Abstract
Objective
To compare the ketoprofen TDS patch with diclofenac gel in the treatment of traumatic acute pain in benign sport‐related soft‐tissue injuries.
Design
7–14 treatment days, prospective, randomised, open study.
Patients
Outpatients aged 18–70 years diagnosed for painful benign sport‐related soft‐tissue injury (sprains, strains and contusions within the prior 48 h), randomised to either ketoprofen patch 100 mg once daily (n = 114) or diclofenac gel 2–4 g three times daily (n = 109).
Intervention
7–14 days of topical non‐steroidal anti‐inflammatory drugs treatment to assess the pain intensity changes (daily activities and spontaneous at rest) in a daily diary (100‐mm Visual Analogue Scale (VAS)).
Main outcome measurement
Pain intensity (VAS).
Results
The ketoprofen patch was not inferior to diclofenac gel in reducing the baseline pain during daily activities (difference of –1.17 mm in favour of ketoprofen patch, 95% CI (–5.86 to 3.52), reducing to the baseline VAS 79%. Ketoprofen patch presented also a higher cure rate (64%) than diclofenac gel (46%) at day 7 (p = 0.004). Patient opinions about the treatment comfort (pharmaceutical shape, application and dosage) were also statistically higher for the ketoprofen patch (>80% of the patients rated as good or excellent the patch removal and skin adherence).
Conclusion
Ketoprofen patches are effective and safe pain relievers for the treatment of sports injury pain with advantages compared with diclofenac gel.
Over the past several decades, although the health benefits of exercise outweigh the risks, occurrences of injuries associated with sports activities have become commonplace. Sports‐related injuries most often result in pain associated with soft‐tissue injuries, such as sprains, strains and contusions.1 Although not serious, in these injuries an inflammatory reaction occurs locally, with resultant swelling and pain, and results in temporary disability.
The use of non‐steroidal anti‐inflammatory drugs (NSAIDs) has proved to be effective in the treatment of soft‐tissue injuries and it has been shown to be of benefit in the early resolution of soft‐tissue injuries because of their ability to inhibit prostaglandin synthetase activity.2,3
Currently, the most widely recommended and used drug treatment for the pain associated with these sporting injuries are orally administered drugs, such as NSAIDs, including aspirin and paracetamol.4 Oral NSAIDs reach the site of activity only after the drug enters the systemic circulation. To have an adequate local effect they must reach relatively high systemic levels and they can cause important systemic side effects.5,6
In contrast, topically applied NSAIDs can provide directed and focal relief without systemic activity. Topical drugs are applied on the skin overlying the injured and painful body region. The drug then penetrates the skin, subcutaneous fatty tissue and muscle in a sufficient amount to exert therapeutic effects, whereas plasma levels remain low, and directly acts within the injured site without the need for systemic activity.7 Topical NSAIDs offer the advantage of local, enhanced drug delivery to affected tissues that can produce clinically meaningful results with a reduced incidence of systemic adverse events, such as peptic ulcer and gastrointestinal haemorrhage and without drug–drug interactions.8 A recent quantitative systemic review of randomised controlled trials concluded that topical NSAIDs are effective in relieving the pain associated with soft‐tissue injuries without systemic adverse reactions.9
A new topical dosage form (patch), containing ketoprofen as the active agent, was jointly developed by Labtec GmbH (Langenfeld, Germany) and Appplied Pharma Research (APR, Balerna, Switzerland). Ketoprofen transdermal delivery system (TDS) patches (size 82×110 mm; surface 90 cm2) are made up of three layers: (1) a backing textile layer of polyester, longwise and crosswise elastic, (2) a matrix of 20% ketoprofen in acrylic pressure sensitive adhesive corresponding to 100 mg ketoprofen per patch and (3) a release liner of polyethylenterephtalat foil, 100 μm, which has one of both sides siliconised. This patch allows release of ketoprofen over 24 h, and a continuous presence of the active substance at the injury sites.
Safety preclinical studies were performed on the ketoprofen TDS patch. There were no dermal reactions, no irritation for the skin or for the eye as a single dose, and no significant irritation in repeated‐dose studies.10,11,12,13 The in vitro percutaneous absorption from the ketoprofen TDS patch was nearly linear over at least 72 h, 14,15 thus showing it was effective as a once daily administration.14 The in vivo transdermal absorption from the ketoprofen TDS patch was evaluated in rabbits with shaved skin and showed an effective delivery from the patch during the application to the skin and a good skin adhesion. The average total systemic exposure, as expressed by the area under the curve, correlated to the amount released from the patch and reached about 10%.16
With all those characteristics, the once a day dosage was likely to ensure a better compliance, in comparison with creams, gels and sprays which often require 3–4 applications per day.17,18,19
The aim of this multicentre study was to assess the efficacy, tolerability and patient's acceptability of a new NSAID drug delivery system, a ketoprofen TDS patch administered once a day, in the treatment of pain associated with acute minor sport‐related soft‐tissue injuries, in comparison with one of the most widely used topical NSAIDs in Spain, diclofenac sodium gel (dolotren gel, FAES Farma S.A., Madrid, Spain) administered three times a day.
Materials and methods
Subjects who showed a painful benign sport‐related soft‐tissue injury (sprains, strains and contusions) of upper or lower limbs, except fingers and toes, which became recently evident (>48 h before the initial study visit) were considered for admission into the study.
Subjects were informed of the procedures, completed a pretest health‐screening questionnaire and provided written informed consent. Experimental procedures were approved by the independent ethics committees of the participating centres in accordance with the Helsinki Declaration (Faculty of Science, Kingson University, London, UK).
The main inclusion criteria were: aged between 18 and 70 years, diagnosed in the previous 48 h maximum with a painful benign sport‐related soft‐tissue injury and with spontaneous pain at rest and pain during daily activities, ⩾35 mm on a 100‐mm Visual Analogue Scale (VAS). Additionally, women of child‐bearing age had to be surgically incapable of pregnancy or using an acceptable method of birth control.
Study design
A phase IIIb, multicentre, open label, active control and randomised parallel‐group study planned for 240 patients with traumatic acute pain in benign sport‐related soft‐tissue injuries. This was a study with direct individual benefit. Patients were randomly allocated to one of the two groups: (a) Ketoprofen TDS patch once daily for 7 or 14 days; or (b) diclofenac gel three times per day for 7 or 14 days.
The duration of the treatment, between 7 and 14 days, was a decision that the investigator made at the time the patient was randomised, based on the severity, location and type of lesion. One of the most useful topical NSAIDs in Spain was used as the active control, diclofenac sodium gel administered three times a day. This active control design followed the recommendations of the Committee for Proprietary Medicinal Products note for guidance of the clinical development of medicinal products for treatment of pain (CPMP/EWP/612/00).
As one of the treatment drugs was administered in a patch and the active control was to be dispensed as a gel, there was no way to blind the drug administration and so an open‐label design was used.
Efficacy measurements included change in pain during daily activities, change in spontaneous pain at rest, onset of the analgesic effect, change in global clinical condition, symptoms of the injury site, daily diary variables (quality of sleep, functional disability, pain intensity, pain relief, use of rescue medication) and global evaluation of the treatment (both investigator and patient). Tolerability measurements included adverse events, physical examination and global evaluation of the tolerability (both investigator and patient).
The study was performed in strict compliance with the Declaration of Helsinki (18th World Medical Assembly, 1964) and its last revision (Edinburgh, October 2000). Additionally, the study was conducted in compliance with the International Conference on Harmonization principles of Good Clinical Practice. The study protocol, the case report form and the informed consent form were approved by the independent ethics committees of the 16 participant centres before the recruitment period.
Statistics
The sample size was determined to show that the ketoprofen patch the showed comparable clinical efficacy with respect to diclofenac gel (“pain during daily activities (on VAS)” difference at day 7 less than Δ = 10 mm), assuming a standard deviation of the mean distribution of no >23 mm, an α level of 5%, and a β level of 10%, giving a statistical power of 90%. With a predicted withdrawal rate of 5%, the estimated necessary sample size was 120 patients for each group.
Demographics and baseline, including all the randomised patients, were compared within the two groups. The numerical efficacy end points were assessed with an analysis of covariance model (with 95% CI least square means), the change in pain being used as the dependant variable and the baseline pain as a covariable and the treatment group as the main factor. A last observation carried forward (LOCF) approach was used to estimate the missing values. The categorical data were analysed with a χ2, Mantel‐Haenszel or Fisher's exact test. Survival analysis (Kaplan–Meier test) was used to test the time to maximum pain intensity difference, time to the analgesic effect and time to the maximum pain relief.
The principal analysis of efficacy was made on the intention to treat (ITT) population, and a secondary analysis on the per‐protocol population (deviations evaluated by the Data Monitoring Committee).
Adverse events were coded for verbatim with the medical dictionary for regulatory activities. The Fisher's exact test was used for all the comparisons if applicable. The overall assessments reported by both the investigator and the patient were analysed by Fisher's exact test.
The statistical analysis was performed using SAS V.8.2 software.
Results
A total of 232 subjects were enrolled and randomised. In all, 180 (77.6%) completed the study protocol. Because no data existed for nine subjects lost to follow‐up, a total of 223 (96.1%) subjects were included in the ITT analysis.
The mean age of subjects was 28.8 (range 18–58) years. In all, 173 were men and 50 women. Moderate to high sporting activity level was practised by 80.3% of subjects and only two subjects did not engage in any sporting activity. A total of 47 subjects had sprains, 60 had strains and 84 had contusions, and the rest had mixed patterns. The two most common sites of injury were ankle (23.3%) and thigh (20.2%). Football (34.5%) and athletics (20.2%) were the most common sports. In all, 14 subjects had had a previous injury at the same site, and 45 subjects had had no previous soft‐tissue injury in the previous 12 months. The mean initial pain score (VAS) in daily activities was 71.98 (range 40–100) mm. Functional disability, loss of passive range of motion, loss of passive isometric contraction and pain on pressure were the most frequent symptoms. There were no statistically significant differences between the groups for any demographic measure (table 1) or for any baseline symptoms assessment (table 2).
Table 1 Demographics and patient characteristics.
| Variable | Ketoprofen | Diclofenac | p Values |
|---|---|---|---|
| Number of patients | n = 114 | n = 109 | |
| Age (years), mean | 29.1 | 28.5 | 0.61* |
| SD | 8.65 | 7.49 | |
| Range | 18–58 | 18–54 | |
| Sex | 0.89† | ||
| Male | 88 (77.2) | 85 (78.2) | |
| Female | 26 (22.8) | 24 (22) | |
| Sporting activity level | 0.72† | ||
| None | 1 (0.9) | 1 (0.9) | |
| Low | 25 (21.9) | 17 (15.6) | |
| Moderate | 39 (34.2) | 39 (35.8) | |
| High | 49 (43) | 49 (43) | |
| Study level | 0.51† | ||
| Primary education | 9 (8) | 13 (11.9) | |
| Secondary education | 38 (33.6) | 31 (28.4) | |
| University studies | 60 (53.1) | 62 (56.9) | |
| Postgraduate studies | 6 (5.3) | 3 (2.8) | |
| Employment | 0.70† | ||
| Active | 100 (90.1) | 101 (93.5) | |
| Unemployed | 2 (1.8) | 1 (0.9) | |
| Other | 9 (8.1) | 6 (5.6) | |
| Nature of injury | 0.83† | ||
| Sprain | 26 (22.8) | 21 (19.3) | |
| Muscle strain | 32 (28.1) | 28 (25.7) | |
| Contusion | 40 (35.1) | 44 (40.4) | |
| Site of injury | 0.63† | ||
| Shoulder | 14 (12.3) | 12 (11) | |
| Knee | 17 (14.9) | 10 (9.2) | |
| Ankle | 28 (24.6) | 24 (22) | |
| Thigh | 20 (17.5) | 25 (22.9) | |
| Calf | 12 (10.5) | 13 (11.9) | |
| Sport | 0.65† | ||
| Athletics | 25 (21.9) | 20 (18.3) | |
| Basketball | 13 (1.4) | 11 (10.1) | |
| Football | 39 (34.2) | 38 (34.9) | |
| Tennis | 10 (8.8) | 6 (5.5) | |
| Precipitant factor | 22 (19.3) | 25 (22.9) | 0.51† |
| Repetitive movement | 6 (27.3) | 11 (45.8) | 0.39† |
| Functional overload | 6 (27.3) | 7 (29.2) | |
| Previous therapeutic treatment | |||
| Ice | 52 (45.6) | 37 (33.9) | 0.08† |
| Treatment | 4 (3.5) | 2 (1.8) | 0.68† |
| Injury last year | 24 (21.1) | 21 (19.3) | 0.74† |
Values are represented as n (%) unless otherwise specified.
*p Values for treatment comparisons from an analysis of variance.
†p Values for treatment comparisons from a χ2 test.
Table 2 Baseline symptoms.
| Variable | Ketoprofen | Diclofenac | p Values |
|---|---|---|---|
| Number of patients | n = 114 | n = 109 | |
| Pain with ADLs (mm), Mean | 72.8 | 71.1 | 0.35* |
| SD | 13.29 | 14.40 | |
| Range | 45–100 | 40–99.9 | |
| Baseline swelling | 0.88† | ||
| Absent | 45 (39.5%) | 38 (34.9%) | |
| Mild | 40 (35.1%) | 42 (28.5%) | |
| Moderate | 25 (21.9%) | 26 (23.9%) | |
| Severe | 4 (3.5%) | 3 (2.8%) | |
| Muscle rigidity or stiffness | 0.52† | ||
| Absent | 48 (42.1%) | 36 (33.0%) | |
| Mild | 31 (27.2%) | 37 (33.9%) | |
| Moderate | 31 (27.2%) | 31 (28.4%) | |
| Severe | 4 (3.5%) | 5 (4.6%) | |
| Bruise | |||
| Absent | 82 (71.9%) | 73 (67.6%) | 0.51† |
| Mild | 16 (14.0%) | 13 (12.0%) | |
| Moderate | 11 (9.6%) | 18 (16.7%) | |
| Severe | 5 (4.4%) | 4 (3.7%) | |
| Functional disability | |||
| Absent | 6 (5.3%) | 6 (5.5%) | 0.42† |
| Mild | 27 (23.7%) | 24 (22.0%) | |
| Moderate | 68 (59.6%) | 59 (54.1%) | |
| Severe | 13 (11.4%) | 20 (18.3%) | |
| Full passive motion | |||
| Absent | 6 (5.3%) | 7 (6.4%) | 0.13† |
| Mild | 29 (25.4%) | 21 (19.3%) | |
| Moderate | 61 (53.5%) | 50 (45.9%) | |
| Severe | 18 (15.8%) | 31 (28.4%) | |
| Passive isometric contraction | |||
| Absent | 18 (15.8%) | 14 (12.8%) | 0.07† |
| Mild | 49 (43.0%) | 37 (33.9%) | |
| Moderate | 39 (34.2%) | 44 (40.4%) | |
| Severe | 8 (7.0%) | 14 (12.9%) | |
| Pain on pressure | |||
| Absent | 1 (0.9%) | 1 (0.9%) | 0.76† |
| Mild | 17 (14.9%) | 13 (11.9%) | |
| Moderate | 55 (48.2%) | 48 (44.0%) | |
| Severe | 41 (36.0%) | 47 (43.1%) |
ADL, activities of daily living.
Values are represented as n (%) unless otherwise specified.
*p Values for treatment comparisons from an analysis of variance.
†p Values for treatment comparisons from a χ2 test.
The ketoprofen patch was not inferior to diclofenac gel in reducing the baseline pain during daily activities, as the adjusted difference between treatments after 7 treatment days was <10 mm (–1.17 mm in favour of ketoprofen patch, with a 95% CI between –5.86 and 3.52). The ketoprofen patch reduced up to 79% of the baseline pain during daily activities after 7 days of treatment, and diclofenac gel reduced 77% of the baseline pain during daily activities. The sensitivity analysis (per protocol population, and LOCF for non‐completers) showed similar results.
We did not observe any statistically significant influence on the pain during daily activities from potential risk factors such as the age, sex, precipitant factors, nature and location of injury, ice usage and soft‐tissue injury in extremities during the past year.
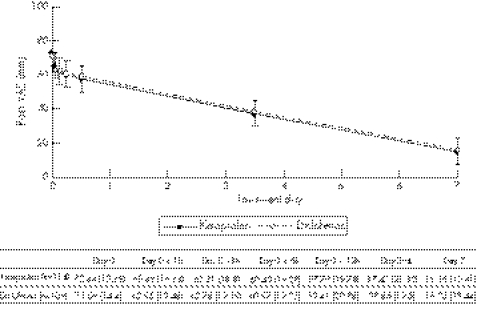
The ketoprofen patch was good at reducing pain both at rest and with activities of daily living (fig 1), similar to diclofenac; the median time to maximum difference in pain (at rest and with activities of daily living) in both groups was 6 days.
Figure 1 Change in pain during daily activities during the study (mean and 95% confidence interval).
The ketoprofen patch had a higher cure rate related to the injury global clinical condition than diclofenac gel at day 7 (p = 0.004), with almost 64% of the patients considered as “cured” with the ketoprofen patch when compared with 46% of those treated with diclofenac gel.
Moreover, the ketoprofen patch provided greater pain relief on full passive motion at day 7 (p = 0.046) and at day 14 (p = 0.040), and greater pain relief on pressure at day 7 (p = 0.010). We observed a similar efficacy profile in both groups for other clinical assessments such as swelling, muscle stiffness, bruising, quality of sleep, functional disability or pain on passive isometric contraction.
The percentage of patients who required rescue drug (paracetamol) was lower in the ketoprofen patch group (20.2%) than in the diclofenac group (31.2%) at days 3‐4 (p = 0.059), but the difference was not statistically significant.
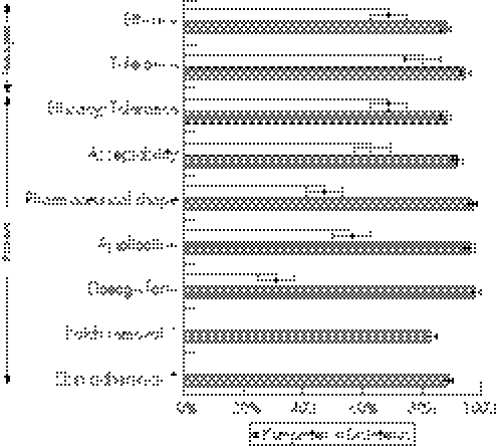
The investigators global assessment of efficacy and tolerance, and the patients global assessment of efficacy/tolerance, acceptability, pharmaceutical shape, application and dosage standard were statistically higher (p = 0.001) for the ketoprofen patch than for the diclofenac gel (fig 2). More than 85% of the patients treated with the ketoprofen patch considered that the treatment had a good or excellent application and dosage form.
Figure 2 Investigators' and patients' global assessment on efficacy, tolerance, accepatability and treatment comfort, and patient opinion about patch removal and skin adherence (percentage of investigators/patients who assessed each domain as good or excellent). *Patch removal and skin adherence only evaluated for the patch form.
In all, >80% of the patients rated the patch removal and skin adherence for the ketoprofen patch as good or excellent.
From the safety point of view, there were no differences between treatment groups in the percentage of patients with adverse events, with a very low incidence: 4.3% for the ketoprofen patch and 0.1% for diclofenac gel. The only related adverse events were two cases of erythema with the ketoprofen patch. There were no serious adverse events.
Discussion
The study has confirmed that the ketoprofen patch (once daily) was not inferior to diclofenac gel (three times per day) in reducing the baseline pain during daily activities, as it did not present an efficacy <10% of that with diclofenac gel. Both treatments produced a significant decrease in pain during daily activities in patients with sport‐related soft‐tissue injuries. In addition, the ketoprofen patch presented a higher efficacy (difference of –1.17 in favour of ketoprofen patch with a 95% CI between −5.86 and 3.52). This difference meant a reduction of up to 79% of the baseline pain during daily activities after 7 days of treatment compared with the 77% reduction obtained with diclofenac gel.
This conclusion was also confirmed with the per protocol analysis and with a LOCF approach to estimate the missing information. Naturally, as the main protocol violation was due to low compliance, the mean difference between treatment groups was higher in the per protocol population than in the ITT population.
The mean age was between 28 and 30 years, and most patients were men performing active work, with >85% usually engaged in moderate to high intensity sporting activity. Football and athletics are the most frequently practiced sports.
Finally, over 95% of the randomised sample were considered for the ITT analysis and almost 80% for the per protocol analysis, giving a sample size large enough to confirm the statistical hypothesis for both analyses.
We observed in this study a pain relief similar to that published in previous studies for the ketoprofen patch,20,21 for diclofenac gel22 and a diclofenac patch,23 but the ketoprofen patch results have been obtained with a single daily dose and after just 1 week of treatment.
Additionally, the change in pain during daily activities from baseline to visit two was analysed according to several baseline variables (age, sex, precipitant factor, nature and location of injury, ice usage and soft‐tissue injury in extremities last year) in order to identify any factor that could have influenced the results. As none of those factors was identified as having a statistically significant influence in the model, the primary efficacy analysis was appropriate to assess the change in pain during daily activities.
For the secondary efficacy variables, the ketoprofen patch presented a good efficacy profile, generally similar to diclofenac gel, in reducing the spontaneous pain at rest and the pain during daily activities, in improving symptoms and signs such as swelling, change in muscle stiffness, bruising, functional disability, pain on full passive motion, pain on passive isometric contraction, pain on pressure, need of rescue drug, sleep quality, pain intensity and pain relief.
Moreover, the ketoprofen patch presented higher efficacy rates than diclofenac gel for the change in functional disability at day 7, in quantitative pain on full passive motion at day 7 and 14, and in pain on pressure at day 7, in addition to a higher cure rate related to the injury global clinical condition, with almost 64% of the patients considered as “cured” with the ketoprofen patch when compared with 46% of those treated with diclofenac gel.
Additional areas where the ketoprofen patch fared better than diclofenac gel were the overall assessments of efficacy and tolerance (both investigator and patient), and the overall assessment of acceptability, treatment comfort related to pharmaceutical shape, application and dosage standard.
Ketoprofen has a good safety profile, similar to that of diclofenac: there was a very low rate of adverse events and clinical findings, with no difference between the two groups.
From an acceptability point of view, >80% of the patients rated the patch removal and the skin adherence as good or excellent.
In summary, the study has confirmed the patients' preference for the patch shown in other studies with topical NSAIDs,20,21,23 not only for efficacy but also for tolerability and acceptability reasons. To our knowledge, there are no published studies comparing the ketoprofen patch with other NSAID patches in this indication; the good pain relief and tolerability shown, obtained with just a single daily ketoprofen patch and after just 7 days of treatment, in addition to the very good compliance obtained with this dosage form, recommend this patch for the treatment of traumatic acute pain in benign sport‐related soft‐tissue injuries.
Conclusion
A ketoprofen patch once daily can be considered as efficacious as diclofenac gel three times per day with an additional better overall assessment of efficacy, tolerability, acceptance and comfort. It is a good option for the treatment of traumatic acute pain in benign sport‐related soft‐tissue injures.
What is already known on this topic
Non‐steroidal anti‐inflammatory drugs (NSAIDs) are effective in the treatment of soft‐tissue injuries.
Topical NSAIDs are effective in relieving the pain associated with soft‐tissue injuries without systemic adverse reactions.
Diclofenac sodium gel administered three times a day is one of the most widely used topical NSAIDs in Spain.
What this study adds
A ketoprofen patch once daily can be considered as efficacious as diclofenac gel three times per day for the treatment of traumatic acute pain in benign sport‐related soft‐tissue injuries, with an additional better overall assessment of efficacy, tolerability, acceptance and comfort.
Acknowledgements
We thank the working group for the acute pain study of SETRADE, and specifically to all the investigators who actively participated in this trial: Centro Médico Juan XIII, Murcia: Dr José Luis Martínez, Dr Juan Francisco Abellán; Clínica CEMTRO, Madrid: Dr Pedro Guillén, Dr Fernando García de Lucas, Dra Isabel Pantin, César Flores; Clínica de la Inmaculada Concepción, Granada: Dr Manuel Zabala, Dra Ana M Oliva Muñoz; Escuela de Medicina de la Educación Física y del Deporte, Universidad de Oviedo: Dr Miguel Enrique del Valle, Dra Nuria Molina, Dra María Murube, Dr Agapito Sánchez; Mutualidad de Futbolistas de Cataluña, Barcelona: Dr Jaume Borrell, Dr Luis Payán, Dr Sergi Sánchez; H Asepeyo Cartuja, Sevilla: Dr Cecilio Neila, Dr José Reyes Fernández; H Monográfico Asepeyo, Madrid: Dr Cristobal Rodríguez, Dr Cristóbal Martínez; H Clínico Universitario San Carlos, Madrid: Dr Luis López‐Durán, Dr Rafael Otero; H Montserrat‐Alianza Médica Leridana, Lleida: Dr Francesc Biosca, Dr Xavier Peirau; H San Rafael, Madrid: Dra Wilma García, Dr Juan Manuel Blanco, Dra M Asunción Bosch; Real Federación Española de Atletismo, Madrid: Dr Juan Manuel Alonso, Dra Josefina Espejo, Dr Jorge González, Dra Carmen León, Dr Cristophe Ramírez; Sanatorio Begoña, Gijón: Dr Luis Rodríguez, Dr Joaquín Carreño; Servicios Médicos Autoridad Portuaria, Valencia: Dr Natalia Giner.
We also thank Dr Lourdes Sunyer and Zambon SA, Barcelona, Spain, and Dr Carlos M Hortelano for the technical contribution to this manuscript preparation.
Finally, we thank the technical contribution of MDS Pharma Services, Madrid, Spain, for the data management, statistical analysis and medical writing activities associated with this study.
Abbreviations
LOCF - last observation carried forward
NSAID - non‐steroidal anti‐inflammatory drug
VAS - Visual Analogue Scale
Footnotes
Funding: The study was sponsored by MDS Pharma Services, Madrid, Spain.
Competing interests: None declared.
References
- 1.Nicholl J P, Coleman P, Williams B T. The epidemiology of sports and exercise related injury in the United Kingdom. Br J Sports Med 199529232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Menasse R, Hedwall P R, Kraetz J.et al Pharmacological properties of diclofenac sodium and its metabolites. Scand J Rheumatol 197822 Suppl5–16. [DOI] [PubMed] [Google Scholar]
- 3.Maier R, Menasse R, Riesterer L.et al The pharmacology of diclofenac sodium (Voltarol). Rheumatol Rehabil 1979Suppl 211–21. [PubMed] [Google Scholar]
- 4.Ogilvie‐Harris D J, Gilbart M. Treatment modalities for soft tissue injuries of the ankle: a critical review. Clin J Sport Med 19955175–186. [DOI] [PubMed] [Google Scholar]
- 5.Burnham R, Gregg R, Healy P.et al The effectiveness of topical diclofenac for lateral epicondylitis. Clin J Sport Med 1998878–81. [DOI] [PubMed] [Google Scholar]
- 6.Galer B S, Rowbotham M, Perander J.et al Topical diclofenac patch relieves minor sports injury pain: results of a multicenter controlled clinical trial. J Pain Symptom Manage 200019287–294. [DOI] [PubMed] [Google Scholar]
- 7.Singh P, Roberts M S. Skin permeability and local tissue concentrations of nonsteroidal anti‐inflammatory drugs after topical application. J Pharmacol Exp Ther 1994268144–151. [PubMed] [Google Scholar]
- 8.Heyneman C A, Lawless‐Liday C, Wall G C. Oral versus topical NSAIDs in rheumatic diseases: a comparison. Drugs 200060555–574. [DOI] [PubMed] [Google Scholar]
- 9.Moore R A, Tramer M R, Carroll D.et al Quantitative systematic review of topically applied non‐steroidal anti‐inflammatory drugs. BMJ 1998316333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.SGS Lab Simon (reference S 100 712). Determination of acute dermal irritation of a ketoprofen TDS patch. Report on file. September 2000
- 11.SGS Lab Simon (reference S 100 713). Determination of acute ocular irritation of a ketoprofen TDS patch. Report on file. October 2000
- 12.SGS Lab Simon (reference S 100 711). Determination of skin sensitization of a ketoprofen TDS patch. Report on file. November 2000
- 13.SGS Lab Simon (reference S 100 714). Determination of repeated skin irritation of a ketoprofen TDS patch. Report on file. December 2000
- 14.Labtec GmbH In vitro percutaneous absorption in hairless mouse skin in comparison with ketoprofen patch (30 mg/70 cm2) and 2.5% ketoprofen gel (Gabrilen®). Report on file. May 1997
- 15.Klaffenbach P, Wollmer U.In vitro percutaneous absorption in human isolated skin: test‐product ketoprofen TDS patch in comparison with ketoprofen gel. Report on file. September 2000
- 16. SGS Biopharma (reference B 100 577). In vivo transdermal absorption of ketoprofen (ketoprofen TDS patch v. ketoprofen gel) after a single administration in 12 rabbits Report on file. March 2001
- 17.Bouchier‐Hayes T A, Rotman H, Darekar B S. Comparison of the efficacy and tolerability of diclofenac gel (Voltarol Emulgel) and felbinac gel (Traxam) in the treatment of soft tissue injuries. Br J Clin Pract 199044319–320. [PubMed] [Google Scholar]
- 18.Radermacher J, Jentsch D, Scholl M A.et al Diclofenac concentrations in synovial fluid and plasma after cutaneous application in inflammatory and degenerative joint disease. Br J Clin Pharmacol 199131537–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ritchie L D. A clinical evaluation of flurbiprofen LAT and piroxicam gel: a multicentre study in general practice. Clin Rheumatol 199615243–247. [DOI] [PubMed] [Google Scholar]
- 20.Mazieres B, Rouanet S, Guillon Y.et al Topical ketoprofen patch in the treatment of tendinitis: a randomized, double blind, placebo controlled study. J Rheumatol 2005321563–1570. [PubMed] [Google Scholar]
- 21.Mazieres B, Rouanet S, Velicy J.et al Topical ketoprofen patch (100 mg) for the treatment of ankle sprain: a randomized, double‐blind, placebo‐controlled study. Am J Sports Med 200533515–523. [DOI] [PubMed] [Google Scholar]
- 22.Patel R K, Leswell P F. Comparison of ketoprofen, piroxicam, and diclofenac gels in the treatment of acute soft‐tissue injury in general practice. General Practice Study Group. Clin Ther 199618497–507. [DOI] [PubMed] [Google Scholar]
- 23.Galer B S, Rowbotham M, Perander J.et al Topical diclofenac patch relieves minor sports injury pain: results of a multicenter controlled clinical trial. J Pain Symptom Manage 200019287–294. [DOI] [PubMed] [Google Scholar]