Abstract
Objective
To study the effects of an acute therapeutic oral intake of β2 agonist on performance and substrate response during supramaximal exercise in women.
Methods
12 healthy moderately trained female volunteers performed a Wingate test after ingestion of placebo (Pla) and salbutamol (Sal; 4 mg) according to a double‐blind randomised crossover study. Blood samples were collected at rest, at the end of exercise and after 5 (r5), 10 (r10) and 15 (r15) min of passive recovery for adrenocorticotropic hormone (ACTH), growth hormone (GH), insulin, blood glucose and lactate measurements.
Results
Peak power (PP) and mean power (MP) significantly increased whereas time to peak power was significantly shorter with Sal than with Pla (p<0.05). No change was observed in the fatigue index. ACTH was not significantly modified but r15 growth hormone significantly decreased (p<0.05) after the intake of Sal. Both blood INS and blood glucose were significantly increased by the intake of Sal during all the experiments (p<0.01). Blood lactate was significantly increased by the intake of Sal compared with that of Pla (p<0.05) after 10 and 15 min of passive recovery.
Conclusion
From these data, acute therapeutic oral intake of Sal seems to induce, irrespective of the subjects' gender, an improvement in performance during a supramaximal exercise—that is, increase in PP and MP. Further studies are necessary to clarify whether the mechanisms involved in the response to intake of Sal are linked to central and/or peripheral pathways.
Numerous studies have demonstrated an increased prevalence of bronchial responsiveness and exercise‐induced bronchoconstriction (EIB) in athletes: a clinical entity that affects approximately 15% of athletes.1,2 Standard treatment involves the use of β2 mimetics, which are widely accepted as the treatment of choice in EIB.1,2,3 However, athletes are now required to provide objective evidence of asthma or EIB when applying to be allowed to use inhaled β2 agonists by the World Anti‐Doping Agency, whereas systemic administration is currently banned both in in‐ and out‐competition doping controls. Indeed, different studies seem to demonstrate an ergogenic effect after oral intake of both acute and chronic β2 agonist or inhalation at supratherapeutic doses in healthy men irrespective of the intensity of the exercise tested.4,5,6,7,8,9,10 There is, however, limited literature on the effects of acute systemic β2 agonist administration during high‐intensity exercise. As a matter of fact, Van Baak et al9 reported a significantly increased isokinetic strength of the knee flexors and extensors in men without asthma after an acute therapeutic oral intake of salbutamol (Sal). Similarly, we found in a precedent study6 that the same acute administration of oral Sal (4 mg) significantly improved peak power (PP) and mean power (MP) while significantly shortening the time to peak power (TTPP) compared with placebo (Pla) in men during a Wingate test. However, to our knowledge, no study of systemic use has focused on women and a specific gender response to β2 agonist can be questioned.
The purpose of this study was therefore to investigate the influence of acute β2 agonist intake at therapeutic doses—that is, 4 mg Sal–on physical performance and blood hormonal and metabolite levels during supramaximal exercise in women.
Methods
Subjects
Twelve recreational trained women (with sport participation between 1 and 3 times per week in various sports such as athletics, weightlifting and basket‐ball for at least 3 years) were invited to participate in this study. Subjects were required to have been taking a low‐dose oral contraceptive (OC) pill continuously in the past 12 months. They were screened with a medical history and physical examination to exclude those subjects with a history of bronchospasm or atopy. Exclusion criteria included respiratory tract infection during the previous month, regular use of tobacco, regular use of any medical drug, recognised asthma or allergy during the 5 years before the study, or a restriction in forced expiratory volume during 1 s of >10% after incremental maximal exercise. Subjects were (mean (SE)) aged 22.3 (0.9) years and weighed 59.1 (4.4) kg.
Subjects were asked to maintain similar exercise patterns and normal food intake and were required to continue taking the OC pill at the same time each day as specified for OC usage throughout the duration of the experiments. They were also to abstain from intense exercise and any caffeine and alcohol 24 h before each trial, which were always performed during the second part of the menstrual cycle.
Subjects were informed of the nature of the study, testing protocols, possible risks or discomforts, and signed a written informed consent. The Human Research Ethics Committee of the Tours Hospital approved the study.
Experimental design
The 30 s Wingate test protocol was performed on a Monark pan load bicycle ergometer (Monark Exercise AB, Varberg, Sweden) with a resistance of 0.075 kg (kg body mass)−1 as recommended.
Before the start of each Wingate test, the saddle height, handlebar height and distance between saddle and handlebar were adjusted to match the patients' leg and arm lengths (comfortable cycling height). These individual bicycle specifications were retained throughout the experiment. Subjects were requested to stay seated during the Wingate trials. They were also instructed to pedal as fast as possible from a dead stop and to maintain maximal pedalling speed throughout the 30 s period. During the test, the revolutions were determined using a magnetic switch and magnets mounted on the wheel of the ergometer. Revolutions were recorded by a computer and used in the calculation of the power variables. The magnets were checked before every test session to make sure that the magnets and switch were producing a signal that was received and recorded by the computer. At the end of the Wingate test, rpm and resistance were used to calculate: PP—that is, the highest power output achieved during the 30 s sprint, the product of force (FPP) and velocity (VPP); MP—that is, the average power output over the 30 s sprint; TTPP—that is, the time between the start of the sprint test and the time at which PP is recorded; and fatigue index—that is, the difference between PP and the lowest power divided by PP.
Familiarisation of subjects with the protocol and reproducibility of the test were improved by asking the subjects to perform an additional supramaximal anaerobic Wingate test trial ride in the month before the actual experiment.
Drugs
The Pla—that is, lactose—and Sal were packaged in identical gelatin capsules to permit a double‐blind administration.
Salbutamol
The choice of the dose of Sal (4 mg) was made in accordance with previous literature.24 Sal (trade name: SALBUMOL 2 mg, tablet, Glaxo‐Wellcome Laboratory, Paris, France) was administered 3 h before the test, when maximal pharmacological activity was expected.
Treatments
Two treatments—that is, Pla (lactose) and Sal—were administered to each subject according to a double‐blind and randomised crossover method. The two trials were separated by a 3–4‐week interval.
Protocol
After a period of familiarisation with this type of exercise in the month before the experiment, each subject performed the supramaximal exercise test twice. The protocol for each trial was identical. Trials were held at the same time of day (9:30–10:30) for each subject in order to prevent diurnal variations in hormonal responses.
On the day of the experiment, subjects reported to the laboratory at 8:30–9:30, 2 h after ingesting a capsule containing either Pla or Sal (4 mg) and 1 h after ingesting a light meal, which was standardised and identical for each trial. Dietary consistency (two slices of bread and butter, 50 g of cheese and 300 ml of unsweetened orange juice totaling to about 2100 kJ) was confirmed through self‐reported diet records and questioning before each trial. After insertion of a catheter into a superficial forearm vein (9:00–10:00), subjects warmed up with light cycling exercise. An accurate record was kept for the duration intensity of the warm‐up on the first trial (about 2 min), which was identical for all trials and was not considered as part of the total exercise time.
The subjects then rested and, between 9:30 and 10:30, performed a 30 s Wingate anaerobic power test. Blood samples were taken at rest, at the end of the Wingate test, and at 5, 10 and 15 min of passive recovery.
Blood analyses
Blood samples (6 ml) were immediately separated into two aliquots, promptly centrifuged, 10 min at 4°C, 3000 rpm, and stored at −72°C until assayed. The first aliquot (3 ml) of blood was placed in a chilled sodium heparinised tube for determining the level of growth hormone (GH) and insulin (Ins) and another 3 ml was placed in a chilled EDTA‐aprotinin tube for analysing ACTH, blood glucose (Glu) and lactate (Lac).
ELISA tests were used for the hormone analyses (kits from Biomedica, San Diego, California, USA, ACTH, DSL, Germany, GH; Bioadvance, Emerainville, France, Ins). Blood Lac and blood Glu were analysed, respectively, by the electro‐enzymatic (Microzym, Biosentec, France) and the classic enzymatic methods. All assays were made in duplicate. Coefficients of variation (inter‐assay and intra‐assay) for all parameters were always <10%.
Statistics
Data are presented as mean (SE) values.
A specific test for crossover trials was used to determine whether significant differences existed between Pla and Sal performance parameters.
Differences in blood parameters between the trials were analysed with a one‐way analysis of variance (ANOVA) with repeated measurements. A post hoc Newman–Keuls test was performed to determine the location of the differences, in the event of an ANOVA revealing a significant main effect. The null hypothesis was rejected at p<0.05.
Results
Performance responses
No rank order effect was detected. PP was significantly increased after the intake of Sal compared with that of Pla (p<0.05) with a significant reduction in TTPP (p<0.05). FPP was significantly increased with Sal (p<0.05) without any significant change in VPP. Similarly, MP was significantly higher with Sal (p<0.05). No change in fatigue index was noted with Sal compared with Pla (table 1).
Table 1 Performance responses after intake of placebo (Pla) and acute salbutamol (Sal) intake during the Wingate test.
| Performance | Pla | Sal |
|---|---|---|
| Peak power (W) | 732.9 (39.9) | 778.8 (44.0)* |
| Peak power (W/kg) | 12.6 (0.7) | 13.2 (0.6)* |
| Mean power (W) | 395.7 (15.8) | 414.4 (17.4)* |
| Force peakpower (N) | 57.6 (3.2) | 65.2 (3.4)* |
| Force peakpower (N/kg) | 0.98 (0.03) | 1.12 (0.06)* |
| Velocity peakpower (rpm) | 129.2 (6.3) | 121.0 (4.2) |
| Time to peak power (s) | 2.65 (0.17) | 1.79 (0.08)* |
| Fatigue index (%) | 63.5 (2.0) | 66.6 (1.7) |
*Significant difference between Pla and Sal (p<0.05).
Values are mean (SE).
Hormonal concentrations
Growth hormone
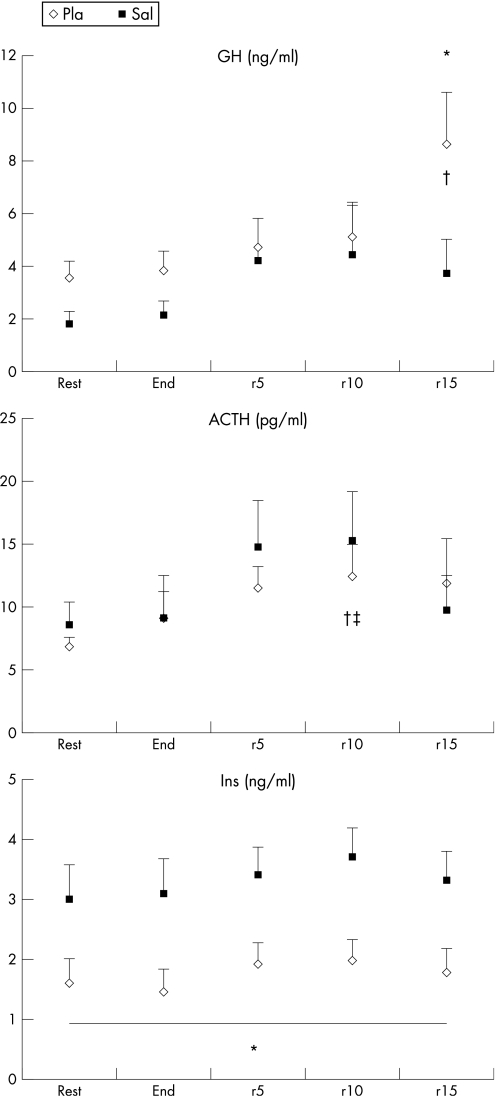
Exercise induced a significant rise in basal GH concentrations (p<0.05) with Pla that started to be significantly higher after 15 min of recovery, whereas no significant change was found compared with resting values with Sal either at the end of the Wingate test or during recovery.
Sal did not cause any significant difference in GH concentrations at rest or at the end of exercise but they were significantly lower compared with Pla (p<0.05) after 15 min of recovery (fig 1).
Figure 1 Growth hormone (GH), adrenocorticotropic hormone (ACTH) and insulin (Ins; mean (SE)) at rest, at the end of the Wingate test and during passive recovery (5, 10 and 15 min after exercise) after the intake of placebo (Pla) and salbutamol (Sal). *Significant difference between Pla and Sal (p<0.05). †Start of significant difference compared with basal values after Pla intake (p<0.05). ‡Start of significant difference compared with basal values after Sal intake (p<0.05).
Adrenocorticotropic hormone
The ANOVA revealed no significant treatment effect on ACTH concentrations.
Exercise induced a significant increase in resting ACTH concentrations (p<0.05) after 10 min of recovery with both treatments.
Insulin
Resting Ins values were significantly increased with Sal compared with Pla (p<0.01).
Exercise did not change the basal Ins concentrations irrespective of the treatment administered. Exercise and recovery Ins values remained significantly higher (p<0.01) with Sal compared with Pla throughout exercise and during recovery.
Metabolic data
Blood glucose
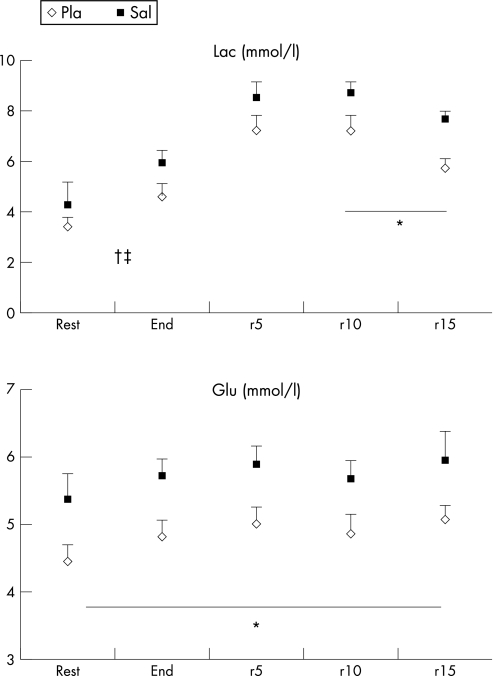
Basal Glu level was not significantly altered during exercise irrespective of the treatment administered. However, blood Glu levels were significantly higher with Sal compared with Pla (p<0.01) throughout the experiment (fig 2).
Figure 2 Mean (SE) glucose (Glu) and lactate (Lac) at rest, at the end of the Wingate test and during passive recovery (5, 10, and 15 min after exercise) after placebo (Pla) and salbutamol (Sal) intake. *Significant difference between Pla and Sal (p<0.05). †Start of significant difference compared with basal values after Pla intake (p<0.05). ‡Start of significant difference compared with basal values after Sal intake (p<0.05).
Lactate
There were no significant differences in basal and end exercise Lac values between Pla and Sal treatments. However, Lac concentrations appeared significantly increased by Sal compared with Pla after 10 and 15 min of passive recovery.
Basal Lac concentrations were significantly higher at the end of exercise irrespective of the treatment administered and remained significantly higher compared with values at rest throughout exercise and during recovery (p<0.05).
Discussion
Our results show that acute therapeutic oral intake of β2 mimetic—that is, 4 mg of Sal—did improve performance during supramaximal exercise in healthy, moderately trained female volunteers. Alterations in hormonal and metabolic parameters—that is, Ins, GH, blood Glu and Lac responses—were recorded after Sal administration.
Few studies have focused on the effects of an acute administration of β2 agonist during maximal or supramaximal exercise.6,9,11,12,13,14,15,16 Most of them did not report any improvement in performance after acute local (inhalation) administration.12,13,14 However, two studies11,15 showed that inhaled Sal exerted an ergogenic effect on power output. Only one of these trials15 tested anaerobic performance in both males and females after Sal inhalation and reported an ergogenic effect of Sal on power output, but it is not known whether gender influenced the results as the data were not analysed by gender. To the best of our knowledge, only two works6,9 investigated the effects of acute systemic therapeutic (oral) administration—that is, administering 10 times the therapeutic inhaled dose on healthy men during this type of exercise, and reported either increased isokinetic strength of the knee flexors and extensors9 or increased PP during a Wingate test.6 However, there is no previous study that investigates the effect of the use of acute systemic Sal on anaerobic performance in women. The results of the present work show that acute Sal intake has an ergogenic effect on power output in recreational trained women. This was demonstrated by the significant positive effect of Sal intake, compared with the use of Pla, on PP, FPP, MP and TTPP measured during a 30‐s Wingate test. There were, however, no significant changes either in the velocity at PP or in the fatigue index. These results support our previous findings in men with similar training status,6 which demonstrated that there was a significant improvement in both anaerobic PP and MP, which is similar to the differences seen in this trial, after acute oral intake of Sal. These findings concur also with the work of Signorile et al15 in that an ergogenic effect on short‐term power output that was independent from the impact on respiratory muscle was observed after Sal inhalation in both male and female non‐asthmatic subjects. The concern regarding the widespread use of Sal is justified, as there are several non‐respiratory mechanisms through which Sal could, hypothetically, have an effect on performance. First, increased contractility and fast‐twitch fibre response have been reported in animal studies. Therefore, although the anabolic effect of β2 agonists after single administration17 can be quickly dismissed, it is suggested that the significant ergogenic effect of Sal could be linked to an increased Ca2+ release from the sarcoplasmic reticulum and/or to an increased Ca2+ sensitivity.15,18,19 In view of the increase in both blood Glu and Lac after Sal intake, a direct role of enhanced glycogenolysis in the performance improvement cannot be ignored. Finally, further studies are necessary to verify the stimulatory effects of β2 agonists on the central nervous system that may enhance performance.3 However, in view of the results obtained in this work, it seems unlikely that β2 agonist drugs have different effects in men and women.
Ingestion of Sal increased both blood Glu and Ins in healthy women throughout the experiment. These results are in agreement with the previous data.5,20,21,22 β2 receptors are involved in glycogenolysis and Ins release and Sal has been shown to increase basal plasma concentrations of Glu and Ins in healthy volunteers as well as in patients with diabetes.21,23 The increase in blood Glu reported here at rest, during exercise and during recovery suggests that this drug stimulates muscle and/or liver glycogenolysis. This would also account for the reported higher recovery of Lac concentrations. As mentioned above and as shown by earlier studies on submaximal exercise,5 blood Lac concentrations were statistically higher in this trial during recovery with Sal compared with Pla. The exact mechanism by which Sal increases blood Lac concentration is unknown but may reflect an increased β2‐adrenergic receptor‐mediated glycolytic flux in skeletal muscle, possibly associated with a change in Lac removal. At the same time, the rise in Ins indicates that Sal has a direct or an indirect stimulatory effect on β‐receptors in the Ins‐secreting cells of the pancreas in response to hyperglycaemia.
We demonstrated a non‐significant decrease in GH by Sal intake at rest and at the end of exercise, with a significant decrease after 15 min of passive recovery. These results are consistent with earlier but limited data on the effects of acute Sal on GH.6,24,25 Trials in experimental animals or in humans have found acute Sal administration either to decrease GH secretion, probably through enhanced somatostatin secretion and/or activity, or to have no effect on GH secretion. In the only study performed during exercise in adult patients with asthmatic bronchitis, Giustina et al24 found that acute β2 stimulation blunts the physiological GH response to maximal exercise. Finally, the results of the present study confirmed the data we obtained for men with the same protocol. This study showed that GH levels tended to fall during the first part of the experiment but the decrease only started to be significant after 10 min of recovery after Sal compared with Pla. It appears, therefore, that acute salbutamol intake affects GH secretion during supramaximal exercise irrespective of the subjects' gender.
What is already known on this topic
The effects of acute, systemic administration of salbutamol as an ergogenic aid during high‐intensity exercise have had little investigation.
Whether this acute use improves performance and/or modifies metabolic responses in women is yet to be determined
What this study adds
Acute systemic administration of salbutamol significantly improves performance in women during supramaximal exercise.
Numerous concomitant changes in metabolic and hormonal responses are induced by this acute oral salbutamol intake.
Although the stimulation of central α 1‐adrenergic mechanisms results in secretion of ACTH in humans, presumably by increased release of a corticotrophin‐releasing factor, β2 adrenergic agonist drugs had no effect on the secretion of ACTH at rest.26 However, to the best of our knowledge, the effects of Sal on ACTH secretion have never been investigated during exercise. In the present study, we found a significant increase in recovery ACTH concentrations compared with basal values, irrespective of the treatment administered. Therefore, it seems that Sal did not alter ACTH secretion during this type of exercise in women.
Conclusion
Our data show that acute Sal intake, as in men, improves cycling performance in women during supramaximal exercise as shown by the significant increase in both PP and MP. However, despite the numerous change in metabolic and hormonal changes induced after systemic acute Sal administration, the mechanisms that could account for this performance gain are, as yet, only speculative and will have to be demonstrated in further work.
Acknowledgements
This project has been carried out with the support of WADA (World Anti‐Doping Agency). We thank the subjects for their dedicated performance. In addition, we also thank Orléans CHR, Pr Candau, Pr Courteix and Dr M. Ferry for their assistance. We also thank the expert technical assistance provided by Mr Régis Bonnefoy.
Abbreviations
ANOVA - analysis of variance
ACTH - adrenocorticotropic hormone
EIB - exercise‐induced bronchoconstriction
GH - growth hormone
Glu - glucose
Ins - insulin
Lac - lactate
MP - mean power
OC - oral contraceptive
Pla - placebo
PP - peak power
Sal - salbutamol
TTPP - time to peak power
Footnotes
Competing interests: None.
References
- 1.Beck K, Joyner M, Scanlon P. Exercise‐induced asthma: diagnosis, treatment, and regulatory issues. Exerc Sport Sci Rev 2002301–3. [DOI] [PubMed] [Google Scholar]
- 2.Fitch K D. The use of anti‐asthmatic drugs. Do they affect sports performance? Sports Med 19863136–150. [DOI] [PubMed] [Google Scholar]
- 3.Price A, Clissold S. Salbutamol in the 1980s. A reappraisal of its clinical efficacy. Drugs 19893877–122. [DOI] [PubMed] [Google Scholar]
- 4.Caruso J, Signorile J, Perry A.et al The effects of albuterol and isokinetic exercise on the quadriceps muscle group. Med Sci Sports Exerc 1995271471–1476. [PubMed] [Google Scholar]
- 5.Collomp K, Candau R, Collomp R.et al Effects of acute ingestion of salbutamol during submaximal exercise. Int J Sports Med 200021480–484. [DOI] [PubMed] [Google Scholar]
- 6.Collomp K, LePanse B, Portier H.et al Effects of acute salbutamol intake during a Wingate test. Int J Sports Med 200526513–517. [DOI] [PubMed] [Google Scholar]
- 7.Le Panse B, Collomp K, Portier H.et al Effects of short‐term salbutamol ingestion during a Wingate test. Int J Sports Med 200526518–523. [DOI] [PubMed] [Google Scholar]
- 8.Martineau L, Horan H, Rothwell N.et al Salbutamol, a β2‐adrenoceptor agonist, increases skeletal muscle strength in young men. Clin Sci 199283615–621. [DOI] [PubMed] [Google Scholar]
- 9.Van Baak M, Mayer L, Kempinski R.et al Effect of salbutamol on muscle strength and endurance performance in nonasthmatic men. Med Sci Sports Exerc 2000321300–1306. [DOI] [PubMed] [Google Scholar]
- 10.Van Baak M, de Hon O, Hartgens F.et al Inhaled salbutamol and endurance cycling performance in non‐asthmatic athletes. Int J Sports Med 200425533–538. [DOI] [PubMed] [Google Scholar]
- 11.Bedi J, Gong H, Horvath S. Enhancement of exercise performance with inhaled albuterol. Can J Sport Sci 198813144–148. [PubMed] [Google Scholar]
- 12.Goubault C, Perault M C, Leleu E.et al Effects of inhaled salbutamol in exercising non‐asthmatic athletes. Thorax 200156675–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lemmer J, Fleck S, Wallach J.et al The effects of albuterol on power output in non‐asthmatic athletes. Int J Sports Med 199516243–249. [DOI] [PubMed] [Google Scholar]
- 14.Meeuwisse W, McKenzie D, Hopkins S.et al The effect of salbutamol on performance in elite nonasthmatic athletes. Med Sci Sports Exerc 1992241161–1166. [PubMed] [Google Scholar]
- 15.Signorile J, Kaplan T, Applegate B.et al Effects of acute inhalation of the bronchodilator, albuterol, on power output. Med Sci Sports Exerc 199224638–642. [PubMed] [Google Scholar]
- 16.Stewart I, Labreche J, McKenzie D. Acute formoterol administration has no ergogenic effect in nonasthmatic athletes. Med Sci Sports Exerc 200234213–217. [DOI] [PubMed] [Google Scholar]
- 17.Reeds P, Hay S, Dorward P.et al The effects of β‐agonists and antagonists on muscle growth and body composition of young rats. Comp Biochem Physiol 198889337–341. [DOI] [PubMed] [Google Scholar]
- 18.Caswell A, Baker S, Boyd H.et al β‐adrenergic receptor and adenylate cyclase in transverse tubules of skeletal muscles. J Biol Chem 19782533049–3054. [PubMed] [Google Scholar]
- 19.Gonzalez‐Serratos H, Hill L, Valle‐Aguilera R. Effects of catecholamines and cyclic AMP on excitation‐contraction coupling in isolated skeletal muscle fibers of the frog. J Physiol 1981315267–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldberg R, Van As M, Joffe B.et al Metabolic responses to selective β adrenergic stimulation in man. Postgrad Med J 19755153–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rolf Smith S, Kendall M. Metabolic responses to β2 stimulants.J R Coll Chest Physiol 198418190–194. [PMC free article] [PubMed] [Google Scholar]
- 22.Smith A, Banks J, Buchenen K.et al Mechanisms of abnormal glucose metabolism during the treatment of acute severe asthma. Q J Med 19928271–80. [PubMed] [Google Scholar]
- 23.Wager J, Fredholm B, Lunell N.et al Metabolic and circulatory effects of intravenous and oral salbutamol in late pregnancy in diabetic and non diabetic women. Acta Obstet Gynaecol Scand 198210841–46. [DOI] [PubMed] [Google Scholar]
- 24.Giustina A, Malerba M, Bresciani E.et al Effect of two β2‐agonist drugs, salbutamol and broxaterol, on the growth hormone response to exercise in adult patients with asthmatic bronchitis. J Endocrinol Invest 199518847–852. [DOI] [PubMed] [Google Scholar]
- 25.Schaub C, Bluet Pajot M, Partouche R. The effects of β adrenergic receptor agonist salbutamol on growth‐hormone release in the rhesus monkey. IRCS Med Sci 198311832–833. [Google Scholar]
- 26.Al‐Damluji S, Perry L, Tomlin S.et al Alpha‐adrenergic stimulation of corticotropin secretion by a specific central mechanism in man. Neuroendocrinology 19874568–76. [DOI] [PubMed] [Google Scholar]