Abstract
An ingestible telemetric temperature sensor for measuring body core temperature (Tc) was first described 45 years ago, although the method has only recently gained widespread use for exercise applications. This review aims to (1) use Bland and Altman's limits of agreement (LoA) method as a basis for quantitatively reviewing the agreement between intestinal sensor temperature (Tintestinal), oesophageal temperature (Toesophageal) and rectal temperature (Trectal) across numerous previously published validation studies; (2) review factors that may affect agreement; and (3) review the application of this technology in field‐based exercise studies. The agreement between Tintestinal and Toesophageal is suggested to meet our delimitation for an acceptable level of agreement (ie, systematic bias <0.1°C and 95% LoA within ±0.4°C). The agreement between Tintestinal and Trectal shows a significant systematic bias >0.1°C, although the 95% LoA is acceptable. Tintestinal responds less rapidly than Toesophageal at the start or cessation of exercise or to a change in exercise intensity, but more rapidly than Trectal. When using this technology, care should be taken to ensure adequate control over sensor calibration and data correction, timing of ingestion and electromagnetic interference. The ingestible sensor has been applied successfully in numerous sport and occupational applications such as the continuous measurement of Tc in deep sea saturation divers, distance runners and soldiers undertaking sustained military training exercises. It is concluded that the ingestible telemetric temperature sensor represents a valid index of Tc and shows excellent utility for ambulatory field‐based applications.
Body core temperature (Tc) measurement is fundamental to the study of human temperature regulation during exercise.1 Core temperature is implicated in heat‐ and cold‐related illnesses2,3,4 and can influence exercise performance.5 Yet, the term “core” does not describe a specific anatomical location, and no single regional internal temperature provides an index of the average internal body temperature; the body interior is not at one uniform temperature and the thermoregulatory centre receives temperature inputs from many internal sites.1,6 The temperature of blood in the pulmonary artery is considered the best representation of the average internal temperature of the body as the mixed venous blood has returned from both the core and periphery and is almost identical to arterial blood.6,7 As this site is not accessible, Tc is often measured at the oesophagus, rectum, mouth or external auditory meatus/tympanic membrane.1,6,7 An ideal site for Tc measurement is one that is convenient, unaffected by environmental conditions, responds rapidly and quantitatively reflects small changes in central blood temperature.1 Oesophageal temperature (Toesophageal) at the level of the left atrium provides the closest agreement with central blood and is considered the best available index of Tc for exercise studies.1,6,7 Rectal temperature (Trectal) is the most widely used index of Tc in exercise studies, yet its slow response to changes in exercise intensity and central blood temperature means that Trectal is only considered an acceptable index of Tc during steady‐state conditions.1,6,7
An alternative method of Tc measurement, particularly suited to field‐based ambulatory applications, is the ingestible telemetric temperature “pill” or “capsule”. An ingestible “radio pill” was first described >45 years ago,8 with technological modifications on this theme continuing to the present.9,10,11 The sensor is ingested and transmits a temperature signal, relative to the surrounding gastrointestinal temperature (Tintestinal), by radio wave to an external receiver for data logging or instant display. Presently, there are two commercially available ingestible temperature sensor and receiver systems: (1) CorTemp (weight = 2.75 g, length = 23 mm, diameter = 10.25 mm) and external ambulatory data receiver (CorTemp HQ Inc., Palmetto, Florida, USA); and (2) VitalSense ingestible telemetric temperature sensor (weight = 1.75 g, length = 21.9 mm, diameter = 8.5 mm) and external ambulatory data receiver (VitalSense, Mini Mitter Co., Inc., Bend, Oregon, USA).
A number of small studies (ie, sample sizes ranging from 4 to 11) have now investigated the validity of the ingestible sensor as an index of Tc and the method is gaining widespread use, particularly in field‐based studies. Therefore, we aim to use Bland and Altman's limits of agreement (LoA) method as a basis for quantitatively reviewing the agreement between Tintestinal, Toesophageal and Trectal across numerous previously published validation studies. Our objective is to provide a clear overview of the results of the numerous validation studies and establish the existence of any consistent differences between measurement sites. We further aim to review factors that may affect agreement and also to review the application of this technology in field‐based studies.
Methods
Ten peer reviewed full publications9,11,12,13,14,15,16,17,18,19 and two abstract publications20,21 comparing Tintestinal with Toesophageal and/or Trectal were reviewed (table 1). LoA was selected as the most appropriate statistical method for assessing agreement between a new measurement technique (eg, ingestible telemetric temperature sensor) and an established technique (eg, oesophageal or rectal probe).22,23 Although only two of the validation studies in table 1 used the LoA method,18,19 individual data were available from three further studies,13,14,17 allowing calculation of LoA for 5 of the 12 validation studies, and allowing a standardised quantitative comparison of agreement across these studies. The method provides a measure of systematic bias (ie, general trend for measurements to be different in a positive or negative direction), evidence of heteroscedasticity (ie, whether differences are related to the magnitude of the measurements) and a 95% random error component (ie, boundaries accounting for 95% of differences between methods).22,23 Thus, LoA represents the largest difference between methods that can be expected for most (ie, 95%) individuals in the studied population. In this review, LoA data form the basis for assessing the level of agreement between Tintestinal, Toesophageal or Trectal before the data are evaluated in the context of findings from the remaining validation studies and conclusions are drawn regarding the relationship of Tintestinal to that of Toesophageal and Trectal. Nine further studies using ingestible sensors in sports or occupational settings involving physical activity were reviewed to assess the utility of the technology for field‐based exercise applications.24,25,26,27,28,29,30,31,32
Table 1 Summary of studies comparing the agreement between core temperature measurements recorded simultaneously from an ingestible telemetric temperature sensor and an oesophageal and/or rectal probe.
| Authors | Comparison | Calibration | Ingestion | Sample | Protocol | Environment | Analysis | Valid |
|---|---|---|---|---|---|---|---|---|
| Gibson et al9 | Oesophageal, rectal | Yes, method NS | 30 min | 7 M | Resting immersion, cycling | W = 41 and 10°C (rest), DB = 25°C (cycling) | Regression, RMSD | Yes |
| Fox et al20 | Oesophageal, rectal | Water bath method NS | NS | NS | Rest, immersion, exercise | DB = NS, W = NS | NS | Yes |
| Livingstone et al12 | Oesophageal, rectal | NS | NS | 5 M | Rest, walking | DB = 24–26°C (rest); DB = −32°C, V = 11 km/h (rest, walking) | NS | Yes |
| Kolka et al13 | Oesophageal, rectal | NS | 2 (0.5) h | 8 M | Cycling | DB = 29.5°C | ANOVA | No |
| Sparling et al14 | Rectal | Water bath, corrections applied | 3–4, 8–9 h | 6 M | Running, cycling | DB = 20.8°C, RH = 56% | t tests | No |
| Kolka et al15 | Oesophageal | Water bath, inaccurate sensors eliminated | 2 (0.5) h | 4 F | Walking | DB = 30°C | Regression | Yes |
| O'Brien et al16 | Oesophageal, rectal | Water bath, regression equation applied | 12 h | 9 (5 F, 4M) | Resting immersion, cycling immersion | W = 18 and 36°C | ANOVA, RMSD | Yes |
| Lee et al17 | Oesophageal, rectal | Water bath, regression equation applied | 6 h | 7 (2F, 5M) | Rest, cycling | NS | ANOVA | Yes |
| Ducharme et al21 | Rectal | NS | 40 min | 11 M | 36 h rest, walking, running | DB = 30°C, RH = 50% | NS | Yes |
| Edwards et al18 | Rectal | NS | 1 h before sleep | 8 M | Circadian monitoring | NS | Correlation, cosinor, LoA | Yes |
| McKenzie and Osgood11 | Rectal | Yes, method NS | NS | 10 (4F, 6M) | Circadian monitoring | NS | Regression, ANOVA | Yes |
| Gant et al19 | Rectal | Water bath, method NS | 10 h | 10 M, 9 M | Intermittent shuttle running | NS | ANOVA, LoA, ICC, CV | Yes |
ANOVA, analysis of variance; CV, coefficient of variation; DB, dry bulb air temperature; F, female; ICC, intraclass correlation coefficient; LoA, limits of agreement; M, male; NS, information not stated; RH, % relative humidity; RMSD, root mean squared deviation; V, km/h wind velocity; W, water temperature.
Values are mean (SD) or range. Comparison, method(s) against which intestinal temperature compared.
Ingestion, timing of sensor ingestion in hours or minutes before start of exercise or data collection.
Validity, decision of authors regarding acceptability of intestinal temperature as a valid index of TC.
Systems used: Custom‐made ingestible temperature‐sensing radio pill and receiver system developed by the National Institute for Medical Research, UK.20 Custom‐made ingestible temperature sensing radio pill and receiver system developed by the Royal Air Force Institute of Aviation Medicine, UK.9 Ingestible temperature‐sensing radio pill and receiver system of unknown origin.12 Commercially available CorTemp system consisting of ingestible telemetric temperature sensor and data recorder (HQ Inc., Palmetto, Florida, USA) developed by the Applied Physics Laboratory, Johns Hopkins University and the National Aeronautics and Space Administration, USA.13,15,17,19 System consisting of CorTemp ingestible telemetric temperature sensor (HQ Inc.) and compact data receiver/logger (BBN Systems and Technologies, Massachusetts, USA).16 Commercially available VitalSense system consisting of Jonah ingestible temperature sensor and telemetric monitor (Mini Mitter Co., Inc., Bend, Oregon, USA).11 System used not stated.21
From the outset, it should be noted that surprisingly few validation studies have delimited an acceptable level of agreement between methods. Gant et al19 delimited a systematic bias of >0.1°C between Tintestinal and Trectal as being practically important in affecting decisions made on an individual's thermal status. Furthermore, these authors stated that 95% of the differences between methods should fall within ±0.3°C.19 Our review indicates the lowest values for 95% LoA to be ±0.37°C for Tintestinal−Toesophageal17 and ±0.22°C for Tintestinal and Trectal.19 Therefore, we delimit an acceptable level of agreement as a bias <0.1°C and 95% LoA within ±0.4°C.
Validity and reliability of the ingestible temperature sensor
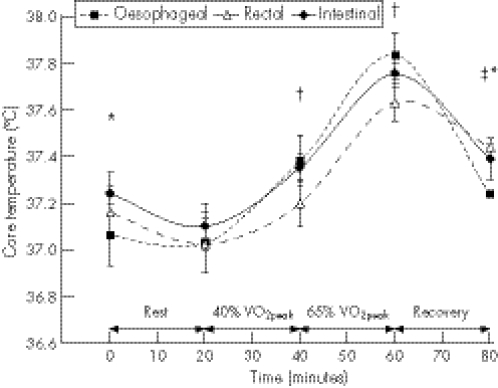
Table 1 shows that 10 of the 12 validation studies (83%) report levels of agreement supporting the conclusion that Tintestinal provides a valid index of Tc.9,11,15,16,17,18,19,20,21 Comparisons have ranged in duration from acute, lasting up to 3 h,17,19,20 to long‐term, lasting for 24 h,18 36 h21 and up to 136 h.11 Agreement has been assessed by simultaneous comparisons of Tc magnitude at discrete time points within a protocol12,13,14,17 and/or by a comparison of Tc responses encompassing the whole protocol.9,11,15,16,18,19 For example, in the study of Lee et al17 shown in figure 1, Tintestinal, Toesophageal and Trectal were compared at several discrete time points throughout an experimental protocol—that is, before and after 20 min of rest, after 20 min of cycling at 40% peak oxygen consumption (VO2peak), after 20 min of cycling at 65% VO2peak, and after 20 min of passive recovery. Several studies have also compared intestinal, oesophageal and rectal sites for their responsiveness (eg, time for a 0.1°C change) and/or rate of change (eg, °C/min) at the start or cessation of exercise or in response to a change in exercise intensity.9,13,17,19
Figure 1 Oesophageal, rectal and intestinal temperatures measured simultaneously during rest, submaximal supine cycling exercise at 40% peak oxygen consumption (VO2peak) and 65% VO2peak, and during passive recovery. Values are mean (SE). *Intestinal temperature significantly higher than oesophageal temperature (p<0.05), †rectal temperature significantly lower than oesophageal temperature (p<0.05), ‡rectal temperature significantly higher than oesophageal temperature (p<0.05). Redrawn from the data of Lee et al17
Oesophageal versus intestinal temperature
Seven studies in table 1 compared Toesophageal with Tintestinal, although LoA data were available for only two studies.13,17 The data of Kolka et al13 showed 95% of Toesophageal readings to fall 0.45°C below to 0.41°C above Tintestinal, similar to the data of Lee et al,18 which showed 95% LoA of −0.40°C to 0.34°C (table 2). Both study data show no significant (p>0.05) systematic bias in the relationship between Toesophageal and Tintestinal (ie, systematic bias of −0.02°C and −0.03°C, respectively). Thus, LoA data show no evidence of a consistent difference between Toesophageal and Tintestinal, with Toesophageal expected to fall within 0.45°C below to 0.41°C above Tintestinal for a new individual from the studied population. Despite our reanalysis of the data of Kolka et al13 showing agreement between Toesophageal and Tintestinal approaching our delimited acceptable level (i.e, <0.4°C), the authors rejected the use of Tintestinal for research purposes. They observed Tintestinal fluctuations of 0.2–0.3°C in two of their eight subjects and hypothesised that the changing anatomical location of the intestinal sensor during gastrointestinal transit could potentially confound the Toesophageal–Tintestinal relationship.
Table 2 Limits of agreement comparison of oesophageal, intestinal and rectal core temperature measurements.
| Reference | Oesophageal vs intestinal | Rectal vs intestinal | Oesophageal vs rectal | |||
|---|---|---|---|---|---|---|
| LoA | Bias ±95% | LoA | Bias ±95% | LoA | Bias ±95% | |
| Kolka et al13 | −0.45 to +0.41 | −0.02 (0.43) | −0.27 to +0.65 | +0.18 (0.47)* | −0.54 to +0.12 | −0.21 (0.33)*† |
| Sparling et al14 | +0.08 to +1.44 | +0.76 (0.68)* | ||||
| Lee et al17 | −0.40 to +0.34 | −0.03 (0.37) | −0.41 to +0.27 | −0.07 (0.34)* | −0.35 to +0.45 | +0.05 (0.40) |
| Edwards et al18‡ | −0.60 to +0.20 | −0.20 (0.40)* | ||||
| Gant et al19 | −0.37 to +0.07 | −0.15 (0.22)* | ||||
LoA, limits of agreement.
It would be expected with 95% probability that for a new individual from the studied population, the difference between two methods of core temperature measurement will fall within these limits.
Bias ±95% represents the mean difference between the two methods of measurement (eg, mean of oesophageal minus intestinal temperatures) and the SD of the differences multiplied by 1.96 represent 95% of differences.
*Significant systematic bias (p<0.05, identified by paired t test) between methods of measurement.
†Significant negative heteroscedasticity (p<0.05, identified by Pearson's product moment correlation coefficient).
‡Data represent the mesor of cosinor analysis—that is, mean of the oscillation over 24 h of circadian measurement.
Supporting the LoA data, Gibson et al9 observed no evidence of a statistically significant systematic bias between Toesophageal and Tintestinal at discrete time points during their experimental protocols, despite evident variability between the two temperatures sites. O'Brien et al16 observed no significant differences between Toesophageal and Tintestinal during 3 h resting and exercise experiments in cold water with overall root mean‐squared deviations (RMSDs) of 0.23°C (0.04)°C and 0.24°C (0.02)°C, respectively. However, during resting and exercise experiments in warm water, Tintestinal was consistently and significantly higher than Toesophageal, although the RMSD (ie, 0.25°C (0.05)°C and 0.26°C (0.03)°C, respectively) remained similar to those observed during the experiments in cold water. Livingstone et al12 also reported Tintestinal to be consistently higher (<0.5°C) than Toesophageal during 90 min protocols of rest in neutral and cold environments, and low‐intensity walking in a cold environment. However, the authors did not report whether this consistent bias was statistically significant. In summary, an acceptable level of agreement has been concluded in six of the seven studies comparing Toesophageal and Tintestinal at discrete time points or when comparing responses over a complete experiment. Level of agreement data and data from varied statistical analyses show variability within ±0.5°C with a tendency for Tintestinal to be higher than Toesophageal. The lowest level of agreement was ±0.37°C with no significant bias, which is within our acceptable limits.
Responsiveness of Toesophageal versus Tintestinal
Table 3 shows the lower rate of change (°C/min) and concomitant longer duration for Tintestinal to achieve a 0.1°C threshold change in temperature than Toesophageal at the onset or cessation of exercise.13,17 Without exception, the data in table 3 show a slower response of Tintestinal versus Toesophageal to changes in exercise intensity; however, differences between the two sites do not always reach statistical significance.
Table 3 A comparison of the time course and rate of core temperature change at the onset or cessation of exercise when measured at oesophageal, intestinal and rectal sites.
| Oesophageal | Intestinal | Rectal | |
|---|---|---|---|
| Time for 0.1°C change from start of exercise (min) | |||
| Kolka et al,13 40% VO2peak | 4.4 (2.7)* | 7.5 (4.8)† | 12.3 (3) |
| Kolka et al,13 80% VO2peak | 1.8 (0.8)* | 3.8 (1.5)† | >5.0 (0) |
| Lee et al,17 40% VO2peak | 10 (1.1) | 14 (1.2) | 15.7 (1.6)‡ |
| Rate of change during exercise (°C/min) | |||
| Kolka et al,14 40% VO2peak | 0.050 (0.013)* | 0.031 (0.014) | 0.018 (0.005) |
| Kolka et al,14 80% VO2peak | 0.112 (0.028)* | 0.066 (0.035)† | 0.018 (0.009) |
| Lee et al,17 40% VO2peak | 0.022 (0.005) | 0.021 (0.004) | 0.016 (0.004) |
| Time to steady‐state temperature during exercise (min) | |||
| Kolka et al,13 40% VO2peak | 18.0 (6.1) | 25.2 (9.1)† | 37.3 (4.6)‡ |
| Time for 0.1°C change from end of exercise (min) | |||
| Kolka et al,13 from 40% VO2peak | 2.3 (0.5)* | 6.5 (3.1)† | 12.2 (3.3) |
| Lee et al,17 from 65% VO2peak | 3.7 (0.4) | 7.1 (1.5) | 10.6 (1.9)‡ |
| Rate of change during recovery (°C/min) | |||
| Lee et al,17 from 65% VO2peak | −0.030 (0.002)* | −0.023 (0.003)† | −0.010 (0.003) |
Values are mean (SD).
*Toesophageal significantly different from Tintestinal and Trectal, p<0.05.
†Tintestinal significantly different from Trectal p<0.05.
‡Trectal significantly different from Toesophageal, p<0.05.
Rectal versus intestinal temperature
Of the 12 studies in table 1, 11 compared Trectal and Tintestinal, with 5 studies using Trectal as the sole criterion measure of Tc.11,14,18,19,21 LoA data were available from five studies,13,14,17,18,19 with each set of study data showing a statistically significant systematic bias between Trectal and Tintestinal (table 2). Mixed findings were revealed regarding the positive or negative direction of the bias. Whereas two earlier studies showed Trectal to read significantly (p<0.05) and consistently higher (ie, 0.18°C (0.47)°C and 0.76°C (0.68)°C) than Tintestinal,13,14 three later studies showed Trectal to read significantly (p<0.05) and consistently lower (ie, −0.07°C (0.34)°C, −0.20°C (0.40)°C and −0.15°C (0.22)°C) than Tintestinal.17,18,19 These consistent findings suggest a practically significant bias (ie, >0.1°C) between Trectal and Tintestinal, which needs to be accounted for when interpreting an individual's thermal status.19
The data of Kolka et al13 suggest that Trectal may range from 0.27°C below to 0.65°C above Tintestinal, whereas the data of Sparling et al14 suggest that Trectal may range from 0.08°C to 1.44°C above Tintestinal. Ducharme et al21 reported absolute differences between Trectal and Tintestinal of magnitude similar to the LoA data of Kolka et al13 during periods of 2 h walking and running exercise in the heat. Rectal temperature was significantly higher than Tintestinal by 0.24 (0.10)°C during exercise, and although a similar trend remained during periods of rest, the absolute difference (ie, 0.12°C (0.09)°C) did not reach significance (p = 0.065). The data of Sparling et al14 suggested a temperature gradient of large magnitude along the gastrointestinal tract, as the six subjects involved in this study exhibited consistently higher Trectal than Tintestinal at rest (0.59°C), during exercise (0.93°C) and during recovery (1.1°C). The only support for Trectal−Tintestinal differences of this magnitude from the remaining literature is the observation of McKenzie and Osgood,11 who reported a Trectal 1.79°C higher than the corresponding Tintestinal in a single subject undertaking strenuous exercise in a 36°C environment. By contrast, the latter three studies in table 2 combined, suggest that Trectal may range from 0.60°C below to 0.27°C above Tintestinal.17,18,19 For example, Gant et al19 observed Tintestinal to be consistently and significantly higher than Trectal on average by 0.15°C throughout the 60 min of intermittent high‐intensity free shuttle running. Such findings are partially supported by O'Brien et al,16 who reported Trectal to be significantly (p<0.05) lower than Tintestinal on average by 0.43°C during one of their four experimental trials—that is, 3 h resting in cold water. However, under three other experimental conditions, no statistically significant differences between Trectal and Tintestinal were observed.16 Similarly, McKenzie and Osgood11 reported no statistically significant difference in the mean of Trectal (36.96°C (0.16)°C) and Tintestinal (36.93°C (0.15)°C) for 10 subjects over 48.6 (35.5) h.
In summary, LoA data indicate a statistically significant bias between Trectal and Tintestinal. The direction and magnitude of this bias has varied from study to study. Support for Tintestinal exceeding Trectal and vice versa is available from the remaining validation studies, as is support for no consistent bias between the two measurement sites. The non‐consistent Trectal–Tintestinal relationship is not reliably explained by experimental factors. Whether the differences represent a true physiological temperature gradient along the gastrointestinal tract remains to be confirmed and the factors affecting this gradient remain to be elucidated. Substantial evidence suggests that the Trectal–Tintestinal agreement is acceptable (ie, <0.4°C).
Responsiveness of Trectal versus Tintestinal
Table 3 shows the consistent finding that Tintestinal is more responsive than Trectal to a change in Tc at the onset or cessation of exercise or to changes in exercise intensity.9,13,17,20 Without exception, the data in table 3 show a slower response of Trectal than Tintestinal to changes in exercise intensity; however, differences between the two sites do not always reach statistical significance.
Toesophageal versus Trectal and Tintestinal
Six of the 12 studies in table 1 simultaneously measured Toesophageal, Trectal and Tintestinal.9,13,16,17,20 This allows for a comparison of the agreement between Toesophageal and Tintestinal in the context of the agreement between Toesophageal and Trectal. Only two study datasets were available for comparison of LoA.13,17 The data of Kolka et al13 showed a statistically significant bias in the comparison of Toesophageal versus Trectal (ie, −0.21°C (0.33)°C), indicating that Trectal was consistently higher than Toesophageal. Furthermore, statistically significant negative heteroscedasticity was evident, indicating that the absolute difference between sites decreased as Tc increased. Conversely, no statistically significant bias (−0.02°C (0.43)°C) or statistically significant heteroscedasticity was evident in the comparison of Toesophageal versus Tintestinal. On the other hand, the data of Lee et al17 show similar non‐significant bias and 95% LoA for Toesophageal–Trectal and Toesophageal–Tintestinal comparisons. Although limited to two datasets, the analysis suggests that the agreement between Toesophageal and Tintestinal is as good as, if not better than, the agreement between Toesophageal and Trectal. Support for this conclusion is provided by O'Brien et al,16 who reported that the RMSD between Toesophageal and Tintestinal was significantly less (p<0.05) than the RMSD between Trectal and Toesophageal during one of their four experimental conditions (ie, 3 h of resting cold‐water immersion) and not statistically different in the remaining experimental conditions.
Responsiveness of Toesophageal versus Tintestinal and Trectal
Table 3 shows that the intestinal site is intermediate to oesophageal and rectal sites in responding to a change in Tc at the onset or cessation of exercise or in response to changes in exercise intensity, reinforcing earlier observations.9,20 For this reason, during dynamic non‐steady‐state Tc situations, Tintestinal may agree more closely with Toesophageal than does Trectal. This is clearly shown in fig 1, where Trectal was significantly lower (p<0.05) than Toesophageal after 20 min of exercise at 40% VO2peak and after a further 20 min at 65% VO2peak, whereas Tintestinal was not significantly (p>0.05) different from Toesophageal at these time points.
Reliability
Only one study from table 1 has directly assessed the reliability of ingestible temperature sensors.19 In that study, nine men performed two 90 min bouts of shuttle running separated by 7 days, with LoA analysis showing no significant bias (ie, −0.01°C (0.23°C); p>0.05) and 95% LoA of −0.24°C to +0.22°C between the two bouts, indicating an acceptable level of reliability.19
Factors affecting agreement
Table 1 shows that a number of key variables (eg, sensor calibration, timing of ingestion) have differed markedly across validation studies. These variables can potentially affect the validity and reliability of Tintestinal and a consideration of their effect will also inform practical application.
Calibration
The precision and accuracy of the manufacturer's calibration can be confirmed by the investigator before use by comparing sensor temperature against a calibrated thermometer across a physiologically valid range of water bath temperatures. For example, Lee et al17 developed individual linear regression equations for each of their seven sensors by comparing sensor and calibrated mercury thermometer temperature at water bath temperatures of 30°C, 34°C, 38°C, and 42°C. Although the composite of 28 comparisons showed a highly linear relationship (ie, R2 = 0.999; °C = 0.997(sensor temperature) + 0.245), sensor temperatures were found to be significantly (p<0.05) and consistently lower than the calibrated thermometer across the range of temperatures. Sparling et al14 highlighted that of the six sensors used in their study, three sensors measured lower (ie, 0.05°C, 0.1°C and 0.1°C) and three sensors measured higher (ie, 0.25°C, 0.25°C and 0.6°C) than a calibrated thermometer. Therefore, it is essential that each sensor is individually calibrated before use. Four studies in table 1 clearly stated their calibration procedures,14,15,16,17 three studies stated that calibration was undertaken but did not elaborate on their procedures,9,11,19,20 whereas five studies did not state whether calibration was undertaken.12,13,18,21
Gastrointestinal motility
The absence of a fixed anatomical position for temperature measurement presents a number of potential problems when using the ingestible sensor, such as the possibility of temperature gradients along the gastrointestinal tract, the acute modifying effects of fluid and food ingestion on Tintestinal, and the uncertainty of sensor transit time.
Gastrointestinal temperature gradients
Table 2 provides evidence of significant (p<0.05) differences between Tintestinal and Trectal, indicating a temperature gradient along the gastrointestinal tract. Kolka et al13 hypothesised that movement of the sensor from the stomach to the small intestine was responsible for temperature variations of 0.2–0.3°C observed in two of their eight subjects. When data collection commenced soon after sensor ingestion, Livingstone et al12 observed a variable Tintestinal–Trectal relationship, which they hypothesised was due to movement of the sensor through the stomach and upper intestine. They observed a more stable and close relationship when the sensor was allowed time (amount not stated) to traverse the gastrointestinal tract.12 O'Brien et al16 had volunteers ingest sensors 12 h before data collection and suggested that this time period should overcome the potential temperature fluctuations associated with sensor transit through the stomach. On the other hand, in a 36 h experiment in which sensor ingestion occurred 40 min before data collection, Ducharme et al.21 reported that Trectal−Tintestinal differences were similar in the first hour (0.15°C (0.11)°C) and in the 36th hour (0.15°C (0.14)°C) of data collection, suggesting no effect of sensor transit on the Trectal–Tintestinal relationship. Sparling et al14 reported no effect on the Trectal–Tintestinal relationship when ingestion occurred 3–4 vs 8–9 h before data collection; however, the comparison was based on only three subjects in each group. Observations in resting animals suggest that the gastrointesinal tract is a major source of heat, exhibiting temperatures significantly higher (p<0.05) than aortic blood.33,34 Furthermore, temperature gradients along the gastrointestinal tract were evident, with the duodenum and ileum exhibiting significantly higher (p<0.05) temperatures than the stomach, large intestine and rectum.34 These findings are in line with the observations of the latter three studies in table 2,17,18,19 showing significantly higher Tintestinal versus Trectal and significantly higher Tintestinal versus Toesophageal.17 This indirect evidence suggests the existence of a temperature gradient along the gastrointestinal tract (ie, from stomach/small intestine/large intestine to rectum), and from the gastrointestinal tract to central blood in humans; however, the magnitude of the gradient does not seem to be affected by movement of the ingestible sensor along the gastrointestinal tract.14,21 Further study is required to determine the magnitude and physiological significance of this temperature gradient.16
Modifying effects of fluid and food
If the sensor is located in the stomach, its temperature will be influenced by the temperature of ingested fluid and food. Fox et al20 stated that observing the effect of a small drink of cold fluid clearly establishes whether the sensor has departed the stomach. If the sensor is ingested in the acute period before data collection and fluid/food is to be ingested, then providing fluid/food at a temperature equivalent to Tc (about 37°C) will minimise this effect. This approach was adopted by Ducharme et al,21 who provided fluid at 37°C throughout their 36 h experiment.
Transit time
Sensor ingestion many hours before data collection (eg, 8–12 h) may ensure departure from the stomach and a more stable Tc; however, there is a risk that the sensor may be expelled before data collection. Indeed, O'Brien et al16 reported a loss of sensor on 3 of 36 (8%) occasions after ingestion at 20:00 h for an experiment at 07:00 h the next day. Kolka et al13 reported mean (SD) transit times for eight men as 30.4 (8.9) h, and McKenzie and Osgood11 reported transit times for six men and four women as 40.8 (26.4) and 62.3 (49.2) h, respectively. Minimum transit times have been reported as 8 h17 and 12.4 h,11 with the maximum reported transit time being 5.6 days.11 McKenzie and Osgood11 noted that the shortest transit times were associated with sensor ingestion just before the evening meal. In line with this observation, ingesting the sensor with a light meal has been used in a number of studies in an attempt to promote sensor transit from the stomach.13,15,17 Lee et al17 proposed a sensor ingestion time of 6 h before data collection, which would seem optimal in avoiding both temperature fluctuations in the upper gastrointestinal tract and sensor expulsion before data collection.
Electromagnetic interference
Reception of the temperature‐dependant low‐frequency radio wave transmitted from the ingestible sensor is susceptible to electromagnetic interference and can result in erroneous or lost data. Mittal et al35 reported a loss of temperature readings when sensors were subjected to interference from an electromagnetic heating device, but accurate readings were obtained immediately after the device was switched off. Interference from computer screens and laboratory equipment monitors can also prevent accurate data recording.17 Sources of interference are more likely to be identified and controlled in a laboratory compared with a field‐based situation where sources are likely to be unpredictable and beyond the researcher's control.
Applications of the ingestible temperature sensor
The earliest application of the ingestible temperature sensor can be traced to the study of Adams et al,36 where it was used as a rectal suppository to measure Trectal during prolonged running in the laboratory and after an outdoor marathon race. Keatinge et al37,38 used the sensor as a rectal suppository and also in its intended ingestible form during case studies of sea swimming in the cold waters of the Bering Strait (water temperature = 7.2–7.4°C) and Beagle Channel (water temperature = 8.3–9°C). An advantage of the ingestible sensor is its ability to obtain Tc measurements on large groups of subjects simultaneously, such as a sample of athletes participating in the same event. For example, Byrne et al28 were able to continuously record Tc simultaneously in 18 runners competing in a 21 km road‐running race. This contrasts with the difficulty faced by Maron et al,39 who made serial Trectal measurements (approximately every 9 min) in two runners during a 42.2 km marathon by pulling alongside the runners in a moving vehicle and connecting their indwelling rectal probe to a measurement device. Although portable data recorders worn by subjects to continuously record Trectal have been described and applied successfully in freely exercising subjects,40 issues regarding the invasive, obtrusive and objectionable nature of rectal thermometry remain unresolved.
We are aware of nine published field‐based studies using ingestible temperature sensors in subjects undertaking sporting or occupational physical activity.24,25,26,27,28,29,30,31,32 Table 4 illustrates these studies and indicates the increasing use of the technology with five of the nine studies published in 2006.28,29,30,31,32 Six field‐based studies have used ingestible sensors to measure Tc during diverse sporting activities.25,27,28,30,31,32 The studies provide unique and ecologically valid Tc data and show the efficacy of the method, as all data were obtained during training sessions27,31 or actual competitive events.28,30,32 Additionally, three studies have used ingestible temperature sensors to investigate potential hypothermia and hyperthermia during occupational activity,24,26,29 such as saturation diving in sea‐water depths up to 160 m26 and during 54 h of sustained Marine Corps cold weather training exercises.29 The utility of the technology is readily shown in providing, in some cases, continuous Tc data in logistically challenging scenarios and often in extreme environments.
Table 4 Field‐based sport and occupational applications of the ingestible telemetric temperature sensor.
| Reference | Application | Characteristics | Sample | Environment | Data collection |
|---|---|---|---|---|---|
| White et al24 | Dry‐suit scuba diving: open water search and rescue activity | Duration, 34–189 minDepth, NS | 20 M | Ice, W = 2.8°CDB, 0.6°CWarm: W = 21°C | Continuous |
| DB = 23°C | |||||
| Leclerc et al25 | Open‐water swimming | Distance, 40 km | 17 | W = 18.3–22.4°C | Intermittent |
| Duration, F = 666 (36) min, M = 628 (40) min | (4 F, 13 M) | DB = 23°C | |||
| Mekjavic et al26 | Thermal suit saturation diving | Duration = 31–450 minDepth = 54–160 m | 30 M | W = 4–6°C | Continuous |
| Fowkes Godek et al27 | American football and cross‐country running | Football, pre‐season twice per day practice | 15 M | DB = 26.1–35°C RH = 36–71% | Intermittent |
| Runners, pre‐season twice per day steady running | (10 football, 5 run) | ||||
| Byrne et al28 | Distance running | Distance = 21 kmDuration = 118 (13) min | 18 M | DB = 26.3–30.6°CRH = 75–90% | Continuous |
| Castellani et al29 | Military field training | Duration = 54 h | 26 | DB = 3.6–21.4°C | Continuous |
| Varied physical and cognitive challenges, sleep deprivation, negative energy balance | (12 F, 14 M) | RH = 72 (21)%V = 1.6 (0.7) m/s | |||
| Edwards and Clark30 | Soccer | Duration, 90 min | 15 M | Amateur | Intermittent |
| Amateur and professional match play | (8 amateur, 7 professional) | DB = 16°CRH = 47% | |||
| Professional | |||||
| DB = 19°C | |||||
| RH = 53% | |||||
| Fowkes Godek et al31 | American football | Twice per day practice | 14 M | DB = 19.4–29.2°C | Intermittent |
| (8 linemen, 6 backs) | |||||
| Laursen et al32 | Ironman triathlon | Distance = 226 km | 9 M | DB = 19–26°C | Intermittent |
| Duration = 611 (49) min | RH = 44–87% | ||||
| Swim = 3.8 km, cycle = 180 km, | |||||
| run = 42.2 km |
DB, dry bulb air temperature; F, female; M, male; RH, relative humidity; V, wind velocity; W, water temperature.
Values are mean (SD) or range.
Data collection problems
Several of the studies in table 4 have reported incidences of poor reliability during sport and occupational applications of the technology. White et al24 reported incomplete or inaccurate data recordings in 7 of 27 (26%) attempts at continuous monitoring of Tc during dry‐suit scuba diving. The authors identified a malfunctioning temperature sensor on one occasion, but the causes of the remaining six failures could not be identified. Similarly, Byrne et al28 could not identify the causes responsible for incomplete data recordings in 4 of 22 (18%) attempts at continuous monitoring of Tc in runners undertaking a half‐marathon. Laursen et al32 obtained Tc readings intermittently during an Ironman triathlon and was successful on 55 of 72 attempts (24% data loss). The authors suggested that human error when running alongside the athletes and/or electromagnetic interference from external radio waves could potentially account for the lost data.32 McKenzie and Osgood11 reported the percentage of missing data points as 6.5% (5.9%) during data collection at minute intervals over 48.6 (35.5) h in 10 subjects. The missing data points were attributed to the sensor and receiver going out of range, such as when sleeping or showering.11 In summary, field‐based use of ingestible sensors have been associated with up to a quarter of the data being incomplete or inaccurate. The principal causes seem to be electromagnetic interference, limitations in sensor transmitting range (ie, VitalSense range = 1 m; CorTemp range = 0.61 m), or experimenter error. However, the potential for data loss must be placed in the context of the objectionable nature of oesophageal or rectal thermometry, where considerable problems with volunteer recruitment and/or drop‐out are likely to be experienced by researchers. Indeed, in a study by one of the authors of this review, an entire cohort of volunteers withdrew from the study after their initial exposure to rectal thermometry.41
Conclusions and recommendations
A quantitative review suggests that the agreement between Tintestinal and Toesophageal can meet our criteria for acceptance as a valid measure of Tc (ie, bias <0.1°C and 95% LoA within ±0.4°C). The agreement between Tintestinal and Trectal shows a significant systematic bias >0.1°C, although the 95% LoA is acceptable. Intestinal sensor temperature responds less rapidly than Toesophageal at the start or cessation of exercise or to a change in exercise intensity, but more rapidly than Trectal. Before use, ingestible sensors should be individually calibrated against a certified thermometer across a physiologically valid range of water‐bath temperatures, enabling the generation of individual regression formula and the correction of raw data. Ingestion of the sensor 6 h before data collection seems optimal to ensure sensor transit beyond the stomach but not expulsion before data collection. Successful data collection by telemetry is susceptible to electromagnetic interference and is limited by the sensor transmission range (ie, <1 m). The ingestible telemetric temperature sensor represents a valid index of Tc that is convenient and shows excellent utility for ambulatory field‐based applications. Benefits of the system over indwelling hard‐wired probes were recognised and summarised 45 years ago by their early pioneers: “The radio pill has the great merit that, once swallowed, the subject is unaware of its presence or of measurements being made. It should prove valuable in field studies, in investigations requiring frequent measurements over long periods, or if the subject needs to be entirely free during the observations.”20
What is already known on this topic
Ingestible telemetric temperature sensors represent an alternative to oesophageal and rectal temperatures as an index of core temperature during exercise.
Variable relationships between these three indices have been observed in validation studies using small sample sizes, varied protocols and varied statistical methods of comparison.
The ingestible sensor method is gaining widespread use, particularly in field‐based studies.
What this study adds
Our quantitative review based on Bland and Altman's limits of agreement suggests the agreement between ingestible sensor and oesophageal temperature can potentially be within our delimitation of acceptable agreement (ie, systematic bias <0.1°C and 95% limits of agreement within ±0.4°C). The agreement with rectal temperature shows a significant systematic bias >0.1°C, with acceptable 95% limits of agreement.
A review of ingestible sensor applications shows excellent utility for ambulatory field‐based uses.
Abbreviations
LoA - limits of agreement
RMSD - root mean‐squared deviation
Footnotes
Competing interests: None declared.
References
- 1.Sawka M N, Wenger C B. Physiological responses to acute exercise‐heat stress. In: Pandolf KB, Sawka MN, Gonzalez RR, eds. Human performance physiology and environmental medicine at terrestrial extremes. Indianapolis IN: Benchmark, 198897–151.
- 2.American College of Sports Medicine Position stand. Heat and cold illnesses during distance running. Med Sci Sports Exerc 1996281–10. [PubMed] [Google Scholar]
- 3.Ainslie P N, Reilly T. Physiology of accidental hypothermia in the mountains: a forgotten story. Br J Sports Med 200337548–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lim C L, Mackinnon L T. The roles of exercise‐induced immune system disturbances in the pathology of heat stroke. The dual pathway model of heat stroke. Sports Med 20063639–64. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez‐Alonso J, Teller C, Andersen S L.et al Influence of body temperature on the development of fatigue during prolonged exercise in the heat. J Appl Physiol 1999861032–1039. [DOI] [PubMed] [Google Scholar]
- 6.Brengelmann G L. Dilemma of body temperature measurement. In: Shiraki K, Yousef MK, eds. Man in stressful environments. Thermal and work physiology. Springfield IL: Charles C Thomas, 19875–22.
- 7.Blatteis C M. Methods of body temperature measurement. In: Blatteis CM, ed. Physiology and pathophysiology of temperature regulation. Singapore: World Scientific, 1998273–279.
- 8.Wolff H S. The radio pill. New Sci 196116419–421. [Google Scholar]
- 9.Gibson T M, Redman P J, Belyavin A J. Prediction of oesophageal temperatures from core temperatures measured at other sites in man. Clin Phys Physiol Meas 19812247–252. [DOI] [PubMed] [Google Scholar]
- 10.Cutchis P N, Hogrefe A F, Lesho J C. The ingestible thermal monitoring system. Johns Hopkins APL Tech Dig 1988916–21. [Google Scholar]
- 11.McKenzie J E, Osgood D W. Validation of a new telemetric core temperature monitor. J Therm Biol 200429605–611. [Google Scholar]
- 12.Livingstone S D, Grayson J, Frim J.et al Effect of cold exposure on various sites of core temperature measurements. J Appl Physiol 1983541025–1031. [DOI] [PubMed] [Google Scholar]
- 13.Kolka M A, Quigley M D, Blanchard L A.et al Validation of a temperature telemetry system during moderate and strenuous exercise. J Therm Biol 199318203–210. [Google Scholar]
- 14.Sparling P B, Snow T K, Millard‐Stafford M L. Monitoring core temperature during exercise: ingestible sensor versus rectal thermistor. Aviat Space Environ Med 199364760–763. [PubMed] [Google Scholar]
- 15.Kolka M A, Levine L, Stephenson L A. Use of an ingestible telemetry sensor to measure core temperature under chemical protective clothing. J Therm Biol 199722343–349. [Google Scholar]
- 16.O'Brien C, Hoyt R W, Buller M J.et al Telemetry pill measurement of core temperature in humans during active heating and cooling. Med Sci Sports Exerc 199830468–472. [DOI] [PubMed] [Google Scholar]
- 17.Lee S M C, Williams W J, Schneider S M. Core temperature measurement during submaximal exercise: esophageal, rectal, and intestinal temperatures. NASA Center for AeroSpace Information Technical Report NASA/TP. 2000;210133
- 18.Edwards B, Waterhouse J, Reilly T.et al A comparison of rectal, gut, and insulated axilla temperatures for measurement of the circadian rhythm of core temperature in field studies. Chronobiol Int 200219579–597. [DOI] [PubMed] [Google Scholar]
- 19.Gant N, Atkinson G, Williams C. The validity and reliability of intestinal temperature during intermittent running. Med Sci Sports Exerc 2006381926–1931. [DOI] [PubMed] [Google Scholar]
- 20.Fox R H, Goldsmith R, Wolff H S. The use of a radio pill to measure deep body temperature [abstract]. J Physiol 196216022–23.13914125 [Google Scholar]
- 21.Ducharme M B, McLellan T M, Moroz D.et al A 36 hour comparison of core temperature at rest and during exercise using rectal probe and pill telemetry [abstract]. Pro Aust Physiol Pharmacol Soc 20013228 [Google Scholar]
- 22.Bland J M, Altman D G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 19861307–310. [PubMed] [Google Scholar]
- 23.Atkinson G, Nevill A M. Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine. Sports Med 199826217–238. [DOI] [PubMed] [Google Scholar]
- 24.White L J, Jackson F, McMullen M J.et al Continuous core temperature monitoring of search and rescue divers during extreme conditions. Prehosp Emerg Care 19982280–284. [DOI] [PubMed] [Google Scholar]
- 25.Leclerc S, Lacroix V J, Montgomery D L. Body temperature homeostasis during a 40 km open water swim. J Swimming Res 20001426–32. [Google Scholar]
- 26.Mekjavic B, Golden F S, Eglin M.et al Thermal status of saturation divers during operational dives in the North Sea. Undersea Hyperb Med 200128149–155. [PubMed] [Google Scholar]
- 27.Fowkes Godek S, Godek J J, Bartolozzi A R. Thermal responses in football and cross country athletes during their respective practices in a hot environment. J Athl Train 200439235–240. [PMC free article] [PubMed] [Google Scholar]
- 28.Byrne C, Lee K W, Chew S A N.et al Continuous thermoregulatory responses to mass‐participation distance running in heat. Med Sci Sports Exerc 200638803–810. [DOI] [PubMed] [Google Scholar]
- 29.Castellani J W, Delany J P, O'Brien C.et al Energy expenditure in men and women during 54 h of exercise and caloric deprivation. Med Sci Sports Exerc 200638894–900. [DOI] [PubMed] [Google Scholar]
- 30.Edwards A M, Clark N A. Thermoregulatory observations in soccer match play: professional and recreational level applications using an intestinal pill system to measure core temperature. Br J Sports Med 200640133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fowkes Godek S, Bartolozzi A R, Burkholder R.et al Core temperature and percentage of dehydration in professional football lineman and backs during preseason practices. J Athl Train 2006418–17. [PMC free article] [PubMed] [Google Scholar]
- 32.Laursen P B, Suriano R, Quod M J.et al Core temperature and hydration status during an ironman triathlon. Br J Sports Med 200640320–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grayson J, Irvine M, Kinnear T. Observations on temperature distribution in the cardiovascular system, thorax and abdomen of monkeys in relation to environment. J Physiol 1966184581–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grayson J, Durotoye A O. Effect of environment on temperatures in the viscera of the dog. Int J Biometeorol 197115176–180. [DOI] [PubMed] [Google Scholar]
- 35.Mittal B B, Sathiaseelan V, Rademaker A W.et al Evaluation of an ingestible telemetric temperature sensor for deep hyperthermia applications. Int J Radiat Oncol Biol Phys 1991211353–1361. [DOI] [PubMed] [Google Scholar]
- 36.Adams W C, Fox R H, Fry A J.et al Thermoregulation during marathon running in cool, moderate, and hot environments. J Appl Physiol 1975381030–1037. [DOI] [PubMed] [Google Scholar]
- 37.Keatinge W R, Nyober J H. Body temperature during a 125‐min swim in Bering Straits in water at 7.2–7.4°C [abstract]. J Physiol 198941242 [Google Scholar]
- 38.Keatinge W R, Neild P J. Use of rectal radiopill to monitor human body core temperature during four mile swim across Beagle Channel, Tierra del Fuego [abstract]. J Physiol 199043093 [Google Scholar]
- 39.Maron M B, Wagner J A, Horvath S M. Thermoregulatory responses during competitive marathon running. J Appl Physiol 197742909–914. [DOI] [PubMed] [Google Scholar]
- 40.Fuller A, Oosthuyse T, Maloney S K.et al Evaluation of miniature data loggers for body temperature measurement during sporting activities. Eur J Appl Physiol 199979341–346. [DOI] [PubMed] [Google Scholar]
- 41.Lim C L, Chung K K, Hock L L. The effects of prolonged passive heat exposure and basic military training on thermoregulatory and cardiovascular responses in recruits from a tropical country. Mil Med 1997162623–627. [PubMed] [Google Scholar]