Abstract
Objective
To examine the effects of a maximal exercise test on cognitive function in recreational athletes.
Design
A repeated‐measures design was used to compare baseline with post‐cognitive function and fatigue symptoms after a maximal exercise test.
Setting
Division 1 American Midwestern University, (Michigan State University, Michigan, USA).
Participants
102 male and female recreational athletes.
Intervention
Participants in the experimental group (n = 54) were asked to perform a maximal treadmill exercise test to maximal oxygen uptake (VO2 max). Participants in the control group were asked to rest for 15 min.
Main outcome measurements
All participants were administered a neuropsychological test battery called Immediate Post‐Concussion Assessment and Cognitive Testing (ImPACT) before and after exercise to measure neurocognitive function and fatigue symptoms.
Results
Results revealed a significant group (control, experimental)×time (baseline, post‐test 1, post‐test 2) interaction for verbal memory composite scores (p = 0.025). Specifically, verbal memory composite scores decreased in the experimental group from baseline to post‐test 1 (p = 0.00). These values returned to baseline 3 days after the VO2 max test (p = 0.00). Further analysis on verbal memory composite scores demonstrated significant differences on immediate recall memory (p = 0.00) and delayed recall memory (p = 0.00). No significant differences were observed for visual memory (p = 0.54), motor processing speed (p = 0.68) and reaction time (p = 0.44) composite scores between the experimental and control groups.
Conclusion
The results of this study suggest that a maximal exercise test attenuated a limiting effect on cognitive function. When utilising a neuropsychological test battery to evaluate a patient who has sustained a head injury, the test should not be administered immediately after a practice or a game session.
Over the past two decades, understanding the cognitive effects of a concussion, and establishing a neuropsychological testing timeline, has become an interest of both researchers and clinicians in the field of sports medicine.1,2 Despite the increased utilisation of computerised neuropsychological testing, considerable debate exists among sports medicine practitioners on the time sequence for baseline and/or follow‐up neuropsychological testing.3 Currently, some sports medicine practitioners administer a baseline and follow‐up neuropsychological test after practice or competition, while athletes are still in an exerted state. Others administer the test either before practice or 24 h after an athlete has sustained a concussion. Clearly, if physical exertion impairs an athlete's cognitive function, then sports medicine professionals should not administer a neuropsychological test until the athlete has recovered from his or her state of exertion.
Immediate Post‐Concussion Assessment and Cognitive Testing (ImPACT) V.2005 (ImPACT Applications, Pittsburgh, Pennsylvania, USA) is a computer‐based program used to assess neurocognitive function and concussion symptoms.4 Computerised neurocognitive testing assists with establishing return‐to‐play criteria5 and allows for tracking of post‐concussive symptoms in athletes who have sustained multiple concussions in their athletic career.1 Pre‐season baseline testing has become a common practice in sport and is imperative to recognise neurocognitive impairments and changes after concussion1; yet, no standard baseline assessment schedule has been agreed on by researchers and clinicians.3
Athletes are often in an state of exertion during the on‐field evaluation of a concussion. Separating exertional effects from physical and cognitive deficits is a challenge for team physicians and certified athletic trainers. Wilkins et al6 reported that fatigue before postural‐stability testing has decreased balance performance. These balance deficits are only one component to evaluation of concussions and return‐to‐play decisions. Therefore, it is important to examine cognitive function and how it is affected by physical exertion. Previous research has been conducted to determine the effects of exertion on cognitive function.7,8,9,10,11,12,13 Despite the plethora of research, results are unequivocal, with some studies suggesting a beneficial or a facilitating effect of exercise on cognitive function,9,10,11,12,13 whereas others show exercise to be detrimental14,15 or having no effect on certain mental tasks.7,16 Results of these studies are difficult to compare owing to varying exercise durations, intensities and a variety of cognitive tests administered. Furthermore, these cognitive tests were administered at different times during and after the exercise test.
To date, only one study has investigated the effects of exertion on a concussion grading system. Leclerc et al17 studied the effects of a 4 min treadmill run at 80% of age predicted maximal heart rate and performance on the McGill Abbreviated Concussion Evaluation in collegiate athletes. Results revealed that athletes did not demonstrate differences in cognitive function from pre‐test to post‐test. Although the McGill Abbreviated Concussion Evaluation is a valid means of baseline and post‐concussion assessment, the ImPACT computerised software has emerged as a leading application to assess the cognitive state of the injured and uninjured athlete. To date, no studies have examined the cognitive effects of a maximal oxygen uptake (VO2 max) exercise on the findings of a computerised neuropsychological test battery—that is, ImPACT. The purpose of the study was to examine the effects of a maximal exercise test on cognitive function, as measured by the ImPACT programme, in recreational athletes.
Methods
Experimental design
A repeated‐measures design was used to compare baseline with post‐cognitive function and fatigue symptoms after a maximal exercise test. The independent variable in this study was a treadmill exercise to the participant's VO2 max and time (baseline, post‐test 1 and post‐test 2). The dependent variables were verbal and visual memory, motor processing speed and reaction time composite scores created with the ImPACT software.
Participants
One hundred and two recreational athletes from a large Midwestern University (Michigan State University, Michigan, USA) volunteered to participate in the study. A recreational athlete was defined as someone who participates in sports or physical activity 2–3 times per week. Participants were aged 18–24 years and reported no lower extremity injury or concussion within the past 12 months. Participants with a history of self‐reported cardiovascular, respiratory illness or colour blindness were excluded from the study.
Instrumentation
To assess cognitive function, the ImPACT V.200518 neuropsychological test battery was used for this study. The ImPACT test takes approximately 25 min to complete and has five different test iterations to minimise the practice effects of taking multiple tests. The ImPACT software records data in three categories. In the first category, athletes use the keyboard and a mouse to input demographic and descriptive information through a series of instructional screens. The demographic section includes experience of playing sports, history of alcohol and drug use, learning disabilities, attention‐deficit hyperactive disorders, major neurological disorders and history of concussion. The second category consists of 22 symptoms that are associated with concussions for which the athletes rate their perception of the presence of the symptom at the time of the test. The items are rated using a 7‐point Likert scale. Athletes self‐rate their concussion symptoms by clicking on a number between 0 (not experiencing) and 6 (severe) using a mouse. This study did not examine athletes who had concussions, therefore, only fatigue was analysed. The third category consists of six neuropsychological test protocols that evaluate the participants' attention processes, verbal recognition memory, visual working memory, visual processing speed, reaction time, numerical sequencing ability and learning.
Previous research has examined the psychometric properties of the ImPACT test battery for both test–retest reliability and validity.19,20 Test–retest reliability for ImPACT was assessed over 8 days across four administrations, yielding correlation coefficients ranging from 0.66 to 0.85 for the verbal memory index, from 0.75 to 0.88 for the processing speed and from 0.62 to 0.66 for the reaction time.4 Using reliable change indices, repeated administrations over a 2‐week period revealed no practice effects.2 Correlations between ImPACT visual and verbal memory composites with the Brief Visual Spatial Memory Test‐Revised total score (r = 0.50) and the delayed recall score (r = 0.85) have been established21; the processing speed composite was shown to correlate with the Trailmaking Tests A (r −0.49) and B (r −0.60), and the Symbol–Digit Modalities test (r = 0.68). More recently, Schatz et al20 examined the sensitivity and specificity of the ImPACT test on individuals diagnosed as having a concussion. Seventy‐two high‐school athletes who sustained a concussion were tested using ImPACT within 72 h of the initial injury. Their scores were then compared with 66 high‐school athletes with no history of concussion. Between‐group comparisons showed a significant multivariate effect of concussion on test performance. Analysis of the battery scores showed 81.9% sensitivity and 89.4% specificity for the assessment of neurocognitive and neurobehavioural sequelae.
Procedure
Before the start of data collection, the University Committee on Research Involving Human subjects approved the study. All participants completed a health history questionnaire and signed a written informed consent form. Following informed consent, all participants were given a practice ImPACT assessment to familiarise them with the software. This practice test was to ensure that participants understood the directions of the test to eliminate potential mistakes on the day of the test. Participants were randomly assigned to either the control or experimental group. Following assignment, participants were asked to sign up for a testing session 3–5 days after the practice session. Participants reported to the Athletic Training Research Laboratory on the day of the test and were administered a baseline ImPACT test. The experimental group was administered a VO2 max treadmill test. Participants in the control group were asked to remain at rest for 15 min. It was determined from pilot data that it takes approximately 15 min to set‐up the athlete on the treadmill and metabolic cart to completion of the VO2 max test.
The treadmill protocol used for the VO2 max test began at a speed of 2.5 mph at 0.0% incline. The speed of the treadmill was increased 0.5 mph/min until 6.0 mph was reached. Once 6.0 mph was achieved, the incline of the treadmill increased 3% per minute until volitional exhaustion occurred. VO2 max was defined as the highest value for VO2 during the test. Achievement of VO2 max was confirmed by a minimum of two of the three following criteria: (1) respiratory exchange ratio (RER) >1.15; (2) >90% of their age predicted maximal heart rate; and (3) plateau of VO2. RER is described as the volume of carbon dioxide produced divided by the volume of oxygen consumed.22
A Sensormedics (Yorba Linda, California, USA) Measurement Cart was used to collect and assess metabolic parameters including VO2, carbon dioxide production, pulmonary ventilation (VE) and RER. Before each test, the apparatus was calibrated against standard commercial gas mixtures. During the test, heart rate was measured every minute using a Polar (Gays Mills, Wisconsin, USA) heart rate monitor.
After the completion of the treadmill test, participants were instructed to immediately begin their follow‐up ImPACT test (post‐test 1). Likewise, 15 min after the baseline test, participants in the control group were instructed to begin their follow‐up test. Three days later (mean 3.20 days) all participants were administered a second post‐test.
Data analysis
The ImPACT test yields individual scores as well as clinical composite scores for verbal memory, visual memory, motor processing speed and reaction time. Athletes with a greater score on verbal and visual memory, and motor processing speed indicate a better performance. Verbal and visual memory scores are presented as a percentage of 100 and motor processing speed as a composite score number. Athletes with a faster score on reaction time, presented in seconds, indicate a better performance. Further analysis investigated performance on the individual modules that make up the verbal memory composite score. The verbal memory score is derived from individual performance on five tasks: (1) immediate recall memory; (2) delayed recall memory; (3) symbol match (with key); (4) symbol match (without key); and (5) 3‐letter recall.
Means (SD) were calculated for participant demographics and VO2 max treadmill test results. A two‐group (experimental, control) × three‐time (baseline, post‐test 1, post‐test 2) analysis of variance with repeated measures was conducted to analyse neuropsychological test scores and fatigue. The level of significance was set at p = 0.05. All analyses were conducted using SPSS V.13.1 for Windows.
Results
There were 48 participants in the control group and 54 in the experimental group (table 1). The average VO2 max for participants in the experimental group was 50.30 (6.45) ml/kg/min. The average RER was 1.16 (0.067) carbon dioxide production/VO2 and maximal heart rate was 190.78 (8.89) bpm.
Table 1 Demographic information of the participants.
| n | Age (years) | Height (inches) | Weight (lbs) | |
|---|---|---|---|---|
| Control | ||||
| Male | 23 | |||
| Mean (SD) | 21.55 (1.87) | 70.69 (2.72) | 197.52 (36.22) | |
| Female | 25 | |||
| Mean (SD) | 20.94 (1.62) | 66.12 (2.57) | 156.40 (24.91) | |
| Total | 48 | |||
| Mean (SD) | 21.25 (1.74) | 68.41 (2.65) | 176.96 (30.57) | |
| Experimental | ||||
| Male | 27 | |||
| Mean (SD) | 20.93 (1.87) | 70.44 (3.14) | 176.18 (23.45) | |
| Female | 27 | |||
| Mean (SD) | 21.07 (1.70) | 64.78 (3.04) | 135.70 (13.29) | |
| Total | 54 | |||
| Mean (SD) | 21.00 (1.79) | 67.61 (3.09) | 155.94 (18.34) |
Self‐reported fatigue
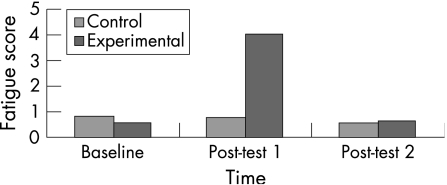
Results showed significant differences in self‐reported fatigue in the experimental group (F(3,52) = 125.77, p<0.00), but not in the control group (F(3,46) = 0.75, p>0.05). Pairwise comparisons for the experimental group revealed significant increases in fatigue scores from baseline to post‐test 1 (p<0.05), and significant decreases in fatigue from post‐test 1 to post‐test 2 (p<0.05; fig 1).
Figure 1 A comparison of self‐reported fatigue score.
Cognitive function following VO2 max
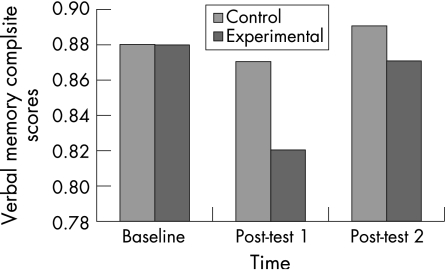
Results revealed a statistically significant group (control, experimental) × time (baseline, post‐test 1, post‐test 2) interaction for verbal memory composite scores (F(2,100) = 3.75, p = 0.00). Specifically, verbal memory composite scores decreased in the experimental group from baseline to post‐test 1 (p = 0.00). These values returned to baseline 3 days after the VO2 max test (p = 0.00; fig 2). There were no significant differences between group × time for visual memory (F(2,100) = 0.090, p = 0.914), motor processing speed (F(2,100) = 2.646, p = 0.074) and reaction time (F(2,100) = 0.334, p = 0.717).
Figure 2 Descriptive means for the verbal memory composite scores.
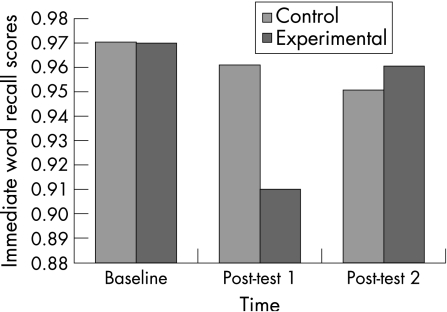
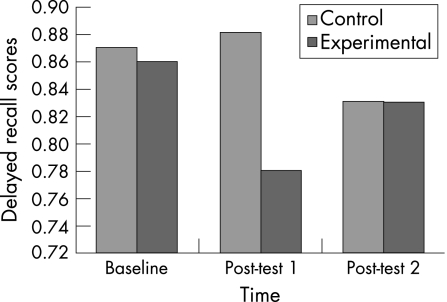
The exercise group had significant impairments in their immediate recall memory scores (F(2,100) = 7.76, p = 0.00). Pairwise comparisons showed a significant deterioration in scores from baseline to post‐test 1 (p = 0.00), and a significant improvement from post‐test 1 to post‐test 2 (p = 0.00; fig 3). Significant differences were also exhibited for the exercise group on delayed recall memory scores (F(2,100) = 5.00, p = 0.00). Analysis of the experimental group using pairwise comparisons revealed a significant deterioration in scores from baseline to post‐test 1, and a significant improvement from post‐test 1 to post‐test 2 (fig 4). There were no main effects or interaction effects for the other three components (symbol match with and without key, three‐letter recall) of the verbal memory composite score. Furthermore, there were no significant differences for visual memory (p = 0.54), motor processing speed (p = 0.68) and reaction time (p = 0.44) composite scores between the experimental and control groups.
Figure 3 Descriptive means for performance on the immediate word recall task.
Figure 4 Descriptive means for performance on the delayed recall word memory task.
Discussion
This study suggests that cognitive impairments on verbal memory composite scores occur after a maximal exercise test and measured by the ImPACT test protocol. Further analysis examining the individual ImPACT test modules that compile the verbal memory composite score revealed a significant deterioration on both the immediate and delayed recall tasks after the exercise intervention. These findings support those of Cian et al23 and Frey et al24 who showed deterioration on verbal memory tasks after a bout of exercise. Cian et al23 attributed the deterioration in performance to dehydration, whereas Frey et al24 suggested a decrease in performance on memory tasks resulting from changes in cortical activity in the brain and hypoxia brought about by exercise.
It is believed that deterioration on the verbal memory composite scores in this study stemmed from fatigue after the maximal exercise test. Immediate recall memory was the first task that participants were exposed to on the post‐exercise ImPACT test. It is possible that the fatigued state of the subject caused by the treadmill intervention led to attentional distraction, possibly leading to difficulty remembering the words presented for immediate word recall, and delayed recall at the end of the test battery (approximately 15 min). The other three components that comprise the verbal memory composite scores (symbol match with and without key, three‐letter recall task) were not significantly affected. It should be noted that these modules were presented later in the test. It seems that participants may have had sufficient time to recover from their fatigue state caused by the maximal exercise test. It is also possible that no cognitive impairments were seen in visual memory, motor processing speed and reaction time composite scores for the same reason. The deterioration in verbal memory composite scores may also be attributed to vascular changes in the brain.25,26 Previous researchers have shown decreased cerebral oxygenation and cerebral cortex activity after an exhaustive bout of exercise.25,26
Neuropsychological testing has become a common method for assessing cognitive function in athletes who have a concussion.1 Sports medicine professionals in the past have relied on self‐reported symptoms and clinical examination. Using computer‐based neuropsychological software allows the sports medicine team to track recovery and symptom resolution. Currently, computerised neuropsychological testing is being used as a means of quantifying post‐concussive symptoms and cognitive deficits in athletes.1 It is becoming common practice in athletic training to administer pre‐season baseline tests. This practice allows clinicians to compare post‐concussion scores with an individualised baseline. At present, there is not enough information to develop a definitive timeline for baseline and post‐concussion neuropsychological testing. On the basis of the findings of this project, it is recommended that these tests should not be administered immediately after practice, competition or after sustaining a concussion owing to the acute physiological effects that exercise may play on an individual's neurocognitive function.
What is already known on this topic
Athletes are often in an exerted state during the on‐field evaluation of a concussion.
Separating exertional effects from physical and cognitive deficits is a challenge for team physicians and certified athletic trainers.
Despite the plethora of research, results are unequivocal, with some studies suggesting a beneficial or a facilitating effect of exercise on cognitive function, whereas others show exercise to be detrimental or having no effect on certain mental tasks.
What this study adds
The results of this study suggest that a maximal exercise test attenuated a limiting effect on cognitive function.
When using a neuropsychological test battery to evaluate a patient who has sustained a head injury, the test should not be administered immediately after a practice or a game session.
Variables such as participants' education level, hydration status and hours of sleep were not controlled in this study. Another limitation was that participant fitness levels were not controlled in this study. This limitation should only have a small effect on the results of this study because the purpose was not to determine a relationship between fitness level and neurocognitive performance. It would have been ideal to randomise the order of the six ImPACT test modules for each recreational athlete. Despite this limitation, ImPACT is programmed to limit practice effects utilising multiple randomised test forms, including different target word lists for each assessment.
In conclusion, this study examined the effects of VO2 max treadmill exercise on neurocognitive function, in attempts to assist sports medicine professionals with developing a timeline for baseline and post‐concussion ImPACT testing. This study is among the first studies to show impairment on the verbal memory composite score following a VO2 max treadmill test on healthy individuals. Additional impairments were seen in immediate and delayed memory composite scores. Further studies should examine the effects of exertion on cognitive function by employing protocols directly related to sports‐specific activity.
Abbreviations
imPACT - Immediate Post‐Concussion Assessment and Cognitive Testing
VO2 max - maximal oxygen uptake
Footnotes
Competing interests: JP was a member of the design team of ImPACT.
References
- 1.Collins M, Grindell S, Lovell M.et al Relationship between concussion and neuropsychological performance in college football players. JAMA 1999282964–970. [DOI] [PubMed] [Google Scholar]
- 2.Iverson G, Lovell M, Collins M.et al Tracking recovery from concussion using ImPACT: applying reliable change methodology. Arch Clin Neuropsychol 200217770 [Google Scholar]
- 3.Schatz P, Zillmer E. Computer‐based assessment of sports related‐concussion. Appl Neuropsychol 20031042–47. [DOI] [PubMed] [Google Scholar]
- 4.Lovell M R, Collins M W, Fu F H.et al Neuropsychologic testing in sports: past, present and future. Br J Sports Med 200135367–372.11579081 [Google Scholar]
- 5.Erlanger D, Saliba E, Barth J.et al Monitoring resolution of post‐concussion symptoms in athletes: preliminary results of a web based neuropsychological test protocol. J Athl Train 200136280–287. [PMC free article] [PubMed] [Google Scholar]
- 6.Wilkins J, Valovich T, Perrin D.et al Performance on the balance error scoring system decreases after fatigue. J Athl Train 200431156–161. [PMC free article] [PubMed] [Google Scholar]
- 7.Fleury M, Bard C. Effects of different types of physical activity on the performance of perceptual tasks in peripheral and central vision and coincident timing. Ergonomics 198730945–958. [DOI] [PubMed] [Google Scholar]
- 8.Gutin B, DiGennaro J. Effect of one‐minute and five‐minute step‐ups on performance of simple addition. Res Quart 1968b3981–85. [PubMed] [Google Scholar]
- 9.Hancock S, McNaughton L. Effects of fatigue on ability to process visual information by experienced orienteers. Percept Mot Skills 198662491–498. [DOI] [PubMed] [Google Scholar]
- 10.Hogervorst E, Riedel W, Jeukendrup A.et al Cognitive performance after strenuous physical exercise. Percept Mot Skills 199683479–488. [DOI] [PubMed] [Google Scholar]
- 11.McGlynn G, Laughlin N, Bender V. Effects of strenuous to exhaustive exercise on a discrimination task. Percept Mot Skills 1977441139–1147. [Google Scholar]
- 12.Paas F, Adam J. Human information processing during physical exercise. Ergonomics 1991341385–1397. [DOI] [PubMed] [Google Scholar]
- 13.Salmela J, Ndoye O. Cognitive distortions during progressive exercise. Percept Mot Skills 1986631067–1072. [Google Scholar]
- 14.Isaacs L, Pohlman E. Effects of exercise intensity on an accompanying timing task. J Hum Mov Stud 199120120–131. [Google Scholar]
- 15.Wrisberg C, Herbert W. Fatigue effects on the timing performance of well practiced subjects. Res47839–844. [PubMed] [Google Scholar]
- 16.Iverson G, Lovell M, Collins M. Validity of ImPACT for measuring the effects of sports‐related concussion. Neuropsychol 200217769. [DOI] [PubMed] [Google Scholar]
- 17.Leclerc S, Hussain S, Johnston K M. Does exertion modify results on the McGill Abbreviated Concussion Evaluation (McGill ACE)? Med Sci Sport Exer 200234S103 [Google Scholar]
- 18.Lovell M, Collins M, Podell K.et alImmediate post‐concussion assessment cognitive testing. Pittsburgh: NeuroHealth Systems, 2005
- 19.Iverson G, Lovell M, Collins M. Validity of ImPACT for measuring processing speed following sport‐related concussion. J Clin Exp Neuropsychol 200527683–689. [DOI] [PubMed] [Google Scholar]
- 20.Schatz P, Pardini J, Lovell M.et al Sensitivity and specificity of the ImPACT Test Battery for concussion in athletes. Arch Clin Neuropsychol 20062191–99. [DOI] [PubMed] [Google Scholar]
- 21.Iverson G L, Franzen M D, Lovell M R.et al Construct validity of computerised neuropsychological screening in athletes with concussion. Clin Neuropsychol 200419961–962. [Google Scholar]
- 22.Howley E, Bassett D, Welch H. Criteria for maximal oxygen uptake: review and commentary. Med Sci Spor Ex 1995271292–1301. [PubMed] [Google Scholar]
- 23.Cian C, Koulmann N, Barraud P A.et al Influence of variations in body hydration on cognitive function: Effects of hyperhydration, heat stress, and exercise‐induced dehydration. J Psychophysiol 20001429–36. [Google Scholar]
- 24.Frey Y, Ferry A, Vom Hofe A.et al effect of physical exhaustion on cognitive functioning. Percep Mot Skill 199784291–298. [DOI] [PubMed] [Google Scholar]
- 25.Shibuya K, Tanaka J, Kuboyama N.et al Cerebral oxygenation during intermittent supramaximal exercise. Resp Physiol Neurobiol 2004140165–172. [DOI] [PubMed] [Google Scholar]
- 26.Shibuya K, Tanaka J, Kuboyama N.et al Cerebral cortex activity during supramaximal exhaustive exercise. J Sports Med Phys Fitness 200444215–219. [PubMed] [Google Scholar]