Abstract
Premature return to play after concussion may have debilitating or even fatal consequences. Computerised neuropsychological test batteries are widely used to monitor recovery, but none meet all specified criteria. One possible alternative is to measure saccadic reaction time or latency. Latency reflects the operation of cerebral decision mechanisms, and is strongly influenced by many agents that impair cortical function. A portable, micro‐miniature device (saccadometer) was used to record the eye movements of amateur boxers before and after competitive bouts. Individual latency distributions were significantly affected after blows to the head, though the effects seemed to be reversible, with recovery over a few days. This quantitative, objective and easy to use technique should perhaps be deployed more widely to evaluate its potential in monitoring the effects of sports‐related head injuries.
Keywords: saccade, brain concussion, traumatic brain injury, athletic injuries, neuropsychological test
The time taken to look at a suddenly presented visual target, saccadic latency, reflects cortical decision time, and has proved a useful measure of the general level of cerebral function, being affected by conditions ranging from sedative levels of anaesthesia to metabolic disorders.1,2,3 Furthermore, because saccades to the left and right are independently controlled by each hemisphere, it can provide information about lateral functional asymmetry—for instance, in monitoring the effects of carotid endarterectomy. Recent technical developments (fig 1) allow the rapid measurement of hundreds of saccades without fatigue, providing highly precise information about cerebral decision processes. We believe this might be a promising approach to the continuing question of how to assess recovery from concussion, as discussed by Randolph et al.4
Figure 1 The saccadometer. Built‐in lasers project targets in front of the subject (left); the hand‐held unit (centre) stores eye‐position data obtained by analysing infrared reflectance from the inner limbus of each eye (right). Informed consent was obtained for publication of this figure.
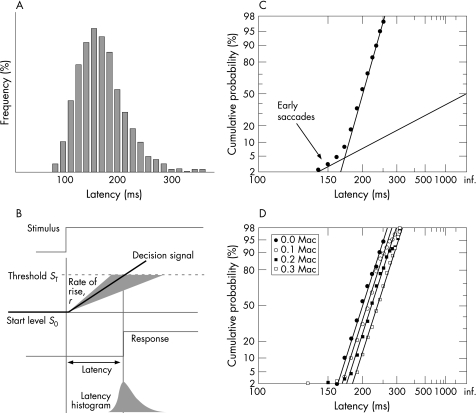
The key to using latency data lies in evaluating entire distributions rather than simply reporting conventional mean values. Although saccades themselves are highly stereotyped movements, latency varies randomly from trial to trial. This characteristic of all reaction times reflects a fundamental aspect of how decisions are made, and by measuring latency distributions we can determine the corresponding parameters of the underlying decision process, using the widely accepted LATER model (fig 2).5,6 This leads to a particular representation of the data—the reciprobit plot—which shows LATER's three parameters in an immediate, graphical form: two describe the mean and standard deviation of the rate of rise of the LATER decision signal, and the third describes the small population of earlysaccades (fig 2) apparent in some subjects as an additional shallower component.7
Figure 2 (A) Latency histograms are skewed. In the LATER model (B), the skew is produced by Gaussian variation in the rate of rise of an underlying linearly rising decision signal. Consequently (C) the reciprocal of latency is itself Gaussian, so that a straight line is produced when cumulative reciprocal latency histograms are plotted using a probit ordinate. Some subjects produce a small additional population of early saccades, with shallower slope. (D) An example of actual plots of this kind: the subject was inhaling subanaesthetic concentrations of sevoflurane as indicated.1
The benefit of applying this quantitative approach in other clinical conditions suggests that it might be useful for evaluating the consequences of sports‐related head injury.
Methods
Twelve boxers in various weight categories, recruited from Cambridge University Amateur Boxing Club and reporting normal vision, participated with informed consent; the general procedure had local ethical committee approval. Saccades were measured within 12 h before and 7 min after a competitive bout lasting a maximum of three rounds of 2 min each. A tally of blows to the head was used as a measure of trauma. Further latency measurements were taken in the days after the bout.
We obtained latency distributions using a portable, micro‐miniaturised head‐mounted saccadometer8 (fig 1), incorporating three lasers projecting high‐contrast red targets in a horizontal line at −10°, 0° and +10° on a wall in front of the seated participant; because the lasers moved with the head, it was not necessary to use a bite‐bar or chin‐rest. Each trial began with the central target that, after a random delay of 0.5–1.5 s, jumped randomly 10° to the left or right; subjects were instructed to follow it with their gaze. Each session, of about 8 min, comprised 240 trials. Using scleral infrared oculometry, the device recorded lateral displacement‐time profiles for each eye, from which latency was computed, with automatic deletion of blinks, movements in the wrong direction or those with an abnormal velocity profile. Individual latency curves (fig 2) were produced and analysed in SPIC,9 which calculated best‐fit LATER parameters by minimisation of the Kolmogorov–Smirnov one‐sample statistic. Kolmogorov–Smirnov two‐sample tests (KS‐2) were used to compare pairs of observed distributions—for instance before and after a bout—and Student's t tests to compare reciprocal medians.
What is already known on this topic
Saccadic reaction time or latency, a useful indicator of many kinds of impairment of brain function, has previously been applied to the study of the effects of concussion.
This work has not led to a clear consensus on whether latency is helpful in this respect, partly because of the intrinsic degree of variability both within and between subjects.
Results
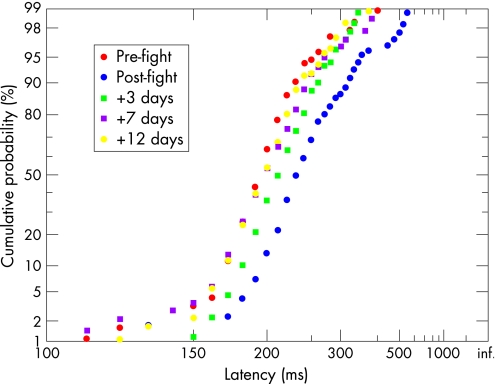
Nine boxers showed a significant latency distribution alteration after their bout (KS‐2, p<0.05); in six cases the median latency was significantly increased (Student's t, p<0.05), in two cases reduced. The four with greatest post‐fight latency increases (20, 26, 34 and 40 ms; fig 3) seemed to have experienced more head insult (assessed by blow tallies and, subjectively, by symptoms), one being deemed concussed at the time (34 ms, p<0.01); for no other participants did the shift exceed 15 ms. In these four boxers, the shift was transient and reversible, with recovery over a few days to a median latency not significantly different from pre‐fight values (fig 3).
Figure 3 Latency distributions for one boxer, showing 40 ms post‐fight increase in median latency, and subsequent recovery.
What this study adds
Here we used a newly developed portable micro‐miniature device to measure a larger number of saccades than was previously possible, in boxers immediately before and after a fight, as well as at a number of days thereafter; this allowed more quantitative comparisons of latency distributions to be made.
We show that after even very mild traumatic brain injury there are obvious alterations in saccadic latency distributions, with increased mean latency, but that they return to pre‐fight levels within a small number of days.
Discussion
It seems that useful comparisons can be made between an individual's baseline and post‐injury saccadic latency distributions, and that large changes are a relatively common outcome of boxing, even in the absence of a period of unconsciousness; those experiencing more head trauma generally showed the largest latency increases. This implies that mild traumatic brain injury impairs cortical decision processes, causing a shift in latency distribution similar, for instance, to that seen in sedative anaesthetic doses (fig 2). The subsequent return of the curve to baseline levels suggests complete recovery of function in the days after injury, reassuring sportsmen that immediate impairment may nevertheless be quite quickly reversible (though because only amateur boxers were included here, the same may not apply to those affected by long‐term professional boxing activity).
Using a similar protocol, but comparing mean saccadic latencies of head‐injured patients with age‐matched controls, Williams et al found reflexive saccadic latency was prolonged by 25 ms (p<0.05): their degree of traumatic brain injury, however, was classified as severe rather than mild.10 More recently, in patients experiencing mild traumatic brain injury, Heitger et al followed up numerous measures of oculomotor performance alongside age‐matched controls.11,12 Though antisaccade latency was prolonged, they found no deficit for simple “reflexive” latency, as measured here. This may be owing to the small number of saccades elicited (44 per patient), and certainly to the diluting effect of comparing patient and control groups rather than using data from before and after concussion in individual subjects (individual latency distributions are idiosyncratic but highly reproducible). Indeed, in the follow‐up study,13 they found a close relationship between mean latency within 1 week and level of self‐perceived recovery within the first 6 months after injury.
Numerous sporting organisations rely on computerised neuropsychological testing for confirmation of recovery from concussion, but few tests claim to demonstrate impairment after the overt symptoms of concussion have resolved.4 We feel that saccadometry may offer certain advantages: the preliminary evidence here is that analysis of distributions may demonstrate large shifts even in asymptomatic subjects (no participant showed symptoms for longer than 24 h). In contrast to relatively complex neuropsychological tasks, saccades do not need to be learnt, permit very high rates of data collection and are relatively immune to distraction. The portable saccadometer measures hundreds of saccades in a matter of minutes, and can be used in the office, the clinic, at the bedside or—literally, in a sporting context—in the field. With the involvement of a wider group of sportsmen, including the boxers, jockeys and rugby players currently being studied, we can begin to correlate the severity of head injuries with changes in latency distributions, and isolate confounding factors such as physical exertion and competition psychology. Precise monitoring of subsequent recovery of latency should allow better return to play decisions to be made, potentially reducing the length of time some players are deemed injured.
Footnotes
Conflict of interest: None declared.
Informed consent was obtained for publication of fig 1.
References
- 1.Nouraei S A, De Pennington N, Jones J G.et al Dose‐related effect of sevoflurane sedation on higher control of eye movements and decision making. Br J Anaesth 200391175–183. [DOI] [PubMed] [Google Scholar]
- 2.Leigh R J, Kennard C. Using saccades as a research tool in the clinical neurosciences. Brain 2004127(part 3)460–477. [DOI] [PubMed] [Google Scholar]
- 3.Ali F R, Michell A W, Barker R A.et al The use of quantitative oculometry in the assessment of Huntington's disease. Exp Brain Res 2006169237–245. [DOI] [PubMed] [Google Scholar]
- 4.Randolph C, McCrea M, Barr W B. Is neuropsychological testing useful in the management of sport‐related concussion? J Athl Train 200540139–152. [PMC free article] [PubMed] [Google Scholar]
- 5.Carpenter R H, Williams M L. Neural computation of log likelihood in control of saccadic eye movements. Nature 199537759–62. [DOI] [PubMed] [Google Scholar]
- 6.Carpenter R H S. LATER. 2006. http://www.cudos.ac.uk/later.htm (accessed 2 July 2007)
- 7.Reddi B A, Carpenter R H. The influence of urgency on decision time. Nat Neurosci 20003827–830. [DOI] [PubMed] [Google Scholar]
- 8.Ober J K, Przedpelska‐Ober E, Gryncewicz W.et al Hand‐held system for ambulatory measurement of saccadic durations of neurological patients. In: Gajda J, ed. Modelling and measurement in medicine. Warsaw: Komitet Biocybernityki i Inzyneierii Biomedycznej PAN, 2003187–198.
- 9.Carpenter R H S. SPIC: a PC‐based system for rapid measurement of saccadic responses. J Physiol 19944804 [Google Scholar]
- 10.Williams I M, Ponsford J L, Gibson K L.et al Cerebral control of saccades and neuropsychological test results after head injury. J Clin Neurosci 19974186–196. [DOI] [PubMed] [Google Scholar]
- 11.Heitger M H, Anderson T J, Jones R D.et al Eye movement and visuomotor arm movement deficits following mild closed head injury. Brain 2004127(Part 3)575–590. [DOI] [PubMed] [Google Scholar]
- 12.Heitger M H, Jones R D, Dalrymple‐Alford J C.et al Motor deficits and recovery during the first year following mild closed head injury. Brain Inj 200620807–824. [DOI] [PubMed] [Google Scholar]
- 13.Heitger M H, Jones R D, Dalrymple‐Alford J C.et al Mild head injury—a close relationship between motor function at 1 week post‐injury and overall recovery at 3 and 6 months. J Neurol Sci 200725334–47. [DOI] [PubMed] [Google Scholar]