Abstract
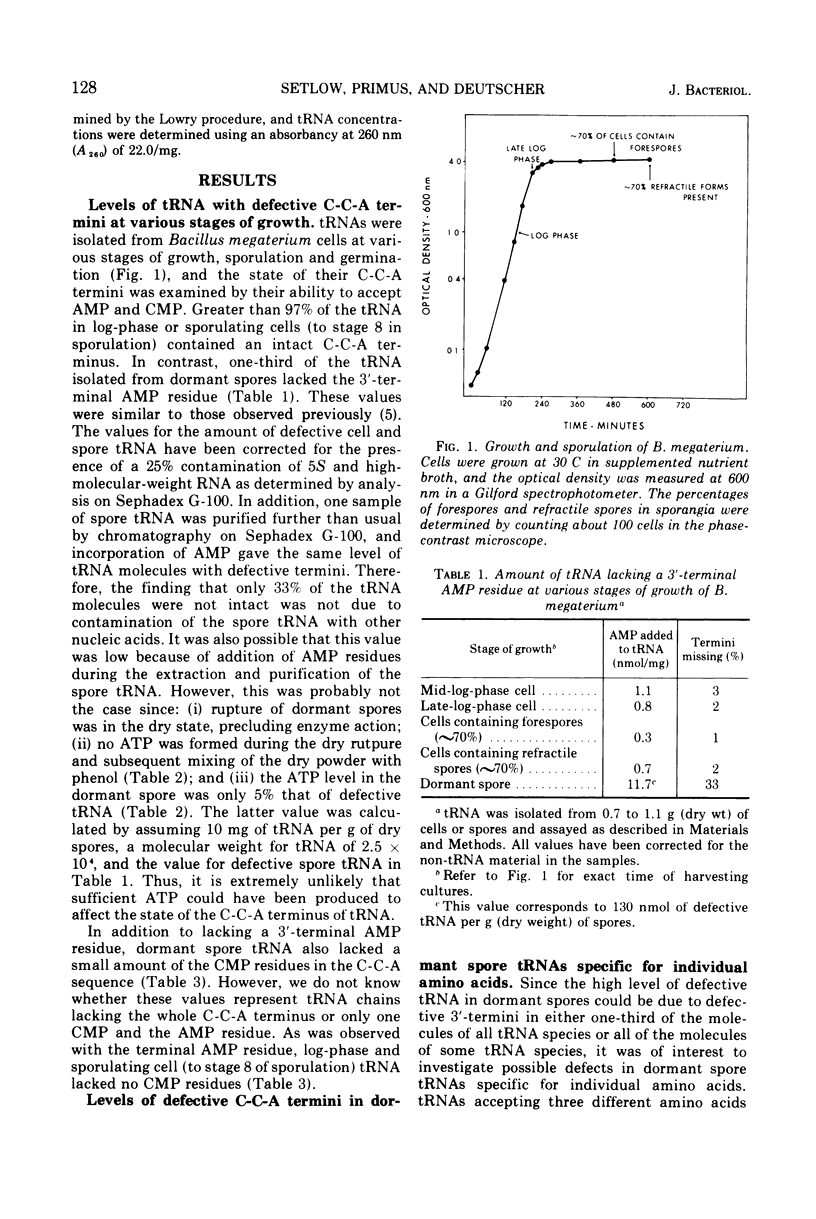
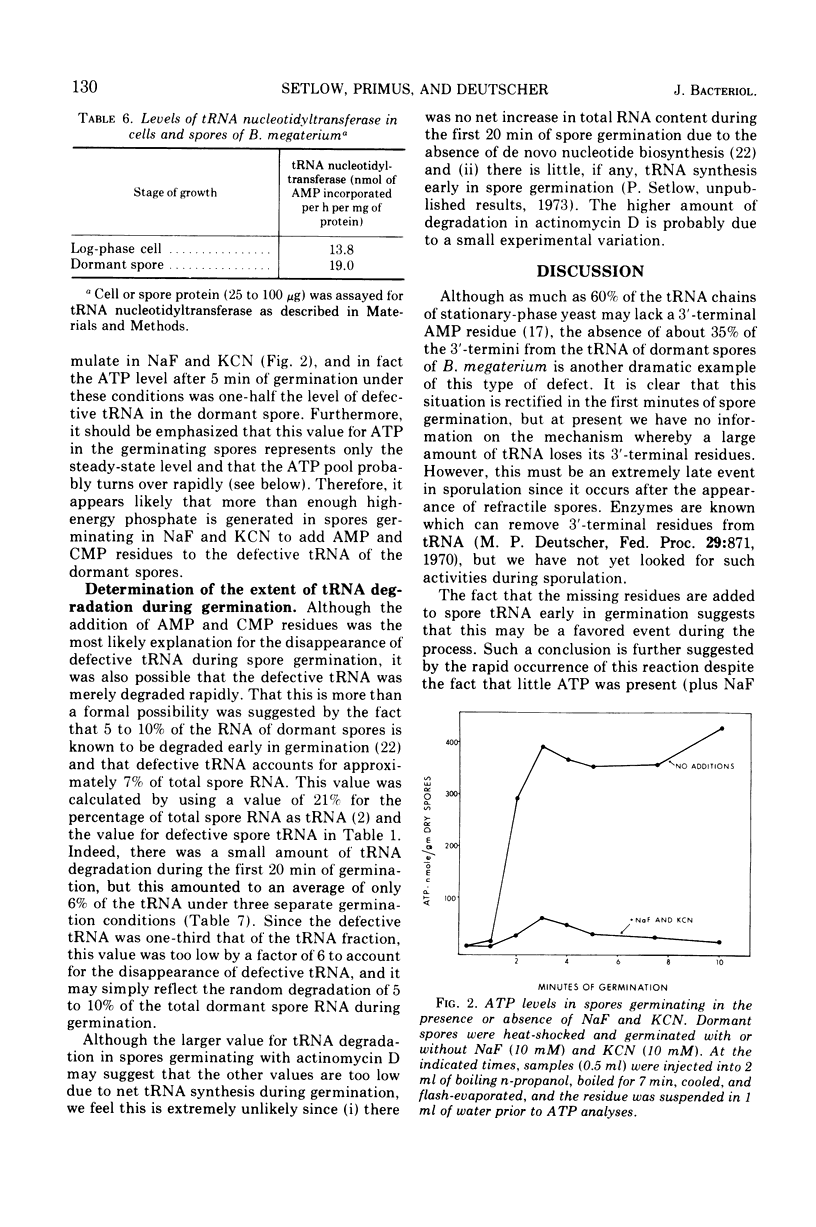
Essentially all (>97%) of the transfer ribonucleic acid (tRNA) in log-phase and sporulating cells of Bacillus megaterium contains a complete 3′-cytidyl-cytidyl-adenosine terminus. However, about one-third of the tRNA in the dormant spore lacks the 3′-terminal adenosine 5′-monophosphate (AMP) residue, and some of the adjacent cytosine monophosphate residues are also missing. Examination of specific tRNAs indicated that those specific for isoleucine, leucine, and methionine are missing 30 to 40% of their terminal residue, whereas tRNAs specific for tyrosine lack 88% of the 3′-terminal AMP. Defective spore tRNA is not degraded during germination, but the missing residues are added back in the first minutes of the process. The enzyme catalyzing the addition reaction, tRNA nucleotidyltransferase, is present in the dormant spore at a level similar to that found in the vegetative cell.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arceneaux J. L., Sueoka N. Two species of Bacillus subtilis tyrosine transfer ribonucleic acid. Biological properties and alteration in their relative amounts during growth. J Biol Chem. 1969 Nov 10;244(21):5959–5966. [PubMed] [Google Scholar]
- Chambon P., Deutscher M. P., Kornberg A. Biochemical studies of bacterial sporulation and germination. X. Ribosomes and nucleic acids of vegetative cells and spores of Bacillus megaterium. J Biol Chem. 1968 Oct 10;243(19):5110–5116. [PubMed] [Google Scholar]
- Deutscher M. P., Chambon P., Konberg A. Biochemical studies of bacterial sporulation and germination. XI. Protein-synthesizing systems from vegetative cells and spores of Bacillus megaterium. J Biol Chem. 1968 Oct 10;243(19):5117–5125. [PubMed] [Google Scholar]
- Deutscher M. P. Reactions at the 3' terminus of transfer ribonucleic acid. II. Purification and physical and chemical properties of rabbit liver transfer ribonucleic acid nucleotidyltransferase. J Biol Chem. 1972 Jan 25;247(2):450–458. [PubMed] [Google Scholar]
- Deutscher M. P. Reactions of the 3' terminus of transfer ribonucleic acid. VI. Properties of the poly(C) polymerase activity associated with rabbit liver transfer ribonucleic acid nucleotidyltransferase. J Biol Chem. 1973 May 10;248(9):3108–3115. [PubMed] [Google Scholar]
- GLISIN V. R., GLISIN M. V. RIBONUCLEIC ACID METABOLISM FOLLOWING FERTILIZATION IN SEA URCHIN EGGS. Proc Natl Acad Sci U S A. 1964 Dec;52:1548–1553. doi: 10.1073/pnas.52.6.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht L. I., Stephenson M. L., Zamecnik P. C. BINDING OF AMINO ACIDS TO THE END GROUP OF A SOLUBLE RIBONUCLEIC ACID. Proc Natl Acad Sci U S A. 1959 Apr;45(4):505–518. doi: 10.1073/pnas.45.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington M. D., Hawtrey A. O. Evidence for the absence of the terminal adenine nucleotide at the amino acid-acceptor end of transfer ribonucleic acid in non-lactating bovine mammary gland and its inhibitory effect on the aminoacylation of rat liver transfer ribonucleic acid. Biochem J. 1970 Feb;116(3):405–414. doi: 10.1042/bj1160405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holms W. H., Hamilton I. D., Robertson A. G. The rate of turnover of the adenosine triphosphate pool of Escherichia coli growing aerobically in simple defined media. Arch Mikrobiol. 1972;83(2):95–109. doi: 10.1007/BF00425016. [DOI] [PubMed] [Google Scholar]
- Idriss J. M., Halvorson H. O. The nature of ribosomes of spores of Bacillus cereus T. and Bacillus megaterium. Arch Biochem Biophys. 1969 Sep;133(2):442–453. doi: 10.1016/0003-9861(69)90474-3. [DOI] [PubMed] [Google Scholar]
- Kaneko I., Doi R. H. Alteration of valyl-sRNA during sporulation of bacillus subtilis. Proc Natl Acad Sci U S A. 1966 Mar;55(3):564–571. doi: 10.1073/pnas.55.3.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzarini R. A. Differences in lysine-sRNA from spore and vegetative cells of Bacillus subtillis. Proc Natl Acad Sci U S A. 1966 Jul;56(1):185–190. doi: 10.1073/pnas.56.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz J., Fain J. N. Stimulation by growth hormone and dexamethasone of labeled cyclic adenosine 3',5'-monophosphate accumulation by white fat cells. J Biol Chem. 1970 Mar 10;245(5):1101–1107. [PubMed] [Google Scholar]
- Preiss J., Berg P., Ofengand E. J., Bergmann F. H., Dieckmann M. THE CHEMICAL NATURE OF THE RNA-AMINO ACID COMPOUND FORMED BY AMINO ACID-ACTIVATING ENZYMES. Proc Natl Acad Sci U S A. 1959 Mar;45(3):319–328. doi: 10.1073/pnas.45.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosset R., Monier R. Instabilité de la séquence 3'-hydroxyle terminale du RNA de transfert chez les microorganismes. I. Renouvellement de l'AMP terminal chez Saccharomyces cerevisiae. Biochim Biophys Acta. 1965 Nov 8;108(3):376–384. [PubMed] [Google Scholar]
- SACKS L. E., BAILEY G. F. DRY RUPTURE OF BACTERIAL SPORES. J Bacteriol. 1963 Mar;85:720–721. doi: 10.1128/jb.85.3.720-721.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P. Deoxyribonucleic acid synthesis and deoxynucleotide metabolism during bacterial spore germination. J Bacteriol. 1973 Jun;114(3):1099–1107. doi: 10.1128/jb.114.3.1099-1107.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P., Kornberg A. Biochemical studies of bacterial sporulation and germination. 23. Nucleotide metabolism during spore germination. J Biol Chem. 1970 Jul 25;245(14):3645–3652. [PubMed] [Google Scholar]
- Setlow P., Kornberg A. Biochemical studies of bacterial sporulation and germination. XVII. Sulfhydryl and disulfide levels in dormancy and germination. J Bacteriol. 1969 Dec;100(3):1155–1160. doi: 10.1128/jb.100.3.1155-1160.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P., Kornberg A. Biochemical studies of bacterial sporulation and germination. XXII. Energy metabolism in early stages of germination of Bacillus megaterium spores. J Biol Chem. 1970 Jul 25;245(14):3637–3644. [PubMed] [Google Scholar]
- Stavy L., Gross P. R. The protein-synthetic lesion in unfertilized eggs. Proc Natl Acad Sci U S A. 1967 Mar;57(3):735–742. doi: 10.1073/pnas.57.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vold B. S. Analysis of isoaccepting transfer ribonucleic acid species of Bacillus subtilis: chromatographic differences between transfer ribonucleic acids from spores and cells in exponential growth. J Bacteriol. 1973 Feb;113(2):825–833. doi: 10.1128/jb.113.2.825-833.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vold B., Minatogawa S. Comparison of procedures for extracting transfer RNA from spores of Bacillus. Arch Biochem Biophys. 1972 Mar;149(1):62–68. doi: 10.1016/0003-9861(72)90299-8. [DOI] [PubMed] [Google Scholar]
- Zachau H. G., Acs G., Lipmann F. ISOLATION OF ADENOSINE AMINO ACID ESTERS FROM A RIBONUCLEASE DIGEST OF SOLUBLE, LIVER RIBONUCLEIC ACID. Proc Natl Acad Sci U S A. 1958 Sep 15;44(9):885–889. doi: 10.1073/pnas.44.9.885. [DOI] [PMC free article] [PubMed] [Google Scholar]



