Abstract
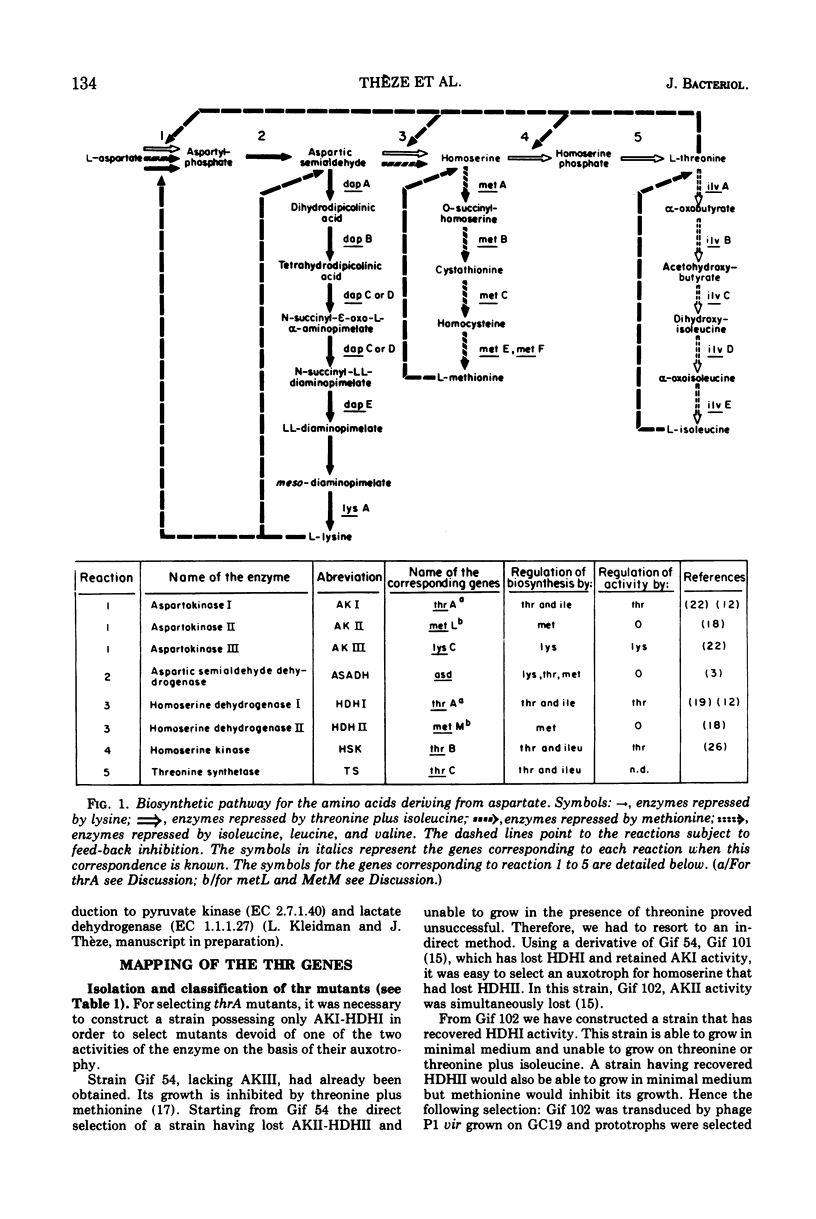
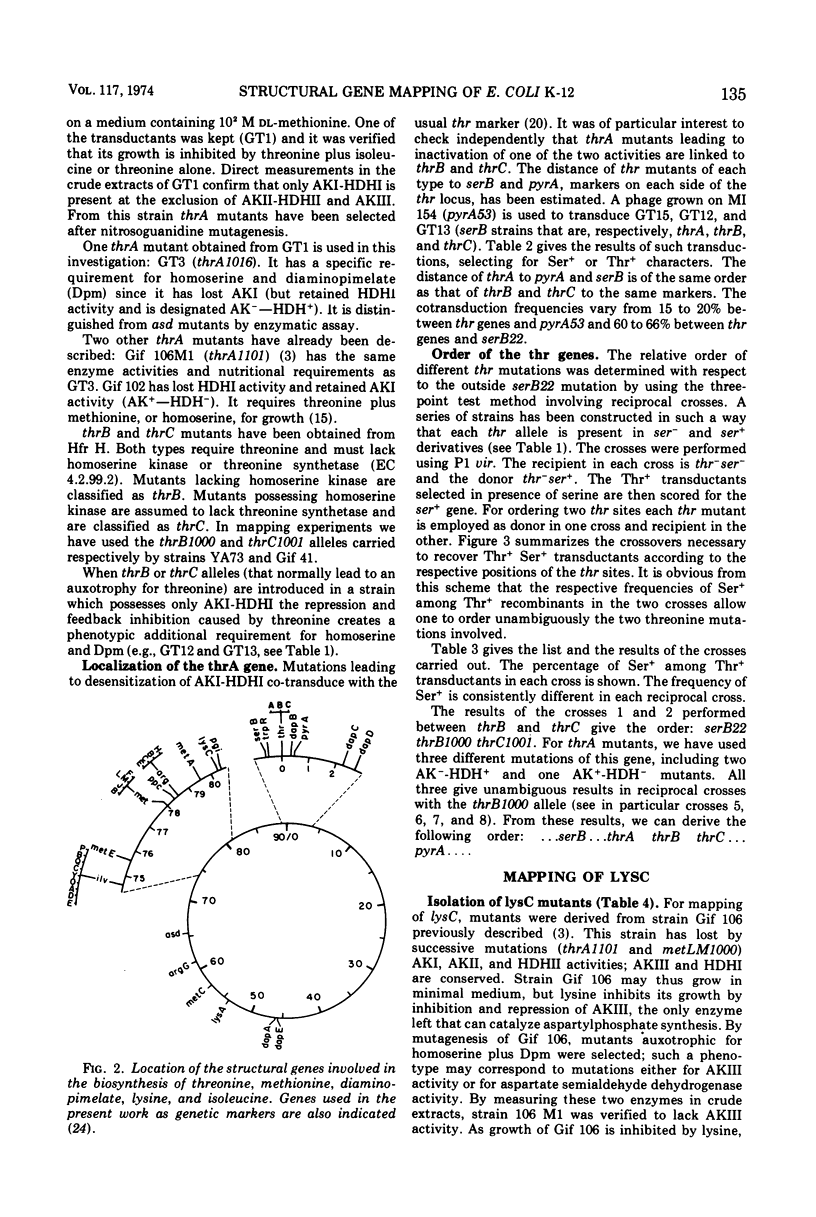
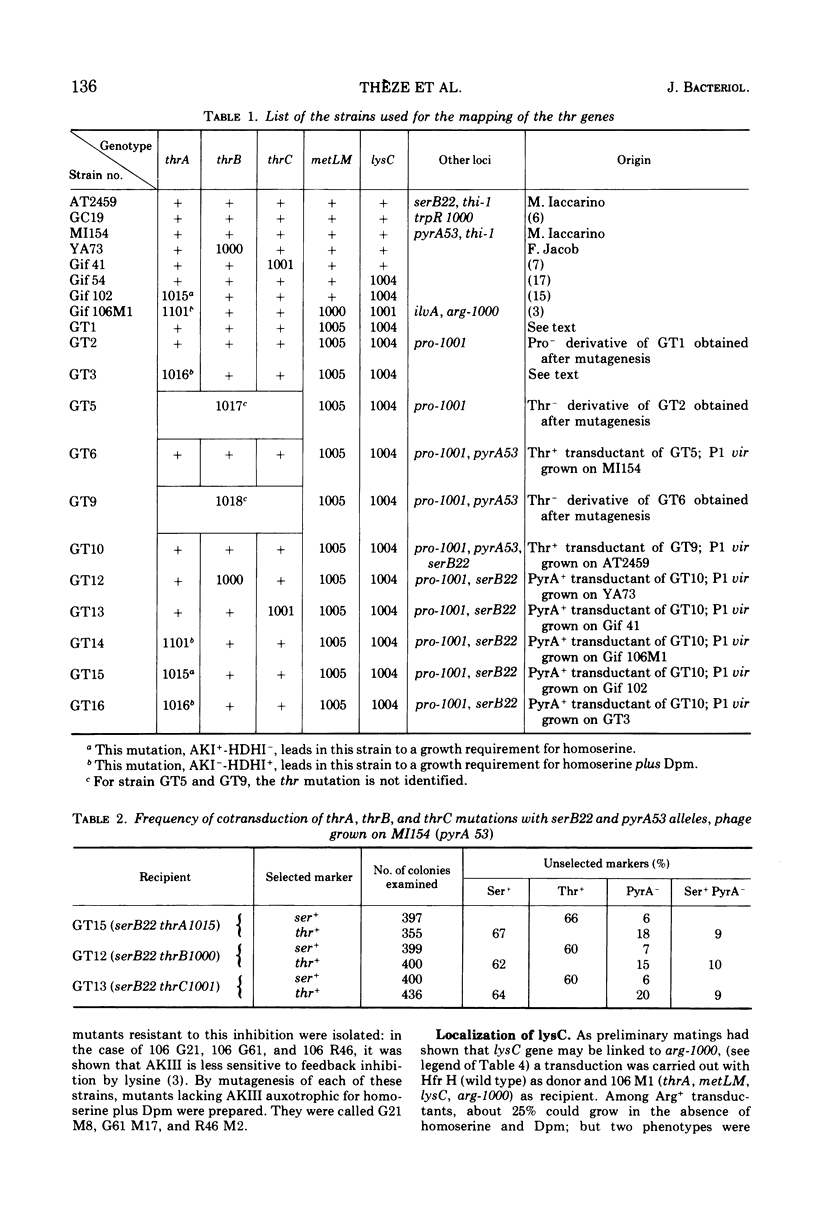
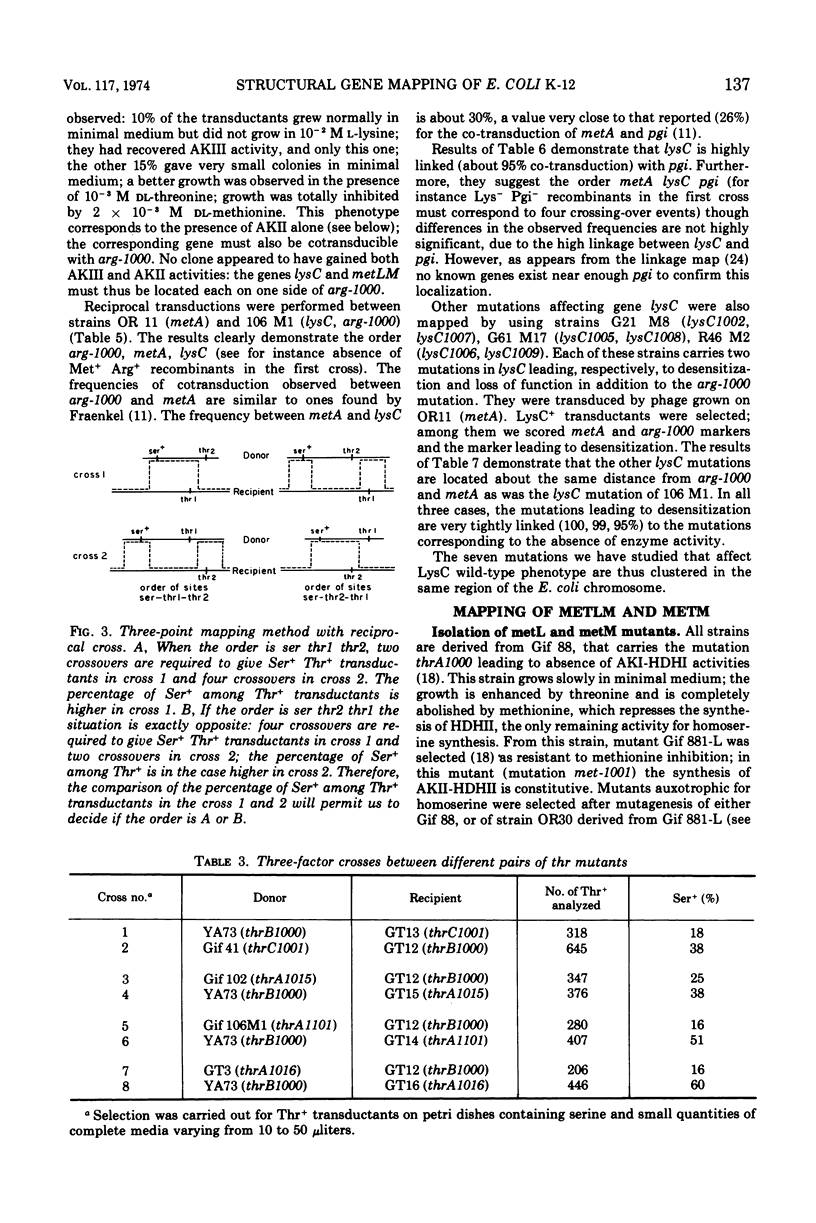
Mutants requiring threonine plus methionine (or homoserine), or threonine plus methionine plus diaminopimelate (or homoserine plus diaminopimelate) have been isolated from strains possessing only one of the three isofunctional aspartokinases. They have been classified in several groups according to their enzymatic defects. Their mapping is described. Several regions of the chromosome are concerned: thrA (aspartokinase I-homoserine dehydrogenase I) is mapped in the same region as thrB and thrC (0 min). lysC (aspartokinase III) is mapped at 80 min, far from the other genes coding for diaminopimelate synthesis. metLM (aspartokinase II-homoserine dehydrogenase II) lies at 78 min closely linked to metB, metJ, and metF.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayling P. D., Chater K. F. The sequence of four structural and two regulatory methionine genes in the Salmonella typhimurium linkage map. Genet Res. 1968 Dec;12(3):341–354. doi: 10.1017/s0016672300011927. [DOI] [PubMed] [Google Scholar]
- Boy E., Patte J. C. Multivalent repression of aspartic semialdehyde dehydrogenase in Escherichia coli K-12. J Bacteriol. 1972 Oct;112(1):84–92. doi: 10.1128/jb.112.1.84-92.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN G. N., RICKENBERG H. V. Concentration spécifique réversible des amino acides chez Escherichia coli. Ann Inst Pasteur (Paris) 1956 Nov;91(5):693–720. [PubMed] [Google Scholar]
- COHEN G., JACOB F. Sur la répression de la synthèse des enzymes intervenant dans la formation du tryptophane chez Escherichia coll. C R Hebd Seances Acad Sci. 1959 Jun 15;248(24):3490–3492. [PubMed] [Google Scholar]
- FREUNDLICH M. Multivalent repression in the biosynthesis of threonine in Salmonella typhimurium and Escherichia coli. Biochem Biophys Res Commun. 1963 Feb 6;10:277–282. doi: 10.1016/0006-291x(63)90430-3. [DOI] [PubMed] [Google Scholar]
- Falcoz-Kelly F., Janin J., Saari J. C., Véron M., Truffa-Bachi P., Cohen G. N. Revised structure of aspartokinase I-homoserine dehydrogenase I of Escherichia coli K12. Evidence for four identical subunits. Eur J Biochem. 1972 Aug 4;28(4):507–519. doi: 10.1111/j.1432-1033.1972.tb01938.x. [DOI] [PubMed] [Google Scholar]
- Falcoz-Kelly F., van Rapenbusch R., Cohen G. N. The methionine-repressible homoserine dehydrogenase and aspartokinase activities of Escherichia coli K 12. Preparation of the homogeneous protein catalyzing the two activities. Molecular weight of the native enzyme and of its subunits. Eur J Biochem. 1969 Mar;8(1):146–152. doi: 10.1111/j.1432-1033.1969.tb00507.x. [DOI] [PubMed] [Google Scholar]
- Fraenkel D. G. Genetic mapping of mutations affecting phosphoglucose isomerase and fructose diphosphatase in Escherichia coli. J Bacteriol. 1967 May;93(5):1582–1587. doi: 10.1128/jb.93.5.1582-1587.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanville E V, Demerec M. Threonine, Isoleucine, and Isoleucine-Valine Mutants of Salmonella Typhimurium. Genetics. 1960 Oct;45(10):1359–1374. doi: 10.1093/genetics/45.10.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janin J., Truffa-Bachi P., Cohen G. N. Subunits of the complex protein carrying the threonine-sensitive aspartokinase activity in a mutant of Escherichia coli K 12. Biochem Biophys Res Commun. 1967 Feb 21;26(4):429–434. doi: 10.1016/0006-291x(67)90564-5. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- PATTE J. C., LE BRAS G., LOVINY T., COHEN G. N. [Retro-inhibition and repression of the homoserine dehydrogenase of Escherichia coli]. Biochim Biophys Acta. 1963 Jan 8;67:16–30. doi: 10.1016/0006-3002(63)91793-1. [DOI] [PubMed] [Google Scholar]
- Patte J. C., Le Bras G., Cohen G. N. Regulation by methionine of the synthesis of a third aspartokinase and of a second homoserine dehydrogenase in Escherichia coli K 12. Biochim Biophys Acta. 1967 Mar 22;136(2):245–247. doi: 10.1016/0304-4165(67)90069-4. [DOI] [PubMed] [Google Scholar]
- Richaud F., Cohen G. N. Selection of Escherichia coli mutants devoid of one or of both the activities carried by a multifunctional protein. Biochem Biophys Res Commun. 1968 Jan 11;30(1):45–49. doi: 10.1016/0006-291x(68)90710-9. [DOI] [PubMed] [Google Scholar]
- Su C. H., Greene R. C. Regulation of methionine biosynthesis in Escherichia coli: mapping of the metJ locus and properties of a metJ plus-metJ minus diploid. Proc Natl Acad Sci U S A. 1971 Feb;68(2):367–371. doi: 10.1073/pnas.68.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Véron M., Falcoz-Kelly F., Cohen G. N. The threonine-sensitive homoserine dehydrogenase and aspartokinase activities of Escherichia coli K12. The two catalytic activities are carried by two independent regions of the polypeptide chain. Eur J Biochem. 1972 Aug 4;28(4):520–527. doi: 10.1111/j.1432-1033.1972.tb01939.x. [DOI] [PubMed] [Google Scholar]
- WORMSER E. H., PARDEE A. B. Regulation of threonine biosynthesis in Escherichia coli. Arch Biochem Biophys. 1958 Dec;78(2):416–432. doi: 10.1016/0003-9861(58)90367-9. [DOI] [PubMed] [Google Scholar]
- Zatti M., Rossi F. Early changes of hexose monophosphate pathway activity and of NADPH oxidation in phagocytizing leucocytes. Biochim Biophys Acta. 1965 Jun 22;99(3):557–561. doi: 10.1016/s0926-6593(65)80213-2. [DOI] [PubMed] [Google Scholar]



