Abstract
Background
Neovascularisation and microcirculatory changes have been reported in Achilles tendinopathy. Cryotherapy and compression, as part of a rest, ice, compression and elevation regimen, are shown to decrease pain and improve function. However, the microcirculatory changes following a given dosage of cryotherapy on mid‐portion Achilles tendon remain unclear.
Study design
Prospective clinical cohort study, level of evidence 2.
Methods
30 people (12 males, 33 (SD 12) years, body mass index 25.6 (5.3) kg/m2) were included in the cohort. 3×10 min KoldBlue ankle‐cooling bandages were applied and microcirculation of Achilles tendon mid‐portion was real‐time and continuously assessed using a laser‐Doppler‐spectrophotometry system (O2C, Germany).
Results
Superficial capillary blood flow was reduced from 42 to 6, 5 and 3 relative units (rU) in the first, second and third cryotherapy periods, respectively (−65%, p = 0.001), with no significant capillary hyperaemia. Deep capillary tendon blood flow was reduced from 180 to 82, 53 and 52 rU (−71%, p = 0.001) within 6–9 min of application without hyperaemia. Superficial tendon oxygen saturation dropped significantly from 43% to 26%, 18% and 11% (p = 0.001) after repetitive cryotherapy, with persisting increase of tendon oxygenation during rewarming (51%, 49% and 54%, p = 0.077) up to 27% of the baseline level. At 8 mm tendon depth, cryotherapy preserved local oxygenation. Relative postcapillary venous tendon filling pressures were favourably reduced from 41 (11) to 31, 28 and 26 rU (−36%, p = 0.001) superficially and from 56 (11) to 45, 46 and 48 rU (−18%, p = 0.001) in deep capillary blood flow during cryotherapy, facilitating capillary venous clearance.
Conclusion
Intermittent cryotherapy of 3×10 min significantly decreases local Achilles tendon mid‐portion capillary blood flow by 71%. Within 2 min of rewarming, tendon oxygen saturation is re‐established following cryotherapy. Postcapillary venous filling pressures are reduced during cryotherapy, favouring capillary venous outflow of the healthy Achilles tendon.
Common cryotherapy, as part of the rest, ice, compression and elevation treatment regimen, is a basic treatment principle of acute soft‐tissue injuries. It is thought to reduce swelling and pain by various mechanisms.1,2 Cryotherapy reduces deep‐tissue temperature in animals and humans, in a time and application‐dependent manner. Vasodilatation, increased capillary permeability with fluid extravasation and swelling, metabolic changes and increased proinflammatory cytokines are associated with soft‐tissue injuries. Although cryotherapy has to penetrate the skin barrier to the injured anatomical structure, which is time‐ and depth‐dependent, compression on the other side is thought to immediately limit bleeding within the injured tissue. However, some studies question the use of cryotherapy and compression in patients following arthroscopic anterior cruciate repair3; but effects on tendon microcirculation have not been studied yet.
Symptomatic Achilles tendinopathy is associated with a certain degree of neovascularisation shown by either colour Doppler or power Doppler ultrasound systems.4 The Achilles tendon and paratendon microcirculation in healthy athletes differ from patients with either insertional or mid‐portion tendinopathy, where capillary blood flow at the point of pain is increased, as determined by laser Doppler flowmetry.5
In a previous study, using a non‐invasive real‐time laser Doppler spectrophotometry system on the ankle to detect the microcirculatory effects of combined cryotherapy and compression as achieved by the AIRCAST Cryo/Cuff device (AIRCAST, Summit, NJ, USA), we found the relevant effects on microcirculation of combined cryotherapy and compression to take place within the first 10 min of application during a total 30 min application time.6 A recent randomised trial has supported this, showing that 10 min of intermittent ice application is superior to a single 20 min cryotherapy treatment with regard to ankle pain on exercise.7 Intermittent ischaemia and reperfusion have been reported to be protective for tissue vitality sustaining an ischaemic event as part of the ischaemia/reperfusion cascade.
We therefore hypothesised that the intermittent 3×10 min cryotherapy changes Achilles tendon microcirculation with regard to tendon capillary blood flow, tendon oxygen saturation and postcapillary venous filling pressure.
Methods
Thirty subjects (12 men, 33 (SD 12) years, body mass index 25.6 (5.3) kg/m2) participated in this study (table 1). Informed consent was given by each subject and institutional review board approval was granted by the local medical school for this study. Regarding physical activity, a total of 23 individuals participated in regular physical activity between 1 and 10 h per week (3.5 (2.9) h/week). Eleven performed jogging, seven underwent fitness training and nine performed cycling. Two participants were competitive athletes in long‐distance running (7–10 h per week). The mean Foot and Ankle Outcome Score (FAOS) ranged from 95.6 to 99.8. Nineteen individuals had reduced results in FAOS domain 1 (symptoms; between 89.3 and 96.4) and only two subjects had reduced FAOS results in FAOS 2 (pain), FAOS 3 (everyday life) and FAOS 4 (sport and leisure, 80–98.5). Seven patients achieved 81.8–93.8 in FAOS 5 (quality of life). Baseline Achilles mid‐portion microcirculation was not different vs the other participants compared with our experience in our trial on Achilles tendinopathy.5 Regarding drug intake at the time of the study, 13 did not take any drugs on a regular basis, 7 were taking oral contraceptives and 4 were treated with β blockers (propanolol and carvedilol) and angiotensin II‐receptor blockers (valsartan and telmisartan) for arterial hypertension. Two participants took analgetics (two non‐steroidal antirheumatics, none aspirin, one ibuprofen and naproxen, one tramadole). No antibiotics, especially chinolones, were used by the participants, which rule out any effect on Achilles tendon microcirculation. During the 60 min measurement, the patients were asked for their subjective assessement of the cryotherapy on a scale of 0–10, where 10 equals the most imaginable coldness (similar to the visual analogue scale for patients' pain assessement).
Table 1 Characteristics of 30 individuals participating in this study.
| Mean (SD) | |
|---|---|
| Male | 12 (40%) |
| Female | 18 (60%) |
| Age (years) | 33 (12) |
| Height (cm) | 173 (5) |
| Weight (kg) | 77 (18) |
| BMI (kg/m2) | 25.6 (5.3) |
| Active smoker | 11 (37%) |
| Pack‐years | 12 (14) |
| Regular sport activity | 23 (77%) |
| Hours/week | 3.5 (2.9) |
| Jogging | 11 (37%) |
| Fitness | 7 (23%) |
| Biking | 9 (30%) |
| FAOS 1 (symptom) | 95.6 (4) |
| FAOS 2 (pain) | 99.5 (2) |
| FAOS 3 (everyday life) | 99.8 (1) |
| FAOS 4 (sport and leisure) | 99 (4) |
| FAOS 5 (quality of life) | 97 (6) |
BMI, body mass index; FAOS, Foot and Ankle Outcome Score.
Exclusion criteria were open wounds at the ankle level, any history of Raynaud's disease or other vasospastic diseases, cold hypersensitivity or diseases with compromised local circulation or evident ulcerations, such as diabetes mellitus. Furthermore, only healthy patients with regard to the Achilles tendon were included. No one had ever had Achilles or patellar tendon pain nor were any sonographic changes of the Achilles tendon evident in conventional ultrasound. Power Doppler identified no neovascularisation among all individuals included. Before the study, potential confounders such as active smoking or decreased FAOS were ruled out to have any influence regarding the microcirculatory effects on the Achilles mid‐portion level in comparison with the other participants as well as in comparison with the patient group of 66 patients in the Achilles tendinopathy trial published.5
Determination of vital parameters of microcirculation
A combined laser Doppler and flowmetry system (Oxygen‐to‐see (O2C system) LEA Medizintechnik, Giessen, Germany, www.lea.de) was used to evaluate microcirculation at two distinct tissue depths non‐invasively. The O2C system has focused areas of determination, which currently cannot be modified manually. Simultaneous measurement at 2 and 8 mm tissue depths was performed. The optical method for measuring blood flow by laser Doppler technique and haemoglobin oxygenation and haemoglobin concentration in tissue by spectrometric techniques has been described in detail elsewhere.6,8,9 The determination of haemoglobin and the principle of blood flow measurement are combined in the O2C system. The local oxygen supply parameters, blood flow, oxygen saturation of haemoglobin sulphur dioxide (SO2; %) and amount of local haemoglobin rHb were recorded by an optical fibre probe (O2COXYGEN‐TO‐SEE, LEA Medizintechnik).
Laser Doppler flowmetry
The tissue was illuminated with coherent laser light of 830 nm and 30 mW from a laser diode through a fibre optic light guide. Backscattered light was collected by the same probe and frequency‐shifted light extracted by the heterodyne light‐beating technique. The power–spectral density of shifted light is a linear function of the average velocity of moving cells within the tissue. As laser Doppler flowmetry detects all the moved particles of certain velocity, it measures blood flow.
Units of O2C system
Arbitrary units (AU) and relative units (rU) were used to measure blood flow and blood flow velocity, respectively. These are units that are chosen arbitrarily by the developer of the device and the reason for the introduction of AU is based on the origin of the values. The measured signals for blood flow are electrical values of frequencies and amplitudes, so the unit is a combination of electrical units. Therefore, a new unit for blood flow is introduced. To calculate the blood flow in ml/min, it would be necessary to compare the electrical signals with a method that measures the blood flow in ml/min (eg, plethysmography, microspheres) for each organ (or organs with similar optical properties). Then the arbitrary units can be converted to ml/min. This “calibration” has to be carried out for the measured organ, as there is no artificial model at the moment that simulates tissue in a realistic way. The same applies for the unit of haemoglobin rHb (relative units).
Reproducibility of the O2C system
The reproducibility of the blood flow values determined using the O2C system has been assessed in a test–retest design in 20 healthy, non‐smoking adults (22–39 years). Multivariate analysis of variance for repeated measures did not show significant differences in the mean blood flow values within and between different days. An average 5% intrasubject variability was calculated.10
KoldBlue application for combined cryotherapy
A KoldBlue cryotherapy device was applied to the Achilles tendon covering the targeted mid‐portion Achilles tendon region according to the manufacturer's recommendation. The O2C probe was placed in every subject at exactly the same site, 4 cm above the Achilles tendon insertion at the mid‐portion tendon level below the KoldBlue ankle bandage where it was fixed. Every subject was lying throughout the 60 min study and was not allowed to change body position in order to rule out any influence of motion. After an initial baseline assessment of parameters of microcirculation, such as microvascular tendon blood flow, tendon oxygen saturation and relative postcapillary venous filling pressures of the Achilles tendon at two distinct tissue depths (2 and 8 mm, which are fixed by the device), measurements were continuously recorded using the O2C system throughout the three periods of 10 min cryotherapy followed by rewarming. All data were collected in real time at 50 Hz, displayed by the machine. We built mean values among every 20 s throughout 60 min of the total study time. Cryotherapy was applied for 10 min, followed by 10 min of rewarming in three cycles (3×10 min cryotherapy and rewarming).
None of the 30 enrolled patients had any skin burns or nerve palsies, such as paresthesias or paresis, following 3×10 min of cryotherapy.
Statistics
The data are presented as mean (SD) for continuous variables or number and percentages for dichotomous variables. Paired t tests were conducted to compare microcirculation SO2, rHb, flow and velocity, and a p value <0.05 was considered to indicate significance. The general linear model with multivariate testing was applied to control the results for the measured difference between the measurements. For the latter, a p value in the Wilks–Lambda score of <0.05 was considered to be of significance. SPSS statistical software V.13.0 OG for Windows was used for statistical analysis.
Results
Subjective cold perception
Before the first cryotherapy, the cold perception value on a scale of 0–10 was on average 0.7 (SD 1.2; range 0–4). During the three consecutive intermittent cryotherapy cycles, the peak score was reached within the first minute during the first application (6.3 (2.4)) and the second application (7.5 (1.7)), and after 2 min during the third application (7.8 (1.7)). During the rewarming without the KoldBlue device, values reached 1.8 (1.7; first period of rewarming), 2.5 (2.3; second period of rewarming) and 2.9 (2.1; third period of rewarming). Nine participants rated their sensibility to coldness as 10 for the KoldBlue cryotherapy periods.
Achilles tendon mid‐portion capillary blood flow
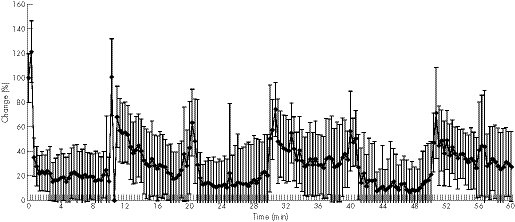
Superficial capillary blood flow at 2 mm of mid‐portion Achilles was 42 rU, which dropped immediately to 15 rU (−65%, p = 0.001) within the first minute during the first cryotherapy cycle. During the second and third cycles, the superficial capillary blood flow decreased to 18 rU (−57% of baseline, p = 0.021) and to 20 rU (−53%, p = 0.006) within 20 s. The lowest capillary flow values were 6 rU (first cryotherapy, −84.8%, p = 0.001), 5 rU (second cryotherapy, −89%, p = 0.001) and 3 rU (third cryotherapy, −93%, p = 0.001) during KoldBlue application (fig 1, table 2). No significant hyperaemia was noted with regard to the superficial mid‐portion Achilles tendon capillary blood flow during the rewarming periods.
Figure 1 Achilles mid‐portion capillary blood flow during three sets of 10 min cryotherapy and rewarming.
Table 2 Parameters of microcirculation, such as tendon oxygen saturation, postcapillary venous filling pressures, capillary tendon blood flow and capillary blood flow velocity at 2 and 8 mm Achilles tendon tissue depth during three repetitive sets of cryotherapy using the Koldblue and consecutive recovery of 10 min each.
| Baseline | First cryotherapy | First recovery | Second cryotherapy | Second recovery | Third cryotherapy | Third recovery | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial first min | Max | Initial first min | Max | Initial first min | Max | Initial first min | Max | Initial first min | Max | Initial first min | Max | ||
| So2 | 43 | 28 (22) | 26 (23) | 48 (23) | 51 (24) | 25 (23) | 18 (19) | 38 (27) | 49 (29) | 20 (20) | 11 (10) | 43 (29) | 54 (26) |
| 2 mm | 19 | p = 0.004 | p = 0.003 | p = 0.09 | p = 0.061 | p = 0.009 | p = 0.000 | p = 0.319 | p = 0.112 | p = 0.000 | p = 0.000 | p = 0.49 | p = 0.077 |
| % | 100 | 67 | 61 | 114 | 120 | 63 | 42 | 93 | 116 | 48 | 25 | 101 | 127 |
| So2 | 68 | 67 (11) | 67 (11) | 69 (10) | 69 (10) | 66 (12) | 66 (12) | 69 (11) | 69 (11) | 65 (11) | 65 (11) | 69 (11) | 69 (11) |
| 8 mm | ±11 | p = 0.129 | p = 0.129 | p = 0.079 | p = 0.079 | p = 0.003 | p = 0.003 | p = 0.239 | p = 0.239 | p = 0.000 | p = 0.000 | p = 0.120 | p = 0.120 |
| % | 100 | 99 | 99 | 102 | 102 | 97 | 97 | 102 | 102 | 96 | 96 | 102 | 102 |
| rHb | 41 | 35 (10) | 31 (10) | 40 (12) | 45 (12) | 34 (12) | 28 (11) | 40 (13) | 46 (14) | 35 (10) | 26 (11) | 41 (13) | 47 (14) |
| 2 mm | ±11 | p = 0.000 | p = 0.000 | p = 0.337 | p = 0.006 | p = 0.000 | p = 0.000 | p = 0.346 | p = 0.010 | p = 0.002 | p = 0.000 | p = 0.469 | p = 0.029 |
| % | 100 | 85 | 78 | 98 | 109 | 83 | 68 | 97 | 112 | 86 | 64 | 100 | 113 |
| rHb | 56 | 47 (9) | 45 (8) | 53 (12) | 59 (14) | 50 (9) | 46 (8) | 53 (12) | 60 (13) | 51 (9) | 46 (8) | 52 (11) | 60 (13) |
| 8 mm | 11 | p = 0.000 | p = 0.000 | p = 0.107 | p = 0.087 | p = 0.000 | p = 0.000 | p = 0.092 | p = 0.008 | p = 0.005 | p = 0.000 | p = 0.040 | p = 0.012 |
| % | 100 | 84 | 81 | 95 | 105 | 89 | 82 | 94 | 108 | 92 | 82 | 94 | 107 |
| Flow | 42 | 15 (13) | 6 (6) | 42 (72) | 59 (83) | 18 (37) | 5 (6) | 31 (38) | 31 (38) | 20 (21) | 3 (3) | 30 (61) | 30 (61) |
| 2 mm | ±46 | p = 0.001 | p = 0.000 | p = 0.256 | p = 0.123 | p = 0.021 | p = 0.000 | p = 0.065 | p = 0.065 | p = 0.006 | p = 0.000 | p = 0.312 | p = 0.312 |
| % | 100 | 35 | 15 | 101 | 141 | 43 | 11 | 74 | 74 | 48 | 7 | 71 | 71 |
| Flow | 180 | 150 (128) | 82 (85) | 163 (174) | 163 (174) | 87 (91) | 53 (56) | 112 (126) | 112 (126) | 95 (120) | 52 (82) | 100 (134) | 100 (134) |
| 8 mm | ±155 | p = 0.034 | p = 0.000 | p = 0.252 | p = 0.252 | p = 0.000 | p = 0.000 | p = 0.001 | p = 0.001 | p = 0.002 | p = 0.000 | p = 0.000 | p = 0.000 |
| % | 100 | 83 | 46 | 90 | 90 | 48 | 29 | 62 | 62 | 52 | 29 | 55 | 55 |
| Velo | 17 | 13 (6) | 10 (8) | 18 (13) | 18 (13) | 13 (8) | 8 (6) | 14 (9) | 14 (9) | 12 (8) | 5 (5) | 13 (10) | 14 (9) |
| 2 mm | ±7 | p = 0.003 | p = 0.000 | p = 0.285 | p = 0.285 | p = 0.001 | p = 0.000 | p = 0.009 | p = 0.009 | p = 0.001 | p = 0.000 | p = 0.011 | p = 0.044 |
| % | 100 | 79 | 59 | 107 | 107 | 74 | 45 | 84 | 84 | 71 | 32 | 75 | 79 |
| Velo | 28 | 25 (15) | 22 (16) | 27 (18) | 27 (18) | 24 (14) | 22 (16) | 25 (17) | 25 (17) | 23 (16) | 21 (16) | 24 (16) | 24 (16) |
| 8 mm | ±15 | p = 0.009 | p = 0.000 | p = 0.203 | p = 0.203 | p = 0.001 | p = 0.000 | p = 0.022 | p = 0.022 | p = 0.000 | p = 0.000 | p = 0.018 | p = 0.018 |
| % | 100 | 89 | 77 | 95 | 95 | 83 | 76 | 90 | 90 | 82 | 73 | 86 | 86 |
Numbers in parentheses are SD.
Flow, capillary tendon blood flow; Max, maximum; rHb, postcapillary venous filling pressure; So2, tendon oxygen saturation; Velo, velocity.
The deep capillary blood flow at 8 mm of the mid‐portion of the Achilles tendon was 180 rU at baseline, dropping significantly to 150 rU (first cryotherapy, −17%, p = 0.034), 87 rU (second cryotherapy, −52%, p = 0.001) and 95 rU (third cryotherapy, −48%, p = 0.002) within the first 40–60 s following KoldBlue application. The lowest levels of deep capillary tendon blood flow were 82 rU (−54%, p = 0.001), 53 rU (−71%, p = 0.001) and 52 rU (−71%, p = 0.001) following 6–9 min of cryotherapy.
Achilles tendon mid‐portion oxygen saturation
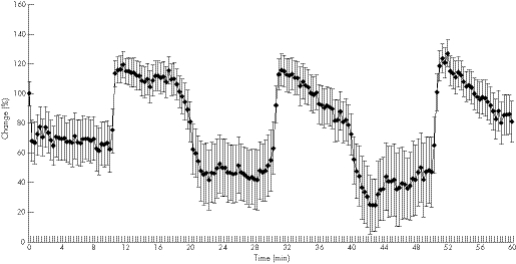
Achilles tendon mid‐portion oxygen saturation was assessed simultaneously at two distinct tissue depths (2 and 8 mm). At baseline, the superficial mid‐portion tendon oxygen saturation at 2 mm was 43 (19%), immediately dropping significantly to 28% (−33%, p = 0.004) during the first cryotherapy cycle (fig 2, table 2).
Figure 2 Achilles mid‐portion tendon oxygen saturation during three sets of 10 min cryotherapy and rewarming.
During the second cycle, Achilles tendon oxygenation was reduced to 25% (−38%, p = 0.009) and 20% (−52%, p = 0.001) during the third standardised KoldBlue application period within the first 2 min. The lowest levels of mid‐portion tendon oxygen saturation were measured after 4–9 min of standardised KoldBlue application during the three application periods (26%, p = 0.003; 18%, p = 0.001; and 11%, p = 0.001, respectively).
Following each cryotherapy cycle, a significant increase of tendon oxygen saturation was noted during the rewarming period beginning 40–60 s after removing the KoldBlue device, reaching the peak levels of 51 (24%; +20% vs baseline, p = 0.061), 49 (29%; +16% vs baseline, p = 0.112) and 54 (26%; +27% vs baseline, p = 0.077) after the three cryotherapy cycles, respectively.
Regarding the deep Achilles tendon mid‐portion oxygen saturation at 8 mm, the baseline was 68% (11%), with a small but significant decrease during the second and third cryotherapy cycles of only 3% and 4% reduction (68% (11%) vs 66% (12%), p = 0.003, and 68% (11%) vs 65% (11%), p = 0.001).
Postcapillary venous filling pressures in mid‐portion Achilles tendon
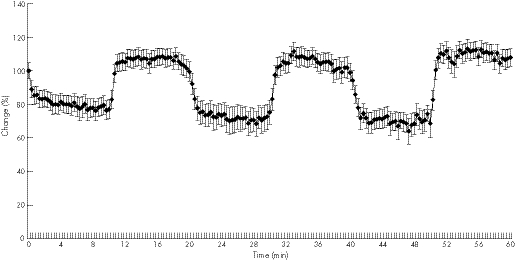
The superficial postcapillary venous filling pressure in the mid‐portion Achilles tendon at baseline was 41 (11) rU, dropping during the three cryotherapy cycles within the first 3 min to 35 (10) rU (−15%, p = 0.001), 34 (12) rU (−17%, p = 0.001) and 35 (10) rU (−14%, p = 0.002, fig 3, table 2). The lowest postcapillary venous filling pressures were noted, 31 (10) rU (−22%, p = 0.001), 28 (11) rU (−32%, p = 0.001) and 26 (11) rU (−36%, p = 0.001), within 6–8 min of cryotherapy using the KoldBlue. Following cryotherapy during rewarming, a small but significant increase of postcapillary venous filling pressure was noted with 45 (12) rU (+9% vs baseline, p = 0.006), 46 (14) rU (+12%, p = 0.01) and 47 (14) rU (13%, p = 0.029), respectively, after 3–5 min.
Figure 3 Achilles mid‐portion postcapillary venous filling pressures during three sets of 10 min cryotherapy and rewarming.
The deep postcapillary venous filling pressure in the mid‐portion Achilles tendon was 56 (11) rU at baseline, decreasing significantly during cryotherapy to 45 (8) rU (first cryotherapy, −19% vs baseline, p = 0.001), 46 (8) rU (second cryotherapy, −18%, p = 0.001) and 46 (8) rU (third cryotherapy, −18%, p = 0.001). During rewarming, postcapillary venous tendon filling pressures returned to the baseline level without a significant change versus baseline in all three rewarming periods.
Discussion
Tendon capillary blood flow
Intermittent cryotherapy of 3×10 min significantly decreases local Achilles tendon mid‐portion capillary blood flow by 71%. To the best of our knowledge, only one working group examined the microvascular effects of sole cryotherapy with significant reduction of microvascular perfusion after 20 min of cryotherapy, an effect that was reversed within 4 h after removal of the ice.11 Intermittent cryotherapy with 10 min intervals has been found to be clinically superior to a single 20 min interval in ankle sprains.7 By contrast, we found immediate changes of the Achilles tendon capillary blood flow within 20 s of cryotherapy using real‐time laser‐Doppler technology at two distinct tissue levels.
Tendon oxygen saturation
Within 2 min of rewarming, tendon oxygen saturation was re‐established following cryotherapy. Increased tendon oxygen saturation represents a beneficial state. In case of ischaemia, it has been found that oxygen saturation measured by the O2C system was reduced significantly up to 90% of the baseline level.9,12 Repetitive short terms of ischaemia and reperfusion are shown to increase oxygen saturation in an experimental study.12 The scheduled repetitive ischaemia/reperfusion cycle named ischaemic preconditioning is supposed to enhance the tissue's capability of withstanding ischaemic damage much better than a tissue that has not been preconditioned. In our model, cryotherapy over 10 min reduced the capillary flow significantly and thus decreased tendon oxygen saturation. Rewarming could enhance tendon oxygen saturation up to 27% higher than the baseline levels before the cryotherapy, although it did not reach statistical significance in this study.
However, the effects of cryotherapy as tested in our setting using KoldBlue is limited to the superficial tendon tissue without any clinical effect on the deep mid‐portion tendon tissue with regard to tendon oxygen saturation.
Cryotherapy alone reduces cellular respiration and enzymatic release of damaged tissue with inflammatory mediators by reducing the energy needs of a cell and thereby decreases the oxygen consumption.13 A 10°C reduction in intra‐articular temperature may decrease the local metabolic activity up to 50%.14 We did not measure intra‐articular or corresponding intratendinous invasive temperature in this completely non‐invasive study, so we cannot comment on the suspected intratendinous temperature following cryotherpy, but we did see changes of superficial tendon oxygen supply without any effect in 8 mm tissue depth on tendon oxygen saturation.
Postcapillary venous filling pressure of the Achilles tendon
Postcapillary venous filling pressures are reduced during cryotherapy, favouring capillary venous outflow. An increased postcapillary filling pressure indicates venous congestion with decreased clearance of local capillary metabolic end products, thus deteriorating the local tendon situation. Facilitation of postcapillary venous clearance of the Achilles mid‐portion is therefore supposed to be beneficial in a microcirculatory view. It could be speculated that in case of increased postcapillary venous filling pressures, such as in deep venous thrombosis or chronic venous insufficiency, a decrease of postcapillary venous filling pressures might be beneficial as well, if local metabolic function is preserved.
Limitations
Our study was designed as a non‐invasive study focusing on cryotherapy as achieved with a KoldBlue device. The conclusions drawn from this study are only valid for the use of cryotherapy in healthy Achilles tendons in the mid‐portion area; no conclusions can be drawn regarding either cryotherapy or compression in combination from this study alone. We decided to study sole cryotherapy in this setting, since it is clinically in use and applied thousands of times in clinical practice. No invasive temperature recordings were performed because this study was a completely non‐invasive trial. Furthermore, we wanted to deal with the issue of dosage such as a repetitive, 3×10 min cryotherapy. Our previous study6 found the main effects occurred within the first 10 min of cryotherapy, a protocol that has recently been tested clinically in a randomised controlled trial on ankle sprains.7 Nevertheless, we do not have any data beyond the intermittent 3×10 min time frame. Furthermore, we studied the tendon microcirculation in healthy subjects without swelling or tendinopathy. To what extent the described microcirculatory effects of sole cryotherapy are evident in patients with tendinopathy will be dealt with in a future study because increased capillary flow is encountered at the point of pain in Achilles tendinopathy. Capillary flow reduction by cryotherapy might be beneficial, especially given these obeservations.
Conclusions
Intermittent cryotherapy of 3×10 min significantly decreases local Achilles tendon mid‐portion capillary blood flow. Within 2 min of rewarming, tendon oxygen saturation is re‐established following cryotherapy. Postcapillary venous filling pressures are reduced during cryotherapy, favouring venous outflow. Cryotherapy exerts beneficial microcirculatory effects on the Achilles tendon mid‐portion.
What is already known on this topic
Cryotherapy is a common therapeutic option of the rest, ice, compression and elevation regimen clinically.
The main microcirculatory changes during cryotherapy are encountered within the first 10 min of application in a 30 min observation period.
What this study adds
Intermittent cryotherapy of 3×10 min significantly decreases local Achilles tendon mid‐portion capillary blood flow.
Within 2 min of rewarming, tendon oxygen saturation is re‐established following cryotherapy.
Postcapillary venous filling pressures are reduced during cryotherapy, favouring venous outflow.
Cryotherapy exerts beneficial microcirculatory effects on the Achilles tendon mid‐portion.
Abbreviations
AU - arbitrary units
FAOS - Foot and Ankle Outcome Score
rU - relative units, rHb‐haemoglobin relative units
Footnotes
Competing interests: None declared.
References
- 1.Knight K L, Londeree B R. Comparison of blood flow in the ankle of uninjured subjects during therapeutic applications of heat, cold, and exercise. Med Sci Sports Exerc 19801276–80. [DOI] [PubMed] [Google Scholar]
- 2.Knight K L. Cryotherapy in sports injury management. Int Perspec Physiother 19894163–185. [Google Scholar]
- 3.Edwards D J, Rimmer M, Keene G C. The use of cold therapy in the postoperative management of patients undergoing arthroscopic anterior cruciate ligament reconstruction. Am J Sports Med 199624193–195. [DOI] [PubMed] [Google Scholar]
- 4.Kristoffersen M, Öhberg L, Johnston C.et al Neovascularisation in chronic tendon injuries detected with colour Doppler ultrasound in horse and man: implications for research and treatment. Knee Surg Sports Traumatol Arthrosc 200513505–508. [DOI] [PubMed] [Google Scholar]
- 5.Knobloch K, Kraemer R, Lichtenberg A.et al Achilles tendon and paratendon microcirculation in mid‐portion and insertional tendinopathy in sportsmen. Am J Sports Med 20063492–97. [DOI] [PubMed] [Google Scholar]
- 6.Knobloch K, Kraemer R, Lichtenberg M.et al Microcirculation of the ankle after Cryo/Cuff application in healthy volunteers. Int J Sport Med 200627250–255. [DOI] [PubMed] [Google Scholar]
- 7.Bleakley C M, McDonough S M, MacAuley D C. Cryotherapy for acute ankle sprains: a randomized controlled study of two different icing protocols. Br J Sports Med 200640700–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frank K H, Kessler M, Appelaum K.et al The erlangen micro‐lightguide spectrophotometer EMPHO I. Phys Med Biol 1989341883–1900. [DOI] [PubMed] [Google Scholar]
- 9.Knobloch K, Lichtenberg A, Pichlmaier M.et al Microcirculation of the sternum following harvesting of the left internal mammary artery. Thorac Cardiovasc Surg 200351255–259. [DOI] [PubMed] [Google Scholar]
- 10.Ghazanfari M, Vogt L, Banzer W.et al Reproducibility of non‐invasive blood flow measurements using laser Doppler spectroscopy. Phys Med Rehab Kuror 200212330–336. [Google Scholar]
- 11.Cohn B T, Draeger R I, Jackson D W. The effects of cold therapy in the postoperative management of pain in patients undergoing anterior cruciate ligament reconstruction. Am J Sports Med 198917344–349. [DOI] [PubMed] [Google Scholar]
- 12.Lichtenberg A, Knobloch K, Pichlmaier M.et al Microcirculation of the human myocardium during ischemic preconditioning. Atherosclerosis 200341P–0003. [Google Scholar]
- 13.Olson J E, Stravino V D. A review of cryotherapy. Phys Ther 197252840–53 [DOI] [PubMed] [Google Scholar]
- 14.Swenson C, Swärd L, Karlsson J. Cryotherapy in sports medicine. Scand J Med Sci Sports 19966193–200. [DOI] [PubMed] [Google Scholar]