricular performance and ultrasonic myocardial quantitative reflectivity in endurance senior athletes: an echocardiographic study. Eur Heart J 199314358–363. [DOI] [PubMed] [Google Scholar]
- 6.Pluim B M, Zwinderman A H, van der Laarse A.et al The athlete's heart. A meta‐analysis of cardiac structure and function. Circulation 2000101336–342. [DOI] [PubMed] [Google Scholar]
- 7.Liu P Y, Death A K, Handelsman D J. Androgens and cardiovascular disease. Endocr Rev 200324313–340. [DOI] [PubMed] [Google Scholar]
- 8.Kuhn C M. Anabolic steroids. Recent Prog Horm Res 200257411–434. [DOI] [PubMed] [Google Scholar]
- 9.Delles C, Schmidt B M, Muller H J.et al Functional relevance of aldosterone for the determination of left ventricular mass. Am J Cardiol 200391297–301. [DOI] [PubMed] [Google Scholar]
- 10.Payne J R, Kotwinski P J, Montgomery H E. Cardiac effects of anabolic steroids. Heart 200490473–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wagman D F, Curry L A, Cook D L. An investigation into the anabolic androgenic steroid use by elite U.S. powerlifters. J Strength Cond Res 19959149–154. [Google Scholar]
- 12.Pesola M K. Reversibility of the haemodynamic effects of anabolic steroids in rats. Eur J Appl Physiol 198858125–131. [DOI] [PubMed] [Google Scholar]
- 13.Kinson G A, Layberry R A, Herbert B. Influences of anabolic androgens on cardiac growth and metabolism in the rat. Can J Physiol Pharmacol 1991691698–1704. [DOI] [PubMed] [Google Scholar]
- 14.Marsh J D, Lehmann M H, Ritchie R H.et al Androgen receptors mediate hypertrophy in cardiac myocytes. Circulation 199898256–261. [DOI] [PubMed] [Google Scholar]
- 15.Behrendt H, Boffin H. Myocardial cell lesions caused by anabolic hormone. Cell Tissue Res 1977181423–426. [DOI] [PubMed] [Google Scholar]
- 16.Rocha R, Funder J W. The pathophysiology of aldosterone in the cardiovascular system. Ann NY Acad Sci 200297089–100. [DOI] [PubMed] [Google Scholar]
- 17.Urhausen A, Albers T, Kindermann W. Are the cardiac effects of anabolic steroid abuse in strength athletes reversible? Heart 200490496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Piccoli B, Giada F, Benettin A.et al Anabolic steroid use in body builders: an echocardiographic study of left ventricle morphology and function. Int J Sports Med 199112408–412. [DOI] [PubMed] [Google Scholar]
- 19.Sachtleben T R, Berg K E, Elias A.et al The effects of anabolic steroids on myocardial structure and cardiovascular fitness. Med Sci Sports Exerc 1993251240–1245. [PubMed] [Google Scholar]
- 20.Deligiannis A, Zahapoulou E, Mandroukas K. Echocardiographic study of cardiac dimension and function in weight lifters and body builders. J Sports Cardiol 1988524–32. [Google Scholar]
- 21.Di Bello V, Girogi D, Bianchi M.et al Effects of anabolic‐androgenic steroids on weight‐lifters' myocardium: an ultrasonic videodensitometric study. Med Sci Sports Exerc 199931514–521. [DOI] [PubMed] [Google Scholar]
- 22.Dickerman R D, Schaller F, Zachariah N Y.et al Left ventricular size and function in elite bodybuilders using anabolic steroids. Clin J Sport Med 1997790–93. [DOI] [PubMed] [Google Scholar]
- 23.Salke R C, Rowland T W, Burke E J. Left ventricular size and function in body builders using anabolic steroids. Med Sci Sports Exerc 198517701–704. [DOI] [PubMed] [Google Scholar]
- 24.Thompson P D, Sadaniantz A, Cullinane E M.et al Left ventricular function is not impaired in weight‐lifters who use anabolic steroids. J Am Coll Cardiol 199219278–282. [DOI] [PubMed] [Google Scholar]
- 25.D'Andrea A, Zeppilli P, Caso P.et al Doppler myocardial imaging in the evaluation of the athlete's heart. Ital Heart J 20034635–644. [PubMed] [Google Scholar]
- 26.Hatle L, Sutherland G R. Regional myocardial function—a new approach. Eur Heart J 2000211337–1357. [DOI] [PubMed] [Google Scholar]
- 27.Sutherland G R, Di Salvo G, Claus P.et al Strain and strain rate imaging: a new clinical approach to quantifying regional function. J Am Soc Echocardiogr 200417788–802. [DOI] [PubMed] [Google Scholar]
- 28.Castro P L, Greenberg N L, Drinko J.et al Potential pitfalls of strain rate imaging: angle dependency. Biomed Sci Instrum 200036197–202. [PubMed] [Google Scholar]
- 29.D'Andrea A, Caso P, Severino S.et al Prognostic value of intra‐left ventricular myocardial delay in young patients with mild hypertrophic cardiomyopathy compared to power athletes. Eur Heart J 200526755–761.15673543 [Google Scholar]
- 30.D'Andrea A, Caso P, Galderisi M.et al Assessment of myocardial response to physical exercise in endurance competitive athletes by pulsed Doppler tissue imaging. Am J Cardiol 2001871226–1230. [DOI] [PubMed] [Google Scholar]
- 31.D'Andrea A, Caso P, Severino S.et al Effects of different training protocols on left ventricular myocardial function in competitive athletes: a Doppler tissue imaging study. Ital Heart J 2002334–40. [PubMed] [Google Scholar]
- 32.Caso P, D'Andrea A, Galderisi M.et al Pulsed Doppler tissue imaging in endurance athletes: relation between left ventricular preload and myocardial regional diastolic function. Am J Cardiol 2000851131–1136. [DOI] [PubMed] [Google Scholar]
- 33.de Simone G, Daniels S R, Devereux R B.et al Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol 1992201251–1260. [DOI] [PubMed] [Google Scholar]
- 34.Gaasch W H, Zile M R, Hosino P K.et al Stress‐shortening relations and myocardial blood flow in compensated and failing canine hearts with pressure‐overload hypertrophy. Circulation 198979872–873. [DOI] [PubMed] [Google Scholar]
- 35.Dubin J, Wallerson D C, Cody R J.et al Comparative accuracy of Doppler echocardiographic methods for clinical stroke volume determination. Am Heart J 1990120116–123. [DOI] [PubMed] [Google Scholar]
- 36.Appleton C P, Hatle L A, Popp R L. Relation of transmitral flow velocity patterns to left ventricular diastolic function: new insights from a combined hemodynamic and Doppler echocardiographic study. J Am Coll Cardiol 198812426–440. [DOI] [PubMed] [Google Scholar]
- 37.Shan K, Bick R J, Poindexter B J.et al Relation of tissue Doppler derived myocardial velocities to myocardial structure and beta‐adrenergic receptor density in humans. J Am Coll Cardiol 200036891–896. [DOI] [PubMed] [Google Scholar]
- 38.Parssinen M, Seppala T. Steroid use and long‐term health risks in former athletes. Sports Med 20023283–94. [DOI] [PubMed] [Google Scholar]