Abstract
Background
The therapeutic efficacy of non‐surgical treatment strategies in Achilles tendinopathy (AT) has not been well clarified. Time‐consuming and costly combinations of treatment for pain, physiotherapy and biomechanical procedures are often applied.
Objective
To analyse the efficacy of single therapeutic regimens commonly used over a short period of 4 weeks.
Methods
31 male runners (mileage >32 km/week) with unilateral, untreated AT completed 4 weeks of either physiotherapy (10 treatments: deep‐friction, pulsed ultrasound, ice, sensory motor training; (P)), wearing custom fit semirigid insoles (I) or remained without treatment (control group C). Before and after treatment, all patients underwent a treadmill test and a plantar flexion strength exercise. Subjective pain (Pain Disability Index, Pain Experience Scale), as well as strength performance capacity (peak torque), was analysed (mean, 95% CI, repeated measures analysis of variance, α = 0.05).
Results
Pain was reduced to <50% of the baseline value after physiotherapy or after wearing insoles (p<0.05). Individual pain reduction was >50% (25%) in 89% (100%) of subjects in I and 55% (73%) in P. Higher eccentric plantar flexion peak torques after treatment were observed in I and P.
Conclusions
Most patients with AT experience a reduction in pain after only 4 weeks of differentiated, non‐surgical treatment consisting of physiotherapy or semirigid insoles.
Problems of Achilles tendon overuse are cited as one of the major pathologies that reduce physical capacity in everyday living, occupation and sports.1,2,3 Owing to the long duration of problems and widely varying, individual responses to treatment, the efficacy of single or combined therapeutic measures still remains a matter of debate.4,5 Thus, treatment is usually complex, time consuming and costly.3,5
It has often been assumed that Achilles tendon problems are caused by an inflammation.6 However, recent histological studies show that inflammatory cells and mediators, usually present after acute mechanical stress, are absent in tendon overuse.7,8,9 On the other hand, high concentrations of glycosaminoglycans and a loss of the hierarchical collagen structure have been found.10,11
The mechanism of pain development is not well understood. Competing explanatory models describe increased mechanical tendon vulnerability, microruptures and the supplanting of collagen type I by type III. Pain is also ascribed to the mechanical irritation of ingrown nerve endings due to neovascularisation.1,9 Reduced perfusion is now considered less important since it was demonstrated that blood supply and oxygen extraction clearly increase during physical exercise.12,13 It thus seems certain that tendon tissue must be considered metabolically active to a far greater extent than has been assumed to date.8,9
Pain reduction has traditionally been the main outcome variable of non‐surgical treatment in Achilles tendinopathy (AT).3 In daily practice, local physiotherapeutic measures such as deep friction massages, ice and ultrasound are usually applied.14,15,16 However, despite broad acceptance and pain reduction in individual cases, scientific evidence of short‐term physiotherapy is still lacking.14 Currently, adjuvant use of sensory motor training18 and eccentric exercises18,19,20 are increasingly being discussed. Shalabi et al21 demonstrated that eccentric exercises led to an improved clinical outcome, reduction in tendon diameter and reduced intratendinous lesions. Alfredson et al18,19 had comparably good clinical results after 12 weeks of predominantly eccentric exercise training.
In addition to physiotherapy and training, custom‐made insoles are frequently used, but evidence of their efficacy is still lacking.15,16 Recent studies using bone pins have shown that the mechanical effect of insoles, understood as an alignment of the skeleton, is unspecific and only slight during walking and running.22 Currently, sensory motor effects of semirigid insoles are being discussed.23,24
The aim of this study was to analyse whether standardised short‐term physiotherapy or wearing individually fitted insoles over a period of 4 weeks reduces pain in patients with unilateral AT.
Methods
Subjects
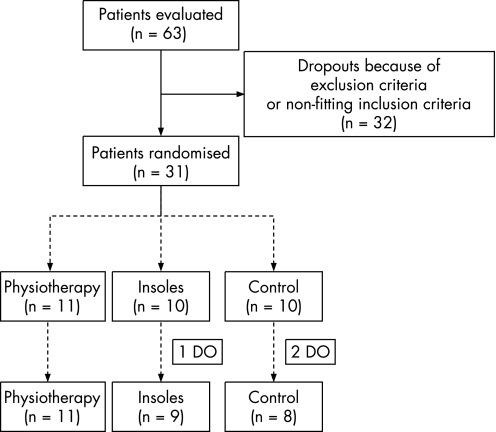
This randomised controlled trial was performed after approval by the local ethics commission in compliance with good clinical practice (Note for Guidance). In all, 63 patients with AT were initially identified as potential subjects at the university outpatient clinic (Medical Clinic, Department of Sports Medicine, University Clinic of Tübingen, Germany). Since it is known that the frequency of AT increases in male runners with a running distance of >32 km/week,16 only men (18–50 years, mileage>32 km/week), with unilateral chronic tendon problems during exercise, untreated for at least 6 months, were evaluated. Diagnosis was always made by the same orthopaedic surgeon (palpable nodes within the tendon itself, pressure pain at the mid‐substance of the tendon). Patients with local inflammation (swelling, redness) or problems at locations other than the mid‐substance of the Achilles tendon were excluded. After validation of inclusion/exclusion criteria, 31 subjects remained (fig 1).
Figure 1 Study flow chart of evaluated patients, participants and dropouts (DO).
Subjects were randomised into one of three groups, according to the planned intervention (control group C, those wearing insoles, I and those who underwent physiotherapy, P). There were three dropouts during the study. One subject (I) did not appear for baseline measurements. One subject (C) had a rupture of the tibialis posterior tendon due to recreational activities. Another subject (C) had a rupture of the Achilles tendon after inclusion. Twenty‐eight subjects completed the study (fig 1, table 1).
Table 1 Anthropometric data of subjects.
| Control | Insoles | PT | |
|---|---|---|---|
| Age (years) | 38 (4.9) | 35 (6.7) | 41 (5.9) |
| Height (cm) | 177 (3.9) | 181 (8.7) | 176 (5.9) |
| Weight (kg) | 70.4 (7.3) | 76.7 (6.7) | 70.7 (7) |
| Mileage (km/week) | 53.1 (10.6) | 50 (13.5) | 50 (13.6) |
| IAT (km/h) | 13 (1) | 13.7 (1.3) | 13.3 (0.9) |
| Pain (months) | 7.9 (6.8) | 13.8 (16.5) | 17.3 (18.7) |
IAT, individual's anaerobic threshold; PT, physiotherapy.
Values are mean (SD).
Study protocol
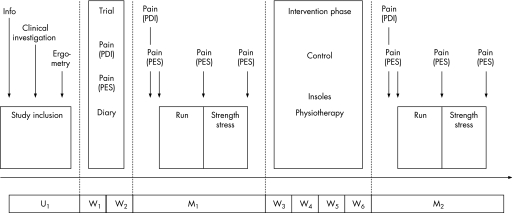
Initially, a clinical examination was performed and study inclusion criteria were checked (U1). Personal data, training data and history of pain were anonymously recorded on a case report form. To achieve individually comparable exercise levels during treadmill measurements (80% of the individual's anaerobic threshold (IAT)), patients underwent treadmill ergometry at the end of U1 (start 8 km/h, increase 2 km/h, increment time 3 min).25
The study protocol consisted of a test–retest procedure (M1, M2), including an intervention phase of 4 weeks. After inclusion and before baseline measurement M1, a 2‐week training and instruction phase (pain diary, training diary) was completed (fig 2). Both pain recordings and training data were taken at M1. M1 began with another clinical examination to determine possible changes compared with U1. Then the subject's pain during activities of daily life (Pain Disability Index, PDI26) followed by a first measurement of current pain experience (Pain Experience Scale, PES27) was recorded. Finally, patients underwent a warm‐up on the treadmill at 80% of the IAT.
Figure 2 Study protocol: baseline examination (U1), 2‐week instructional phase (W1, W2), measurement 1 (M1) and measurement 2 (M2) separated by a 4‐week intervention phase (W3–W6). PDI, Pain Disability Index; PES, Pain Experience Scale. Presentation of methods used and study schedule.
Exercises consisted of a 20 min treadmill run (80% IAT) to elicit their running‐specific pain and a subsequent plantar flexion strength test to increase further load on the Achilles tendon. At the end of each exercise, PES was recorded. Strength exercise was carried out concentrically and eccentrically (supine position, 30° knee flexion, Lido‐Active, Loredan Biomedical, California USA). After cool‐down (treadmill, stretching), the 4‐week treatment was explained, a pain and training diary was given to the patients and post‐treatment measurement M2 was scheduled. In M2, pain and training diaries were checked for completeness before recording the PDI. Protocols M1 and M2 were analogous. Each subject underwent a thorough clinical examination.
Ttreatment and intervention
Standardised physiotherapy consisted of 10 single 30 min sessions (2 or 3/week over 4 weeks). Each included deep‐friction massages at the mid‐substance of the Achilles tendon, local pulsed ultrasound (1.5 W/cm2), ice application and sensory motor training. This training consisted of three sets of 15 repetitions of balance and stabilisation exercises on a stability pad and eccentric exercises (loading of the calf muscles by lowering the heel standing with the forefoot on stairs, drop‐jumps and counter‐movement jumps). Each patient was informed and instructed by a physiotherapist.
Individually fitted, semirigid insoles with bowl‐shaped heels, moulded, longitudinal arch support and detorsion wedge were provided on the basis of a dynamic plantar pressure distribution measurement (EMED SF; Novel, Munich, Germany). Pedography and fitting were always carried out by the same technician. Insoles had to be worn for all physical activities during the treatment phase.
Subjects in C continued their activities without limitations, and no intervention was applied.
Quantities and methods applied
The PDI and PES were used to sum up patients' problems. PDI describes pain during various activities of daily life (family, rest, social activities, sexual activities, independence and life‐maintaining activities) based on visual analogue scales. The sum of pain in all categories was calculated.26 PES was used to quantify the current pain before and after exercises in M1 and M2 (fig 2). PES describes the sensory and affective pain qualities, which were recorded using a simple descriptive scale. A summary score of each sensory and affective quality was calculated. Finally, data were t transformed in relation to a reference group with chronic musculoskeletal pain.9
Strength performance capacity (ankle plantar flexors) was measured during maximum stress (50° range of motion; concentrically and eccentrically, Lido‐Active, Loredan); peak torque (mean of the three highest torques within five repetitions) was calculated.
Statistics
Data were stored in duplicate and compared for plausibility and correctness. After deblinding, data were assessed descriptively (means, SD, 95% CI; Statistical Discovery Software package JMP V.5.0.1.a, SAS Institute). Effects of treatment were analysed by either taking the difference between M2 and M1 or calculating the relative change in M2 compared with M1 ((M2–M1)×100%). In addition, the number of patients who responded to treatment was calculated.
The main quantity for statistical analysis of the effects of therapy on subjective pain was defined as the reduction of the PDI sum score (PDIsum) between M1 and M2. Analysis of the equality of groups was performed according to Brown–Forsythe. PDIsum was analysed statistically using a two‐factor analysis of variance (α = 0.05) for repeated measures with respect to the factors “group” (C, I, P) and “exercise” (M1, M2).
Results
Subjective pain
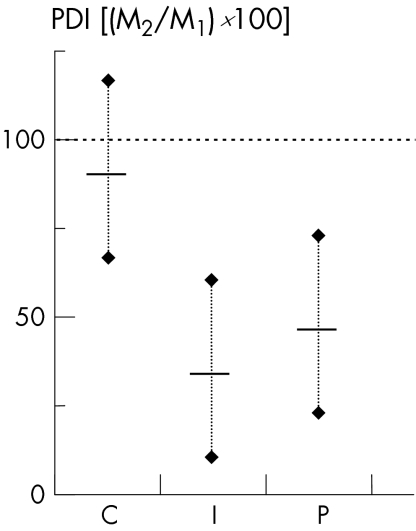
PDI reduction was statistically significant after intervention in I and P (p<0.05, fig 3). The statistical model applied explained 93% of the variances; a significant interaction effect (group×exercise; p<0.05) was obvious.
Figure 3 Differences in subjective pain (Pain Disability Index (PDI)) after therapy in relation to baseline (M2/M1×100). Presentation of group means with 95% CI. C, control group; I, group wearing insoles; M1 and M2 measurements 1 and 2; P, group undergoing physiotherapy.
Regarding individual responses, 21 of 28 patients (9/9 (100%) patients in I, 8/11 (73%) patients in P, and 4/8 (50%) patients in C) reported reduction in pain of >25% compared with baseline level. In all, 14 of 28 patients (8/9 (89%) patients in I, 6/11 (55%) patients in P, and 0/8 (0%) patients in C) reported reduction in pain of >50% compared with baseline level.
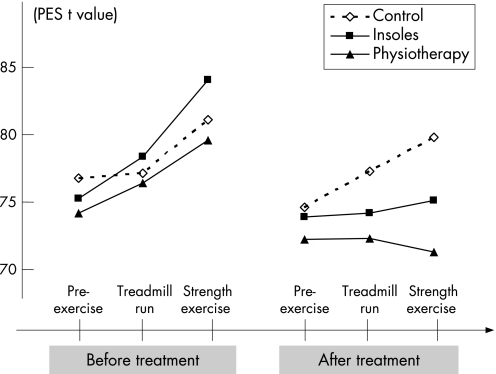
Sensory and affective pain (PES) increased in all groups during M1 up to a maximum pain following strength stress (fig 4). After treatment (M2), pain increased only in C (fig 4).
Figure 4 Results of the Pain Experience Scale (PES; t values, mean) pre‐exercise, after treadmill run and after strength exercise during measurements 1 and 2 (M1 and M2).
Peak torque
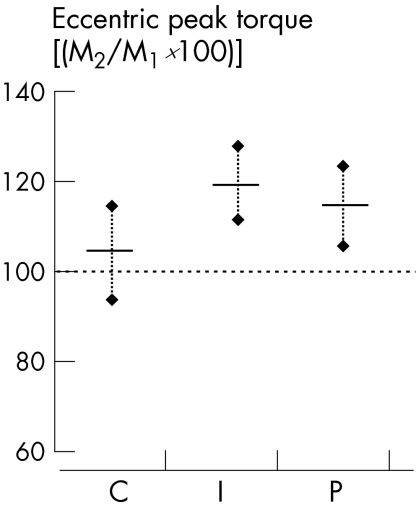
After treatment, no increase in concentric peak torques (mean >10%) could be observed in any group, whereas higher eccentric peak torques (mean >10%) were obvious in I and P (fig 5).
Figure 5 Strength performance capacity of plantar flexion after treatment (M2) in relation to baseline (M1): isokinetic‐eccentric peak torque (group means with 95% CI). C, control group; I, group wearing insoles; P, group undergoing physiotherapy.
Discussion
Present randomised controlled trial results show pain reduction at rest and during exercise, following a short‐term treatment of, 4 weeks of physiotherapy and fitting with insoles.
Patients with AT might benefit from non‐surgical treatment.3,15 However, about 25–30% of patients were found to be resistant to treatment and had to undergo surgical intervention.3,16,28 In an 8‐year follow‐up, Paavola et al28 reported reduction in pain and full functional recovery in 55 of 83 patients with subacute or subchronic AT. Nevertheless, the effectiveness of measures applied (modified rest, muscle stretching, local injections, oral non‐steroidal anti‐inflammatory drugs, orthosis, physical therapy) could not be differentiated, since treatment was performed individually and was not standardised.28
The recent reduction in pain after only 4 weeks of standardised physiotherapy shows that even short‐term non‐surgical treatment is effective in most patients. Possible mechanisms are nevertheless open to debate. Deep‐friction massages can be understood as soft‐tissue mobilisation, mechanotherapy (release of adhesions), fibroblast proliferation and stimulation of collagen synthesis.29,30 Additional sensory motor and eccentric training seem to be effective as local load management (eg, increase in collagen synthesis) and possibly because of modulation of afferent input to influence neuromuscular regulation.31 Optimisation of dynamic joint stability and postural control is rational, even though the spinal and central neurophysiological mechanisms of training effects remain largely unclear.17 Furthermore, increased eccentric peak torques indicate treatment‐specific sensory motor effects. Modulation of nociception and a reduction in spinal inhibitions might be one explanation.
Training regimens with primarily eccentric stress have been used to treat AT.18,19,20,21 Alfredson et al19 prescribed heavy‐load eccentric exercises for 12 weeks in 15 patients resistant to treatment. After treatment, all patients resumed their usual running mileage, with considerably reduced pain. Further studies confirmed these results.18,32 As a mechanism for reduction in pain, it is postulated that substance P‐positive nerve endings,33 which have grown during neovascularisation, are destroyed mechanically. However, the histological proof is still missing. Using colour Doppler sonography, a reduction in neovascularisation—and thereby a reduction in nerve endings—homogenisation of tendon structures and a decrease in tendon thickness could be demonstrated.32,34 By contrast, Khan et al35 pointed out that the 12‐month clinical outcome cannot be predicted using ultrasound. Power and colour Doppler sonography did not improve performance.35 Taking the recent study design into account, it remains a matter of debate whether these mechanisms are already available after 4 weeks and whether a persistent, long‐term effect could be attained.
Several studies confirm possible reduction in pain using insoles. However, differentiated, pathology‐related ascriptions of valid mechanisms have not yet been successful. To date, the dominant opinion of mechanical aligning of the skeleton is being increasingly criticised.24,25 The effect varies individually, and, on average, is unspecific on segmental movements.22 Consequently, recent discussions focus on optimisation of muscular‐regulated joint stability.25 In this sense, a combination of longitudinal arch support with rear foot stabilisation possibly led directly (compression of the peroneal tendon) or indirectly (stimulation of the proprioceptors by altering joint position) to modulation of the afferent input. However, it must be remembered that within the recent study design it could not be clarified whether these effects are ascribable to reduction in pain itself, or whether an altered movement regulation (and thus load management) leads secondarily to the reduction of problems.
Conclusion
A short‐term treatment of only 4 weeks of deep friction and ultrasound in combination with sensory motor and eccentric training, as well as insoles with longitudinal arch support might result in reduction in pain in most patients with AT, even without reducing running mileage. As most of the patients had little time to spare, these non‐invasive short‐term measures could possibly be considered valid treatment options in AT.
What is already known on this topic
Achilles tendinopathy is common in athletes.
Owing to individual responses and the long duration of problems, non‐surgical treatment is time consuming (weeks to months).
Effectiveness of single measures is not clarified.
What this study adds
Physiotherapy (deep‐friction massages, ultrasound, sensory motor and eccentric training) for 4 weeks and individually fitted insoles (moulded, longitudinal arch support, bowl‐shaped heel) are effective in runners with Achilles tendinopathy.
Maintaining running mileage and reduction in pain during activities of daily life as well as during exercise and higher eccentric plantar flexion strength capacity can be observed.
Acknowledgements
This study was supported by the Fortune‐Science Grant Program, University of Tübingen, Germany, IETEC, Fulda, Germany, and Schuhstudio Türk, Freudenstadt, Germany.
We thank our former workgroup at the Medical Clinic, Department of Sports Medicine, University of Tübingen, Germany, for help with measurements.
Abbreviations
AT - Achilles tendinopathy
C - control group
I - group wearing insoles
IAT - individual's anaerobic threshold
P - group undergoing physiotherapy
PDI - Pain Disability Index
PES - Pain Experience Scale
Footnotes
Competing interests: None.
References
- 1.Maffulli N, Ewen S W, Waterston S W.et al Tenocytes from ruptured and tendinopathic Achilles tendon produce greater quantities of type III collagen than tenocytes from normal Achilles tendons. An in vitro model of human tendon healing. Am J Sports Med 200028499–505. [DOI] [PubMed] [Google Scholar]
- 2.Maffulli N, Kader D. Tendinopathy of tendo achillis. J Bone Joint Surg Br 2002841–8. [DOI] [PubMed] [Google Scholar]
- 3.Paavola M, Kannus P, Järvinen T.et al Current concepts review. Achilles tendinopathy. J Bone Joint Surg Am 2002842062–2076. [DOI] [PubMed] [Google Scholar]
- 4.Alfredson H. Chronic midportion Achilles tendinopathy: an update on research and treatment. Clin Sports Med 200322727–741. [DOI] [PubMed] [Google Scholar]
- 5.Rees J D, Wilson A M, Wolman R L. Current concepts in the management of tendon disorders. Rheumatology (Oxford)200645508–521. [DOI] [PubMed] [Google Scholar]
- 6.Khan K M, Cook J L, Bonar F.et al Histopathology of common tendinopathies. Sports Med 199927393–408. [DOI] [PubMed] [Google Scholar]
- 7.Alfredson H, Thorsen K, Lorentzon R. In situ microdialysis in tendon tissue: high levels of glutamate but not prostaglandin E2 in Achilles tendon pain. Knee Surg Sports Traumatol Arthrosc 19997378–381. [DOI] [PubMed] [Google Scholar]
- 8.Benjamin M. Tendons are dynamic structures that respond to changes in exercise levels. Scand J Med Sci Sports 20021263–64. [DOI] [PubMed] [Google Scholar]
- 9.Kjaer M. Adaptation of tendons to physical exercise. Dt Z Sportmed 200455148–151. [Google Scholar]
- 10.Movin T, Gad A, Reinholt P.et al Tendon pathology in long‐standing achillodynia – biopsy findings in 40 patients. Acta Orthop Scand 199768170–175. [DOI] [PubMed] [Google Scholar]
- 11.Riley G. The pathogenesis of tendinopathy. A molecular review. Rheumatology (Oxford)200443131–142. [DOI] [PubMed] [Google Scholar]
- 12.Boushel R, Langberg H, Olesen J.et al Monitoring tissue oxygen availability with near infrared spectroscopy (NIRS) in health and disease. Scand J Med Sci Sports 200111213–222. [DOI] [PubMed] [Google Scholar]
- 13.Langberg H, Bülow J, Kjaer M. Blood flow in the peritendinous space of the human Achilles tendon during exercise. Acta Physiol Scand 1998163149–153. [DOI] [PubMed] [Google Scholar]
- 14.Brosseau L, Casimiro L, Milne S.et al Deep transverse friction massage for treating tendinitis (Cochrane Review). In: The Cochrane Library, Issue 3, Chichester, UK: John Wiley & Sons, Ltd 2004
- 15.Kader D, Saxena A, Movin T.et al Achilles tendinopathy: some aspects of basic science and clinical management. Br J Sports Med 200236239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McLauchlan G J, Handoll H H G. Interventions for treating acute and chronic Achilles tendinitis (Cochrane Review). In: The Cochrane Library, Issue 1 Chichester, UK: John Wiley & Sons 2004
- 17.Holm I, Fosdahl M A, Frijs A.et al Effect of neuromuscular training on proprioception, balance, muscle strength, and lower limb function in female team handball players. Clin J Sports Med 20041488–94. [DOI] [PubMed] [Google Scholar]
- 18.Alfredson H, Lorentzon R. Intratendinous glutamate levels and eccentric training in chronic Achilles tendinosis: a prospective study using microdialysis technique. Knee Surg Sports Traumatol Arthrosc 200311196–199. [DOI] [PubMed] [Google Scholar]
- 19.Alfredson H, Pietilä T, Jonsson P.et al Heavy‐load eccentric calf muscle training for the treatment of chronic Achilles tendinosis. Am J Sports Med 199826360–366. [DOI] [PubMed] [Google Scholar]
- 20.Roos E M, Engstrom M, Lagerquist A.et al Clinical improvement after 6 weeks of eccentric exercise in patients with mid‐portion Achilles tendinopathy—a randomized trial with 1‐year follow‐up. Scand J Med Sci Sports 200414286–295. [DOI] [PubMed] [Google Scholar]
- 21.Shalabi A, Kristoffersen‐Wilberg M, Svensson L.et al Eccentric training of the gastrocnemius‐soleus complex in chronic Achilles tendinopathy results in decreased tendon volume and intratendinous signal as evaluated by MRI. Am J Sports Med 2004321286–1296. [DOI] [PubMed] [Google Scholar]
- 22.Stacoff A, Reinschmidt C, Nigg B M.et al Effect of foot orthoses on skeletal motion during running. Clin Biomech 20001554–64. [DOI] [PubMed] [Google Scholar]
- 23.Mundermann A, Nigg B M, Humble R N.et al Orthotic comfort is related to kinematics, kinetics, and EMG in recreational runners. Med Sci Sports Exerc 2003351710–1719. [DOI] [PubMed] [Google Scholar]
- 24.Nawoczenski D A, Ludewig P M. Electromyographic effects of foot orthotics on selected lower extremity muscles during running. Arch Phys Med Rehabil 199980540–544. [DOI] [PubMed] [Google Scholar]
- 25.Roecker K, Schotte O, Niess A M.et al Predicting competition performance in long‐distance running by means of a treadmill test. Med Sci Sports Exerc 1998301552–1557. [DOI] [PubMed] [Google Scholar]
- 26.Tait R C, Chibnall J T, Krause S. The pain disability index: psychometric properties. Pain 199040171–182. [DOI] [PubMed] [Google Scholar]
- 27.Heuser J, Geissner E. Computerized version of the pain experience scale: a study of equivalence. Schmerz 199812205–208. [DOI] [PubMed] [Google Scholar]
- 28.Paavola M, Kannus P, Paakala T.et al Long‐term prognosis of patients with Achilles tendinopathy. An observational 8‐year follow‐up study. Am J Sports Med 200028634–642. [DOI] [PubMed] [Google Scholar]
- 29.Gehlsen G M, Ganion L R, Helfst R. Fibroblast responses to variation in soft tissue mobilization pressure. Med Sci Sports Exerc 199931531–535. [DOI] [PubMed] [Google Scholar]
- 30.Ramirez A, Schwane J A, McFarland C.et al The effect of ultrasound on collagen synthesis and fibroblast proliferation in vitro. Med Sci Sports Exerc 199729326–332. [DOI] [PubMed] [Google Scholar]
- 31.Aagaard P. Training‐induced changes in neural function. Exerc Sport Sci Rev 20033161–66. [DOI] [PubMed] [Google Scholar]
- 32.Öhberg L, Lorentzon R, Alfredson H. Eccentric training in patients with chronic Achilles tendinosis: normalised tendon structure and decreased thickness at follow up. Br J Sports Med 2004388–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schubert T E, Weidler C, Lerch K.et al Achilles tendinosis is associated with sprouting of substance P positive nerve fibres. Ann Rheum Dis 2005641083–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zanetti M, Metzdorf A, Kundert H P.et al Achilles tendons: clinical relevance of neovascularisation diagnosed with power Doppler US. Radiology 2003227556–560. [DOI] [PubMed] [Google Scholar]
- 35.Khan K M, Foster B B, Robinson J.et al Are ultrasound and magnetic resonance imaging of value in assessment of Achilles tendon disorders? A two year prospective study. Br J Sports Med 200337149–153. [DOI] [PMC free article] [PubMed] [Google Scholar]