Abstract
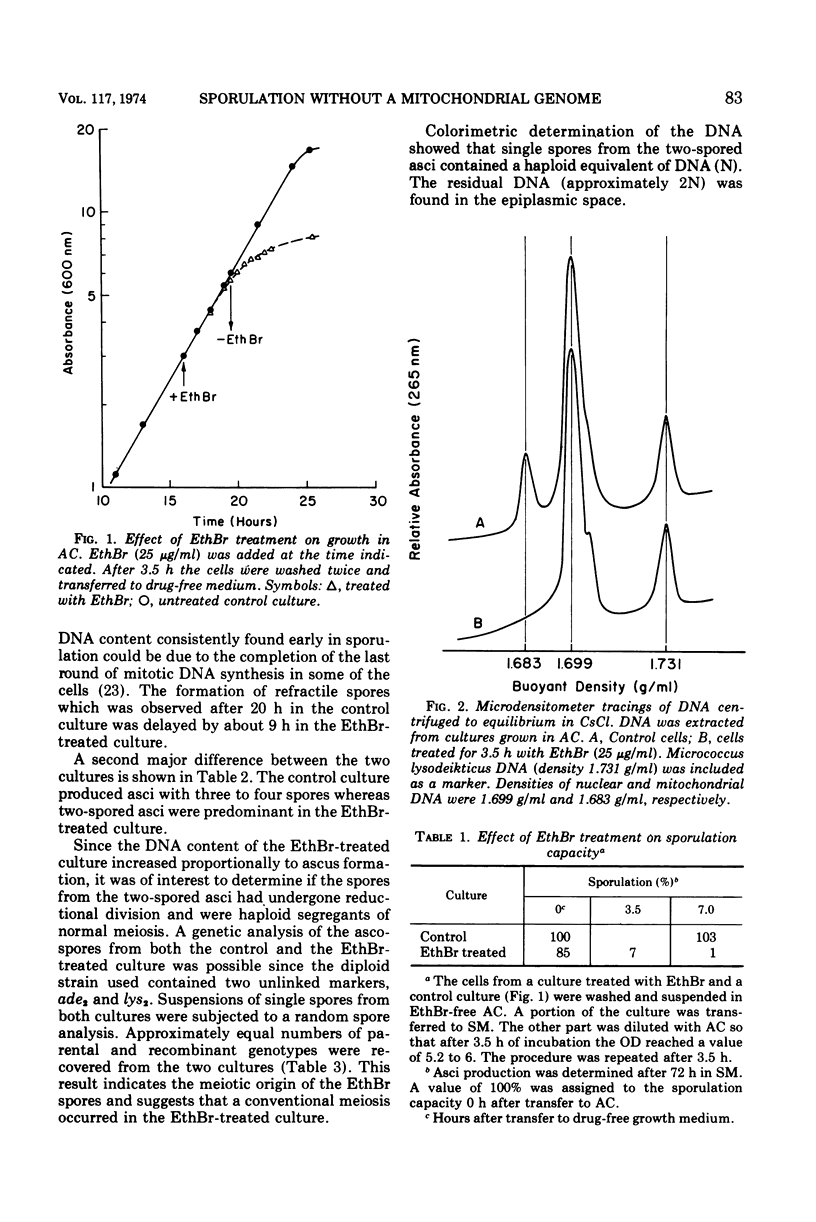
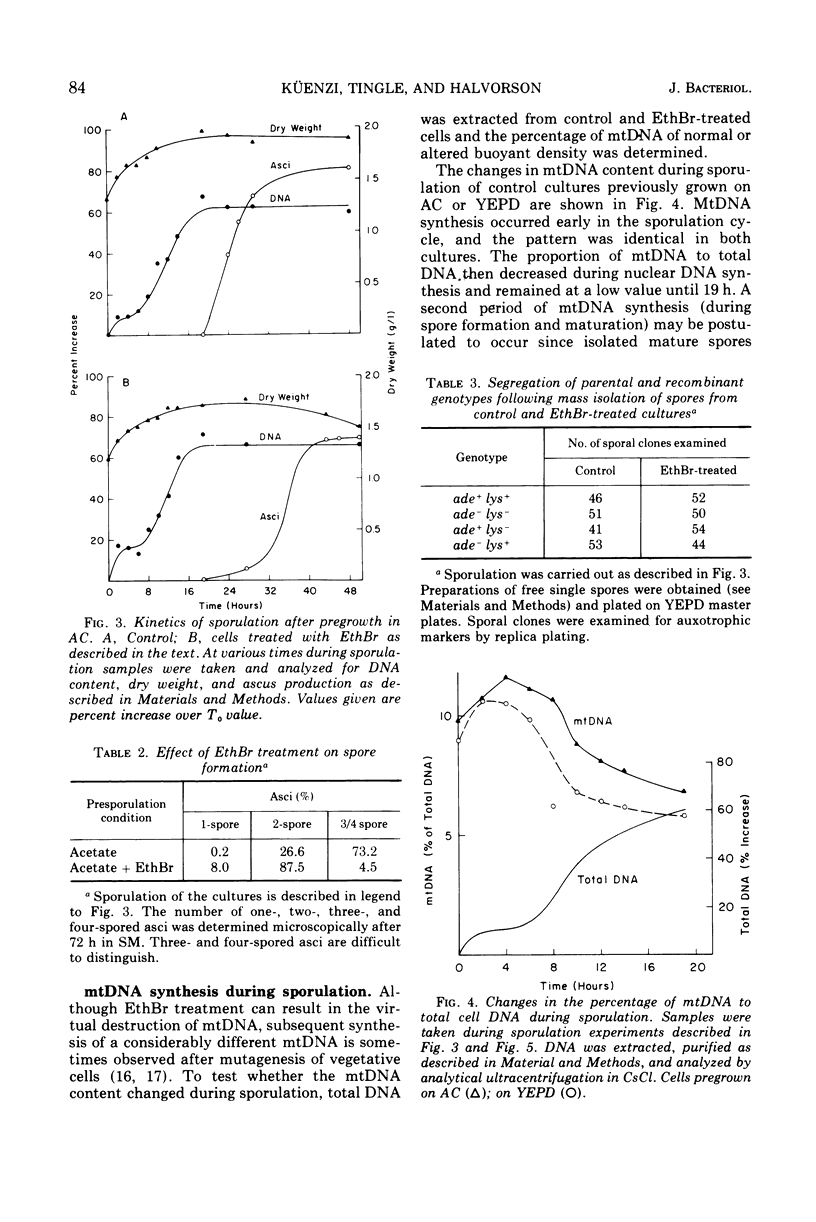
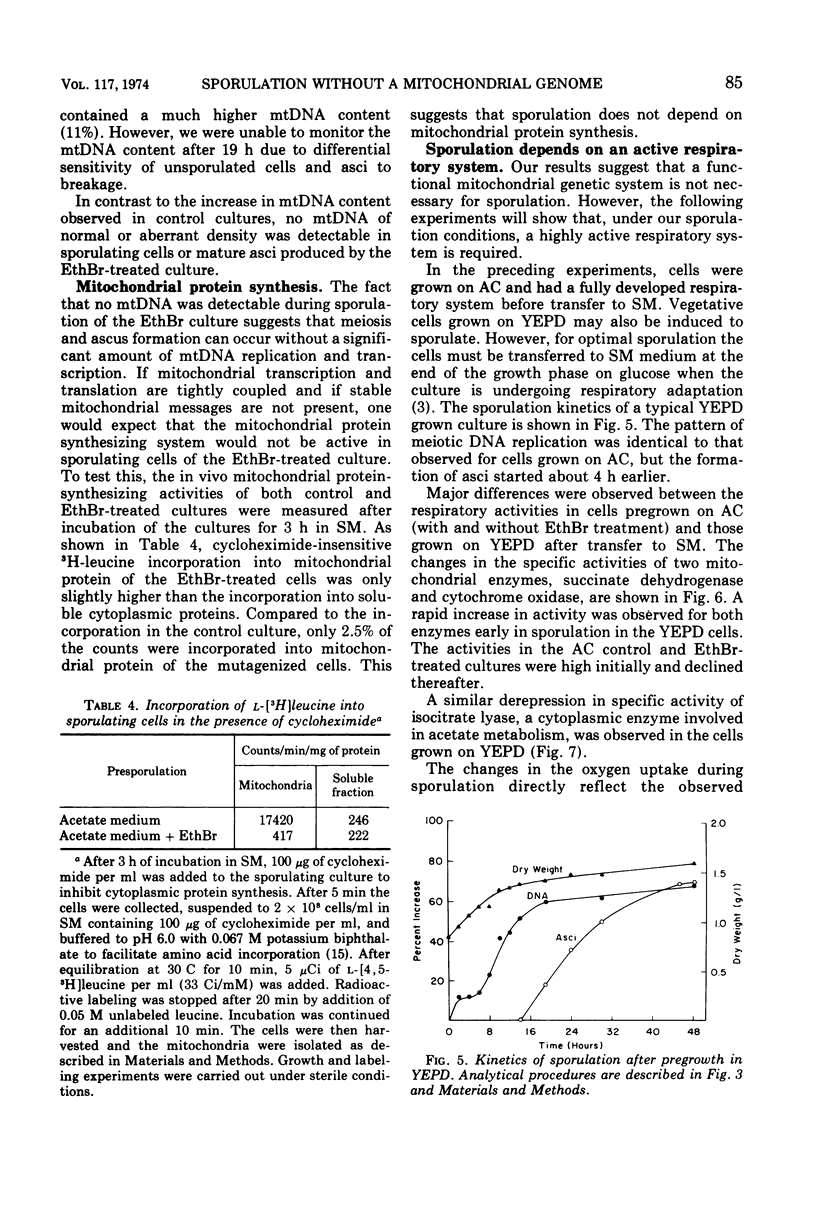
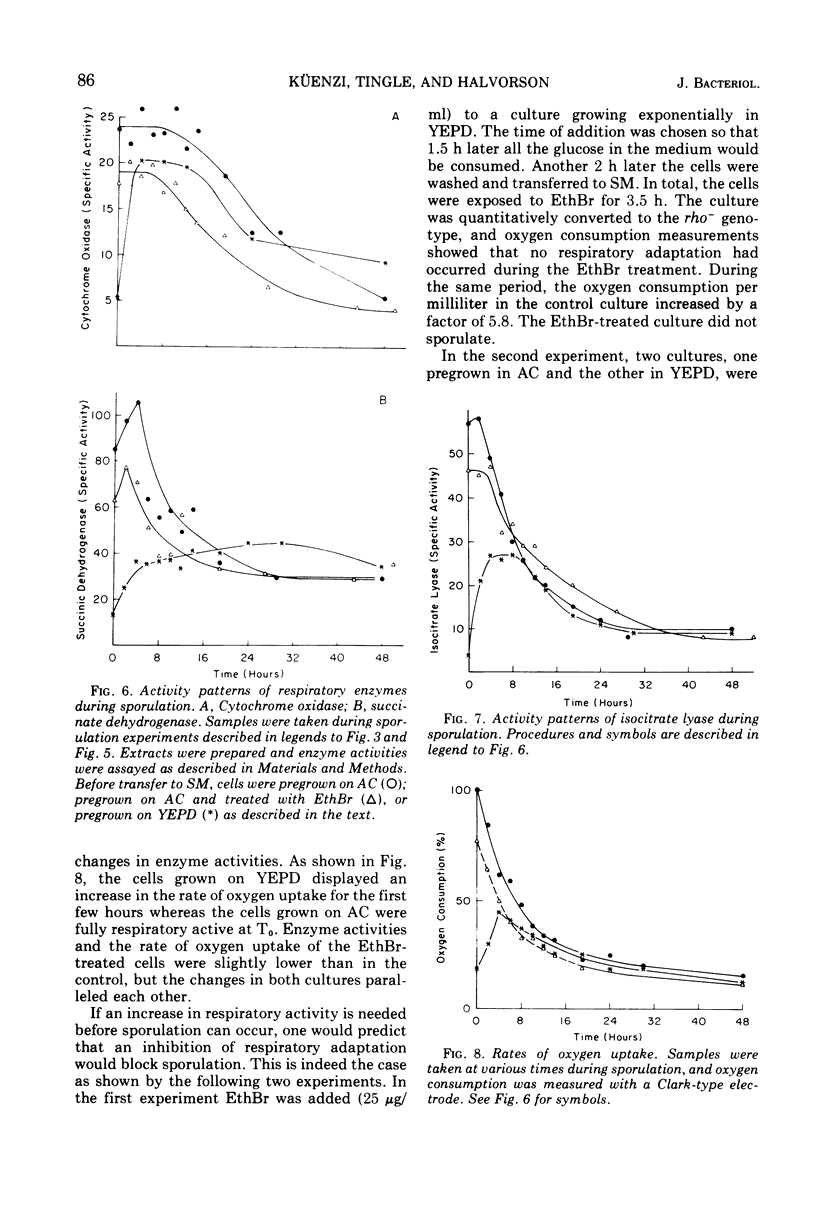
The role of the mitochondrial system during sporulation of Saccharomyces cerevisiae was studied. Addition of ethidium bromide (EthBr) to cells growing in acetate medium resulted in the quantitative (>98%) conversion of the culture to the petite genotype in one generation. The cells were respiratory active (derepressed) but contained no mitochondrial deoxyribonucleic acid (mtDNA) as demonstrated by analytical ultracentrifugation in CsCl. When transferred to acetate sporulation medium, the culture sporulated. Ascus production was only slightly below that of the control culture. Synthesis of mtDNA occurred during sporulation in the control but not in the EthBr-treated culture. Mitochondrial protein synthesis was virtually eliminated in the EthBr-treated culture. Therefore, completely derepressed cells can sporulate without a functional mitochondrial genetic system. When partially repressed cells were treated with EthBr, no ascus formation was observed after transfer to sporulation medium. Control cultures underwent respiratory adaptation in sporulation medium and then sporulated. Extensive derepression of the respiratory system is thus required for sporulation, and this adaptation is dependent on a functional mitochondrial system. Our results suggest that once the cells are fully derepressed no mitochondrial genetic information has to be expressed during meiosis and ascus formation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bulder C. J. Anaerobic growth, ergosterol content and sensitivity to a polyene antibiotic, of the yeast Schizosaccharomyces japonicus. Antonie Van Leeuwenhoek. 1971;37(3):353–358. doi: 10.1007/BF02218505. [DOI] [PubMed] [Google Scholar]
- Esposito M. S., Esposito R. E., Arnaud M., Halvorson H. O. Conditional mutants of meiosis in yeast. J Bacteriol. 1970 Oct;104(1):202–210. doi: 10.1128/jb.104.1.202-210.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito R. E., Frink N., Bernstein P., Esposito M. S. The genetic control of sporulation in Saccharomyces. II. Dominance and complementation of mutants of meiosis and spore formation. Mol Gen Genet. 1972;114(3):241–248. doi: 10.1007/BF01788893. [DOI] [PubMed] [Google Scholar]
- Goldring E. S., Grossman L. I., Krupnick D., Cryer D. R., Marmur J. The petite mutation in yeast. Loss of mitochondrial deoxyribonucleic acid during induction of petites with ethidium bromide. J Mol Biol. 1970 Sep 14;52(2):323–335. doi: 10.1016/0022-2836(70)90033-1. [DOI] [PubMed] [Google Scholar]
- HALPERN C., MILLER J. J. The metabolism of yeast sporulation. I. Effect of certain metabolites and inhibitors. Can J Microbiol. 1956 Oct;2(6):519–537. doi: 10.1139/m56-064. [DOI] [PubMed] [Google Scholar]
- Haber J. E., Halvorson H. O. Cell cycle dependency of sporulation in Saccharomyces cerevisiae. J Bacteriol. 1972 Mar;109(3):1027–1033. doi: 10.1128/jb.109.3.1027-1033.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber J. E., Halvorson H. O. Regulation of sporulation in yeast. Curr Top Dev Biol. 1972;7:61–83. doi: 10.1016/s0070-2153(08)60069-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mahler H. R., Perlman P. S. Effects of mutagenic treatment by ethidium bromide on cellular and mitochondrial phenotype. Arch Biochem Biophys. 1972 Jan;148(1):115–129. doi: 10.1016/0003-9861(72)90122-1. [DOI] [PubMed] [Google Scholar]
- Mills D. Effect of pH on adenine and amino acid uptake during sporulation in Saccharomyces cerevisiae. J Bacteriol. 1972 Oct;112(1):519–526. doi: 10.1128/jb.112.1.519-526.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagley P., Linnane A. W. Biogenesis of mitochondria. XXI. Studies on the nature of the mitochondrial genome in yeast: the degenerative effects of ethidium bromide on mitochondrial genetic information in a respiratory competent strain. J Mol Biol. 1972 Apr 28;66(1):181–193. doi: 10.1016/s0022-2836(72)80015-9. [DOI] [PubMed] [Google Scholar]
- Perlman P. S., Mahler H. R. Molecular consequences of ethidium bromide mutagenesis. Nat New Biol. 1971 May 5;231(18):12–16. [PubMed] [Google Scholar]
- Puglisi P. P., Zennaro E. Erythromycin inhibition of sporulation in Saccharomyces cerevisiae. Experientia. 1971 Aug;27(8):963–964. doi: 10.1007/BF02135775. [DOI] [PubMed] [Google Scholar]
- Roth R. Carbohydrate accumulation during the sporulation of yeast. J Bacteriol. 1970 Jan;101(1):53–57. doi: 10.1128/jb.101.1.53-57.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth R., Fogel S. A system selective for yeast mutants deficient in meiotic recombination. Mol Gen Genet. 1971;112(4):295–305. doi: 10.1007/BF00334431. [DOI] [PubMed] [Google Scholar]
- Schatz G., Saltzgaber J. Protein synthesis by yeast promitochondria in vivo. Biochem Biophys Res Commun. 1969 Dec 4;37(6):996–1001. doi: 10.1016/0006-291x(69)90230-7. [DOI] [PubMed] [Google Scholar]
- Slonimski P. P., Perrodin G., Croft J. H. Ethidium bromide induced mutation of yeast mitochondria: complete transformation of cells into respiratory deficient non-chromosomal "petites". Biochem Biophys Res Commun. 1968 Feb 15;30(3):232–239. doi: 10.1016/0006-291x(68)90440-3. [DOI] [PubMed] [Google Scholar]
- Tingle M. A., Küenzi M. T., Halvorson H. O. Germination of yeast spores lacking mitochondrial deoxyribonucleic acid. J Bacteriol. 1974 Jan;117(1):89–93. doi: 10.1128/jb.117.1.89-93.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


