Abstract
Objective
To investigate the changes in temperature of human muscle during microwave hyperthermia.
Methods
Skin surface and muscle temperatures were measured in 11 healthy adult men (mean (SD) age 24.3 (2.2) years; height 174.2 (6.1) cm; weight 70.0 (5.3) kg) during a 30 min exposure of the thigh to 434 MHz microwave hyperthermia. Skin temperature was maintained at the pilot temperature of 40°C, and the temperature of the water in the bolus was 38°C. The peak power output was set at 60 W and controlled automatically to maintain the pilot temperature. The temperature was measured in the vastus lateralis muscle at an average muscle depth of 2.0 (0.2) cm, using a 23 G Teflon‐shielded thermocouple. Biopsy specimens were obtained for light microscopy from three subjects. A muscle‐equivalent phantom was used to evaluate the vertical heating pattern.
Results
Both skin and muscle temperatures increased from baseline, and muscle temperature was higher than skin temperature (skin temperature 39.2 (0.5)°C, temperature rise 5.0 (1.5)°C; muscle temperature 43.7 (0.8)°C, temperature rise 8.9 (1.4)°C). At the end of the hyperthermia treatment, muscle temperature decreased to 39.8 (0.9)°C, but was still 4.8 (1.5)°C higher than the baseline. No signs of muscle damage were observed on the basis of the blood creatine kinase activity and histological sections.
Conclusions
The results show that the 434 MHz microwave hyperthermia treatment increased and maintained muscle temperature locally by 6.3–11.4°C without muscle damage. These findings suggest that the microwave hyperthermia system provides effective and safe treatment.
For many years, heat treatment has been used as a therapy for muscle tissue injured in sports. There are several heating modalities, including silicon gel hot packs, whirlpools, paraffin baths, ultrasound and electromagnetic waves.1 Most of these modalities are classified as superficial heating, and primarily cause a temperature increase in structures up to 1 cm below the skin surface. Ultrasound and electromagnetic waves (eg, radiofrequency and microwaves) are used for deep heating at tissue depths of 3–5 cm.2
To gain therapeutic benefit, the target tissue depth and temperature should be considered when choosing the appropriate thermal modality. When deep skeletal muscle (over a small region) is targeted for heat treatment, both ultrasound and microwaves are used clinically because both modalities are thought to effectively heat the target tissue. Garrett et al3 evaluated the changes in gastrocnemius muscle temperature induced by short‐wave diathermy, and found that 20 min of microwave exposure induced an increase of 4.6°C in muscle temperature. Another study reported that ultrasound produced a mean temperature rise of 3.5°C in the gastrocnemius–soleus complex.4 A few comparative studies have examined the use of heat modalities on living tissue, such as muscle, and reported that electromagnetic waves are more effective than ultrasound.3,5 In general, the therapeutic range for sports medicine is assumed to be from 41°C to 45°C.6 However, the increase in temperature may depend on the thermal modality.
Recently, 434 MHz hyperthermia has been introduced in physical medicine and rehabilitation. Hyperthermia treatment with this equipment was reported to be a highly innovative and reliable modality for treating acute muscle injuries in sports.5,7,8 These reports mentioned that 434 MHz hyperthermia treatment significantly reduces the pressure pain and pain on active contraction, but they did not determine muscle temperature during treatment. To our knowledge, only one report9 has measured the changes in temperature, and it used a phantom. The therapeutic benefits of hyperthermia treatment are related to the target‐tissue temperature and changes in temperature; so the change in temperature with treatment should be measured to assess the therapeutic benefits. Measuring the temperature can also provide evidence for the safety of the treatment. Nevertheless, the changes in human muscle temperature have not been reported because it is difficult to measure muscle temperature in a clinical setting. Therefore, we investigated the changes in the temperature of human muscle induced by a 434 MHz microwave hyperthermia system to provide evidence for its clinical effectiveness and safety.
Hyperthermia system
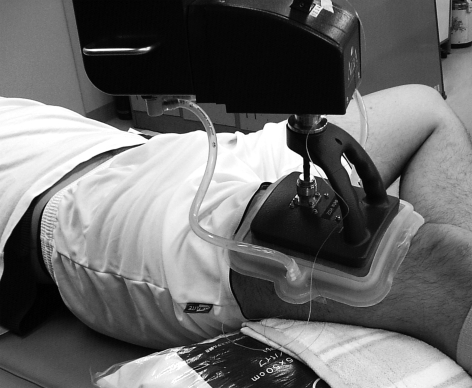
A direct‐contact microwave hyperthermia device that uses an integral system of flowing deionised water to cool the skin while simultaneously heating the deeper tissues with microwaves was applied to the human thigh. The system was an ALBA Hyperthermia System (Restek SRL, Rome, Italy; fig 1) equipped with a 434 MHz microwave generator with a maximum power output of 100 W, a microstrip antenna applicator with a curve shape specific for semicylindrical joint volumes, 20–30 cm in diameter (50% specific absorption rate of the surface is 96 cm2), and a 0.5 cm thick silicon bolus filled, with thermostatic water to prevent overheating of the superficial tissues near the radiant source. The water temperature remained between 30°C and 42°C, and the temperature of the skin in contact with the water bolus was measured using at least one thermocouple. The skin temperature was maintained at the target temperature by automatically decreasing/increasing the power output. The accuracy of the temperature control system in the treatment area was ±0.2°C. The microwave power source was set to turn on/off periodically as the default setting, and the temperature data were measured and stored during the power‐off phase.
Figure 1 The applicator for the hyperthermia system positioned on a human thigh. Informed consent was obtained for publication of this figure.
Experimental procedure
Measuring muscle temperature during hyperthermia treatment
The subjects were 11 healthy adult men (mean (SD) age 24.3 (2.2) years; height 174.2 (6.1) cm; weight 70.0 (5.3) kg; table 1). The skin subcutaneous thickness, including the skin surface layer, was measured using an ultrasound diagnostic scanner (SSD‐900, Aloka, Mitaka, Tokyo, Japan). The subjects underwent 30 min of hyperthermia treatment at 434 MHz. The hyperthermia treatment was administered at a power of 60 W, a skin pilot temperature of 40°C and a bolus water temperature of 38°C. In all subjects undergoing the hyperthermia treatment, the treatment settings were not changed. With the subject lying supine, the applicator was placed on the lateral side of one thigh. The water bolus thickness ranged from 1.0 to 1.5 cm. The thermocouple for determining the pilot temperature was placed on the belly of the vastus lateralis muscle. To minimise the effect of the electromagnetic field, the thermocouple was placed on the skin perpendicular to the electromagnetic field.
Table 1 Physical characteristics of the subjects.
| Subject ID | Age (years) | Height (cm) | Weight (kg) | ST (cm) |
|---|---|---|---|---|
| 01 | 26 | 168.1 | 72.2 | 0.8 |
| 02 | 23 | 178.9 | 74.5 | 0.4 |
| 03 | 24 | 174.6 | 69.2 | 0.8 |
| 04 | 23 | 163.8 | 69.9 | 0.4 |
| 05 | 28 | 173.3 | 69.2 | 0.4 |
| 06 | 23 | 170.1 | 67.2 | 0.9 |
| 07 | 28 | 184.0 | 81.6 | 0.9 |
| 08 | 24 | 167.5 | 59.0 | 0.5 |
| 09 | 25 | 182.5 | 71.5 | 0.6 |
| 10 | 21 | 177.3 | 70.0 | 0.7 |
| 11 | 22 | 174.6 | 65.7 | 0.4 |
| Mean | 24.3 | 174.2 | 70.0 | 0.6 |
| SD | 2.2 | 6.1 | 5.3 | 0.2 |
ST, subcutaneous thickness including skin surface layer.
The vastus lateralis muscle temperature was measured at a depth of 2.0 (0.2) cm during hyperthermia treatment. A 60% lidocaine tape (Penles, Wyeth KK, Chuo‐ku, Tokyo, Japan) was applied to the intended site for at least 30 min before the treatment. A 22 G indwelling cannula with a needle and catheter was inserted as a guide for the thermocouple. After the needle was extracted, a 23 G thermocouple (0.23 mm diameter; IT‐23, Physitemp Instruments, Clifton, New Jersey, USA) was inserted into the catheter. When the thermocouple reached approximately 2 cm, the catheter was also extracted, and the thermocouple alone was left inside the muscle. The water in the bolus was preheated to 38°C and the centre of the applicator position was adjusted to the position of the thermocouple. The measured temperatures were calibrated using a digital thermometer (PTW‐301, Unique Medical, Komae, Tokyo, Japan). The room temperature and humidity were controlled at 24.5 (0.3)°C and 55.9 (8.2)%, respectively. All procedures described were performed with the approval of the Juntendo University Human Ethics Committee and complied with the Declaration of Helsinki. All of the subjects entering the study gave written informed consent.
Assessing muscle tissue disruption induced by hyperthermia treatment
We measured the creatine kinase (CK) activity from fingertip blood samples obtained before and 1, 3, 6, 12 and 24 h after treatment in all subjects (Reflotron Plus, Roche Diagnostics, Basel, Switzerland). In addition, to assess whether the muscle fibre was damaged by hyperthermia treatment, muscle tissue was taken from the intended site in three subjects. At 24 h after the treatment, biopsy specimens were obtained from the belly of the vastus lateralis muscle at 2 cm depth under local anaesthesia using a 14 G disposable biopsy instrument (Max Core, CR Bard, Covington, Georgia, USA). The muscle tissues were immediately frozen in liquid nitrogen and stored at −85°C for subsequent analysis. Sections were cut in a cryostat at a thickness of 9 μm and stained with H&E for light microscopy. The presence of muscle damage (eg, swelling, distortion and loss of staining elements) was evaluated qualitatively by medical pathologists.
Determining the depth of the maximum heating point
To determine the depth of the maximum heating point, we evaluated the heating patterns induced under experimental conditions using a muscle‐equivalent phantom.10 The phantom was a cylinder 14 cm in diameter and 38 cm in length, with an electrical conductivity and permittivity at 434 MHz of 1.56 S/m and 54.73, respectively. The temperature at 2 cm depth was also measured using the 23 G thermocouple during hyperthermia treatment. After the hyperthermia treatment, the temperature distributions on the vertical and horizontal cutting surfaces of the phantom were recorded immediately using a thermal camera (Thermo Tracer TH71000, NEC San‐ei Instruments, Tachikawa, Tokyo, Japan).
Statistical analysis
A paired t test was used to analyse the differences. Correlation analysis was used to quantify the association between values. p<0.05 was considered significant, and the statistical analysis was performed using StatView, V.5.0.
Results
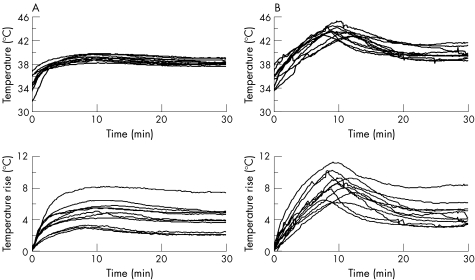
There were no complaints of pain or discomfort in any subject throughout the experiment. Skin temperature before treatment (baseline) was 34.2 (1.4)°C (range 31.6–35.9°C; fig 2A), and it had increased significantly (p<0.001) to 39.2 (0.5)°C (range 38.2–39.8°C, temperature increase 5.0 (1.5)°C) at 10 min. Power output control occurred automatically in four subjects because their skin temperature approached 40°C. However, this was for <5 min, and, even when the power output was kept at 60 W continuously or recovered to 60 W, the skin temperature gradually decreased after the peak value. At the end of treatment, skin temperature decreased to 38.3 (0.4)°C.
Figure 2 Changes in skin (A, upper panel) and muscle (B, upper panel) temperature and the temperature rise (lower panels) during exposure to microwave hyperthermia. Skin temperature was maintained at the pilot temperature of 40°C.
The vastus lateralis muscle temperature before treatment (baseline) was 35.0 (1.3)°C (range 33.2–37.3°C; fig 2B). The peak muscle temperature was 43.7 (0.8)°C (range 42.8–45.4°C), and the temperature rise in the muscle was 8.9 (1.4)°C (range 6.3–11.4°C) at 9.9 (1.4) min after the onset of treatment (p<0.001 vs baseline, table 2). Muscle temperature in one subject exceeded 45°C, but this was only for 1.2 min. After reaching the peak value, muscle temperature decreased to 39.8 (0.9)°C (range 38.7–41.8°C).
Table 2 Peak temperatures at the skin surface and in muscle induced by microwave hyperthermia.
| Baseline (°C) | Peak (°C) | Temperature rise (°C) | Duration (s) | |
|---|---|---|---|---|
| Skin surface | ||||
| Mean (SD) | 34.2 (1.4) | 39.2 (0.5)* | 5.0 (1.5) | 10.7 (1.6) |
| Range | 31.6–35.9 | 38.2–39.8 | 3.1–8.2 | 8.4–13. 7 |
| Muscle | ||||
| Mean (SD) | 35.0 (1.3) | 43.7 (0.8)* | 8.9 (1.4) | 9.9 (1.4) |
| Range | 33.2–37.3 | 42.8–45.4 | 6.3–11.4 | 8.4–12.5 |
Duration, time to peak temperature.
*p<0.01 vs baseline.
Both the skin surface and muscle peak temperatures were significantly increased from baseline.
There was no significant difference in CK activity before and after treatment in all subjects (table 3). In addition, three subjects underwent severe, but standard, hyperthermia treatment at a high power of 70 W to raise muscle temperature close to 45°C. The muscle fibres did not show any damage (swelling, distortion or loss of staining elements) induced by severe hyperthermia treatment.
Table 3 Blood creatine kinase activity before and after hyperthermia treatment.
| Baseline | Time from the end of treatment | |||||
|---|---|---|---|---|---|---|
| 1 h | 3 h | 6 h | 12 h | 24 h | ||
| 108.7 (42.5) | 110.6 (41.9) | 112.3 (41.0) | 109.8 (39.7) | 119.9 (33.9) | 107.5 (27.7) | |
Values are mean (SD) expressed as µ/l.
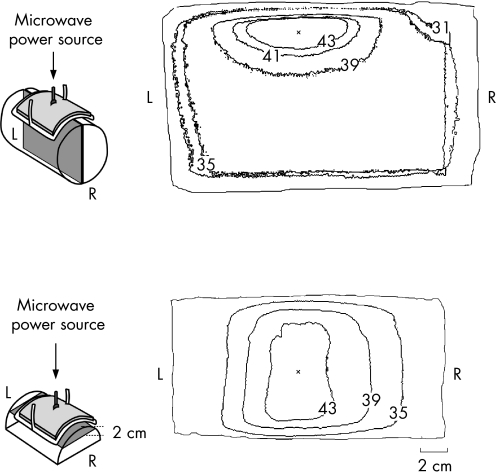
Figure 3 shows the heating pattern in the phantom. The temperature was >43°C at depths of 1–4 cm, and the maximum heating point was at a depth of approximately 2 cm. The temperature difference between the thermograph and thermocouple was <0.5°C.
Figure 3 The thermal distributions (°C) on the vertical (upper panel) and horizontal (lower panel) cut surfaces of the phantom. The maximum heating point appeared at a depth of approximately 2 cm from the surface. L, left; R, right.
Discussion
There are reports of the short‐term effectiveness and safety of hyperthermia in controlled clinical trials.7,8 These reports also state that 434 MHz hyperthermia treatment significantly reduces the pressure pain and pain on active contraction. However, they did not determine muscle temperature during treatment.
In general, the therapeutic range for sports medicine is assumed to be 41–45°C,6 because this range causes the maximum increase in local blood flow.11 Muscle temperatures in our subjects reached at least 42.8°C, and the maximum muscle temperature was 45.4°C. Therefore, the hyperthermia treatment was considered to provide effective therapeutic conditions. Conversely, from the perspective of safety, the possibility that treatment above 45°C causes muscle damage cannot be ignored. There were no complaints of pain and no changes in CK activity in any subject. In addition, to assess the possibility of muscle damage induced directly by hyperthermia treatment, we examined muscle damage in severe hyperthermia treatment using light microscopy. No damage to muscle tissue was observed in any subject undergoing severe hyperthermia treatment that resulted in an increase in muscle temperature over 45°C. The duration of heat treatment is also a concern in hyperthermia therapy.12 For instance, in oncology, patients receive hyperthermia therapy at 45°C for 15 min if they do not feel pain.12 The muscle temperature in one subject exceeded 45°C, but it was only for 1.2 min with no complaint of pain. Consequently, because of the brief duration of exposure to temperature >45°C, we assume that the treatment did not cause muscle tissue damage.
We also examined the depth of the maximum heating point induced by the 434 MHz hyperthermia system. It is impossible to evaluate the maximum heating area accurately using the phantom because it cannot simulate the cooling effect of blood perfusion in muscle. However, the depth of the maximum heating point in human muscle might not differ from that in the phantom. The depth of muscle temperature measurement was considered adequate because the result of the phantom experiment showed that the maximum heating point was at a depth of approximately 2 cm. Therefore, we assume that the hyperthermia treatment did not damage the tissue. The effect of the subcutaneous thickness (including the skin layer) could be ignored because there was no significant correlation between subcutaneous thickness and peak muscle temperature. In addition, skin temperature was maintained at approximately 40°C by the water in the bolus, although muscle temperature increased by 6–11°C. Our results confirm reports that 434 MHz hyperthermia treatment provides effective and safe treatment for human muscle.
After reaching the peak value, muscle temperature decreased in all subjects, although the microwave power output was not changed in seven subjects. This was considered to be caused by local thermal washout. At temperatures >41.5°C and up to 45°C, the increase in tissue temperature enhances local blood flow.6,11,13 At the critical temperature, when the highest local muscle temperature first exceeds a threshold of 42–45°C, rapid perfusion of the cooling blood flow is induced in the high‐temperature region.14 This thermal washout reduces the temperature to save the muscle from overheating. Thermal washout certainly caused the decrease in temperature in seven subjects, whereas in four subjects, the temperature was also controlled by a decrease in power output.
What is already known on this topic
In a clinical situation, 434 MHz hyperthermia treatment has been reported to be a highly innovative and reliable modality for treating acute sport muscle injuries. However, muscle temperatures during treatment have not been measured.
What this study adds
Our results show that the 434 MHz microwave hyperthermia treatment increased and maintained local muscle temperature without muscle damage. These findings support the safety and effectiveness of 434 MHz hyperthermia treatment reported in previous clinical studies.
The ameliorating effects of heated tissue are associated with the increased blood flow. Experimental and clinical studies have demonstrated the limitations of some heating modalities or the mechanisms required to produce a significant increase in blood perfusion.14,15,16 The mechanism that influences the healing of damaged tissue may be highly dependent on the transport of blood‐nurturing substances and on the removal of toxic waste products.17,18 Our results suggest that the hyperthermia treatment caused increased blood flow, although we did not measure the blood flow. The blood flow response of damaged muscle might differ from that of normal muscle, depending on the symptoms. Therefore, the default setting should be decided carefully in a clinical situation according to the severity of symptoms. The mechanisms responsible for the changes in muscle temperature and blood flow, as well as their correlation, should be considered in further studies.
Another possible healing mechanism is the induction of heat shock proteins (HSPs). We previously reported that HSPs were generated by an increase in muscle temperature above 41°C induced by microwave hyperthermia.19 Increased HSP levels increase protein synthesis and decrease protein degradation.20 In addition, HSPs induced by heat stress preceding eccentric contraction may prevent skeletal muscle breakdown during exercise.21,22 We cannot reach a conclusion regarding the mechanism in this study because we measured only muscle temperatures; however, our findings suggest that hyperthermia treatment is effective not only in muscle trauma but also for exercise preconditioning.
In conclusion, we measured the changes in muscle and skin temperatures in the human thigh during 434 MHz hyperthermia treatment. Our results show that muscle temperature increased to the therapeutic temperature range while maintaining skin temperature. These findings support the effectiveness of 434 MHz hyperthermia treatment reported in previous clinical studies. In future, the mechanisms responsible for the changes in muscle temperature should be studied.
Acknowledgements
The skilful technical assistance of Dr K Saitoh and Dr H Kobayashi is gratefully acknowledged. This work was partly supported by a grant from Juntendo University and grants‐in‐aid for scientific research (16300212 and 12480011 to HN) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Abbreviations
CK - creatine kinase
HSP - heat shock protein
Footnotes
Competing interests: None declared.
References
- 1.Prentice W E.Therapeutic modalities in sports medicine. 3rd edn. St Louis, MO: Times Mirror/Mosby, 1994151–287.
- 2.Michlovitz S L.Thermal agents in rehabilitation. Philadelphia, PA: FA Davis, 1986141–212.
- 3.Garrett C L, Draper D O, Knight K L. Heat distribution in the lower leg from pulsed short‐wave diathermy and ultrasound treatments. J Athl Train 20003550–55. [PMC free article] [PubMed] [Google Scholar]
- 4.Draper D O, Ricard M D. Rate of temperature decay in human muscle following 3 MHz ultrasound: the stretching window revealed. J Athl Train 199530304–307. [PMC free article] [PubMed] [Google Scholar]
- 5.Giombini A, Di Cesare A, Safran M R.et al Short‐term effectiveness of hyperthermia for supraspinatus tendinopathy in athletes: a short‐term randomized controlled study. Am J Sports Med 2006341247–1253. [DOI] [PubMed] [Google Scholar]
- 6.Lehmann J F, de Lateur B J. Therapeutic heat. In: Lehmann JF, ed. Therapeutic heat and cold. 3rd edn. Baltimore, MD: Williams & Wilkins, 1982404–562.
- 7.Giombini A, Casciello G, Di Cesare M C.et al A controlled study on the effects of hyperthermia at 434 MHz and conventional ultrasound upon muscle injuries in sport. J Sports Med Phys Fitness 200141521–527. [PubMed] [Google Scholar]
- 8.Giombini A, Di Cesare A, Casciello G.et al Hyperthermia at 434 MHz in the treatment of overuse sport tendinopathies: a randomised controlled clinical trial. Int J Sports Med 200223207–211. [DOI] [PubMed] [Google Scholar]
- 9.Marini P, Guiot C, Baiotto B.et al Measures of specific absorption rate (SAR) in microwave hyperthermic oncology and the influence of the dynamic bolus on clinical practice [Italian]. Radiol Med (Torino) 2001102159–167. [PubMed] [Google Scholar]
- 10.Okano Y, Ito K, Ida I.et al The SAR evaluation method by a combination of thermographic experiments and biological tissue‐equivalent phantoms. IEEE Trans Microwave Theory Tech 2000482094–2103. [Google Scholar]
- 11.Sekins K M, Lehmann J F, Esselman P.et al Local muscle blood flow and temperature responses to 915 MHz diathermy as simultaneously measured and numerically predicted. Arch Phys Med Rehabil 1984651–7. [PubMed] [Google Scholar]
- 12.Perez C A, Scott C, Emami B.et al Evaluation of 45 degrees C hyperthermia and irradiation. II. A phase I clinical trial in humans by the Radiation Therapy Oncology Group. Am J Clin Oncol 199316477–481. [PubMed] [Google Scholar]
- 13.Astrom M, Gentz C F, Nilsson P.et al Imaging in chronic Achilles tendinopathy: a comparison of ultrasonography, magnetic resonance imaging and surgical findings in 27 histologically verified cases. Skelet Radiol 199625615–620. [DOI] [PubMed] [Google Scholar]
- 14.Sekins K M, Emery A F, Lehmann J F.et al Determination of perfusion field during local hyperthermia with the aid of finite element thermal models. J Biomech Eng 1982104272–279. [DOI] [PubMed] [Google Scholar]
- 15.Sekins K M, Dundore D, Emery A F.et al Muscle blood flow changes in response to 915 MHz diathermy with surface cooling as measured by Xe133 clearance. Arch Phys Med Rehabil 198061105–113. [PubMed] [Google Scholar]
- 16.Song C W. Effect of local hyperthermia on blood flow and microenvironment: a review. Cancer Res 198444(Suppl)4721s–30s. [PubMed] [Google Scholar]
- 17.Lehmann J F, Dundore D E, Esselman P C.et al Microwave diathermy: effects on experimental muscle hematoma resolution. Arch Phys Med Rehabil 198364127–129. [PubMed] [Google Scholar]
- 18.Weinberger A, Fadilah R, Lev A.et al Treatment of articular effusions with local deep microwave hyperthermia. Clin Rheumatol 19898461–466. [DOI] [PubMed] [Google Scholar]
- 19.Ogura Y, Naito H, Tsurukawa T.et al Microwave hyperthermia treatment increases heat shock proteins in human skeletal muscle. Br J Sports Med 200741453–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naito H, Powers S K, Demirel H A.et al Heat stress attenuates skeletal muscle atrophy in hindlimb‐unweighted rats. J Appl Physiol 200088359–363. [DOI] [PubMed] [Google Scholar]
- 21.McArdle A, Dillmann W H, Mestril R.et al Overexpression of HSP70 in mouse skeletal muscle protects against muscle damage and age‐related muscle dysfunction. FASEB J 200418355–357. [DOI] [PubMed] [Google Scholar]
- 22.Koh T J. Do small heat shock proteins protect skeletal muscle from injury? Exerc Sport Sci Rev 200230117–121. [DOI] [PubMed] [Google Scholar]