Abstract
Background
In the rat brain, heat‐stroke‐induced damage to cerebral neurons is attenuated through heat‐shock‐induced overexpression of heat‐shock protein 72 (HSP72).
Objective
To ascertain whether progressive exercise preconditioning induces HSP72 expression in the rat brain and prevents heat‐stroke‐induced cerebral ischaemia and injury.
Methods
Male Wistar rats were randomly assigned to either a sedentary group or an exercise group. Those in the exercise group progressively ran on a treadmill 5 days/week, for 30–60 min/day at an intensity of 20–30 m/min for 3 weeks. The effects of heat stroke on mean arterial pressure, cerebral blood flow, brain ischaemia markers (glutamate, lactate/pyruvate ratio and nitric oxide), a cerebral injury marker (glycerol) and brain neuronal damage score in the preconditioned animals were compared with effects in unexercised controls. Heat stroke was induced by exposing urethane‐anaesthetised animals to a temperature of 43°C for 55 min, which caused the body temperature to reach 42°C.
Results
Three weeks of progressive exercise pretreatment induced HSP72 preconditioning in the brain and conferred significant protection against heat‐stroke‐induced hyperthermia, arterial hypotension, cerebral ischaemia and neuronal damage; it also prolonged survival.
Conclusions
Exercise for 3 weeks can improve heat tolerance as well as attenuate heat‐stroke‐induced cerebral ischaemia in rats. The maintenance of mean arterial pressure and cerebral blood flow at appropriate levels in the rat brain may be related to overexpression of HSP72.
Keywords: hyperthermia, heat stress, brain damage, exercise training, heat shock protein 72
Owing to the greenhouse effect, the temperature in many parts of the world is increasing. As a result, heat stroke (HS) is becoming a serious clinical problem when humans and mammals are exposed to high ambient temperatures. In more than 50% of victims, the symptoms of HS include hyperthermia, central nervous system disorders and multiple organ failure.1 No pharmacological agent has been found to be beneficial in the treatment of HS.2 Immediate whole‐body cooling is the therapeutic strategy for a patient suffering from HS, but there may still be some sequelae.3 In rodent HS models, arterial hypotension, intracranial hypertension, disseminated intravascular coagulopathy, cerebral ischaemia, brain neuronal damage and overload of cerebral dopamine, cytokines, nitric oxide (NO) and glutamate are observed relative to normothermic controls.4,5,6,7,8 Therefore, the acute phase and most severe level of human HS is closely reflected by the rodent model of HS.9
Experimental HS models have shown that induction of heat‐shock protein 72 (HSP72) in the striatum and nucleus tractus solitarii (NTS) of the rat brain by heat shock or the creation of transgenic mice that overexpress HSP72 alleviate these HS factors.10,11,12,13 We have also previously shown that HSP72 can be detected in vital peripheral organs from rats with progressive exercise training and that HSP72 attenuates HS‐induced hyperthermia, circulatory shock and overproduction of tumour necrosis factor‐α.14 However, induction of HSP72 expression in the rat brain after progressive exercise training for 3 weeks has not been systematically studied. It also remains unclear whether physical exercise protects rats against HS‐induced cerebral ischaemia and damage to cerebral neurons.
In this study, we sought to close this knowledge gap by: (a) comparing the temporal profiles of mean arterial pressure (MAP), cerebral blood flow (CBF), heart rate (HR), NO concentrations and markers of cellular injury and cerebral ischaemia (including glycerol, glutamate and lactate/pyruvate ratio) in the brain of rats with HS with or without physical exercise training; (b) determining whether HSP72 overexpression occurs in the rat brain after 3 weeks of progressive exercise training.
Methods
Experimental animals
Male Wistar rats weighing 250–350 g were purchased from the National Science Council. Pelleted rat chow and tap water were allowed ad libitum. All experimental procedures were conducted in compliance with the NIH Guide for the care and use of laboratory animals. The animals were randomly assigned to one of two major groups: (1) a sedentary control group (SED) and (2) a 1 day after 3‐week exercise training group (3wk1D). After 3 weeks of preconditioning (or no conditioning in control rats), the rats of these two groups were randomly assigned to a normothermic control (NT) subgroup or a heat exposure (HE) subgroup.
Exercise training protocol
The exercise training protocol was implemented according to previously described methods.14 In brief, rats were trained to run on a treadmill (Simplex II; Columbus Instruments, Columbus, Ohio, USA) 5 days a week for 3 weeks. Initially, the rats were acclimatised to run for 15 min at 20 m/min, 0% slope for 3 days. Electrical shocks (1.0 mA) were needed initially to force animals to run forward. Subsequently, they ran without electrical stimulation. The duration and intensity of the exercise were then increased progressively so that the animals were running for 30 min at 20 m/min, 30 min at 30 m/min and 60 min at 30 m/min after 1, 2 and 3 weeks of training, respectively. The work rate of rats on this training protocol is about 70–75% of their maximal oxygen consumption.15 Animals that could not cope with the intensity and duration of this training protocol were withdrawn from the study. Sedentary animals were placed daily on a stationary treadmill and were given electrical stimulation in a manner identical with that used for the exercise group.
HS induction
After urethane anaesthesia (1.4 g/kg intraperitoneally), the HE subgroup was induced by exposure to a blanket temperature (TB) of 43°C for 55 min.14 The moment at which MAP and CBF decreased from their peak values was taken as HS onset. The TB was then returned immediately to 24°C. The survival time (interval between HS onset and cardiac arrest) was obtained for each group. In contrast, the NT subgroup was exposed to 24°C for at least 160 min, with colonic temperature (TCO) maintained at 36±0.1°C using an electric thermal mat.
Different groups of animals were used for three experiments: (1) determination of HSP72 expression in the brain in SED and 3wk1D rats; (2) determination of TCO, MAP, HR, CBF, cerebral NO and extracellular ischaemia markers in SED and 3wk1D rats throughout HS; (3) determination of scores for damage to brain neurons in SED and 3wk1D rats after HS onset.
Western blot
For experiment 1, the animals were killed by decapitation 24 h after the 3‐week period of exercise training. Different brain regions were dissected, including frontal cortex, stratum, hippocampus, hypothalamus and NTS. HSP72 was analysed by western blotting with monoclonal antibody to HSP72 (SPA 810; StressGen, Victoria, British Columbia, Canada).14 Actin was used as the internal control. Blot bands were quantified using an optical scanner and ImageMaster TotalLab 1D Elite software (V.2.01; Amersham Pharmacia, Piscataway, New Jersey, USA).
Monitoring of physiological variables
For experiment 2, physiological monitoring included TCO, TB, MAP, HR, CBF and ischaemia markers in the rat brain. Under general anaesthesia, the femoral artery was cannulated with polyethylene tubing. MAP and HR were continuously monitored using a polygraph (model 2107; Gould, Cleveland, Ohio, USA). TCO was monitored continuously with a thermocouple.
The animal's head was mounted on a stereotaxic apparatus with two burr holes in the cranium allowing insertion of a separate laser–Doppler flowmeter (Laserflo BPM2; Vasamedics, St Paul, Minnesota, USA) and microdialysis probes (CMA12; Carnegie Medicine, Stockholm, Sweden), as described previously.16 The former (length 40 mm) and latter (length 2 mm) were inserted into the right and left striatum, respectively, according to the atlas and coordinates of Paxinos and Watson.17 The microdialysis probe, which allows 13–26% recovery, was perfused with artificial cerebrospinal fluid at 1.2 μl/min. A stabilisation period of 3 h without sampling was allowed after probe implantation.
Dialysate (5 μl portions) was injected into a microdialysis analyser (CMA600; Carnegie Medicine) for measurement of glutamate, glycerol, pyruvate and lactate. The detection thresholds of the CMA600 were 1 μmol/l for glutamate and glycerol, 10 μmol/l for pyruvate and 0.1 mmol/l for lactate. Total nitrate (NO3−) and nitrite (NO2−) in the dialysates were determined using the NO metabolite (NOx−) analysis system (ENO‐10; Eicom, Kyoto, Japan).
Brain neuronal damage score
For experiment 3, rats were perfused with saline followed by 4% paraformaldehyde buffer 15 min after HS onset. Serial sections (4 μm) through the frontal cortex, stratum, hippocampus and hypothalamus were stained with H&E for microscopic evaluation. Neuronal damage in these brain regions was scored on a 0–3 integer scale, indicating normal and <25%, 25–60%, and >60% of neurons damaged, respectively.16 Each hemisphere was evaluated independently, with the examiner blind to the experimental conditions.
Statistical analysis
Experimental data are expressed as mean (SEM) for each point. All variables in fig 1 are continuous. The damage score presented in table 1 is an ordinal variable. Analysis of variance with repeated measures followed by Duncan's multiple‐range test was used for post hoc multiple comparisons of means in fig 1. Neuronal damage scores were evaluated by the Wilcoxon test. Student's t test was used for analysis of data for fig 3. Statistical significance for all tests was defined as p<0.05.
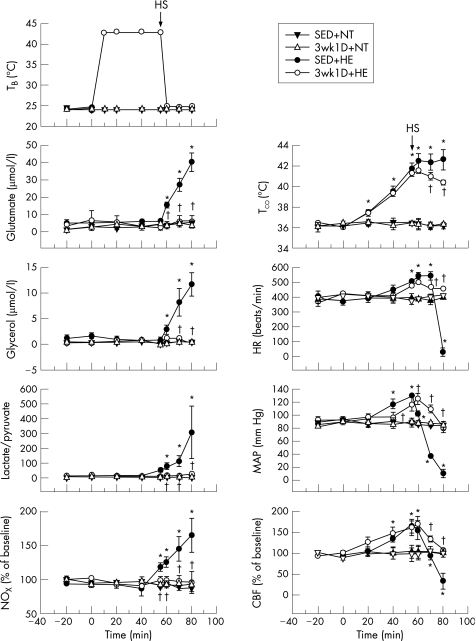
Figure 1 Effects of heat exposure (43°C) on TCO, MAP, HR, CBF, the extracellular concentrations of glutamate, glycerol and NO, and the lactate/pyruvate ratio in rat brain in different groups. Points are mean (SEM) for eight rats per group. NT, rats kept normothermic (24°C); HE, rats kept at 43°C for 55 min; HS, heat stroke onset; SED, sedentary rats; 3wk1D, 1 day after 3‐week‐exercise‐training group; TB, blanket temperature; TCO, colonic temperature; MAP, mean arterial pressure; HR, heart rate; CBF, cerebral blood flow; NOx, nitric oxide metabolite. *p<0.05 compared with the SED+NT group. †p<0.05 compared with the SED+HE group (analysis of variance for repeated measures, followed by Duncan's test).
Table 1 Neuronal damage scores for different brain structures from normothermic control rats and rats in different heat exposure groups.
| Treatment | Neuronal damage score (0–3) | |||
|---|---|---|---|---|
| Striatum | Cortex | Hippocampus | Hypothalamus | |
| SED+NT | 0 (0, 0.75) | 0 (0, 0.5) | 0 (0, 0) | 0 (0, 0) |
| 3wk1D+NT | 0 (0, 1) | 0 (0, 0.75) | 0 (0, 0.75) | 0 (0, 0) |
| SED+HE | 2 (2, 2)* | 2 (2, 2)* | 2 (2, 2)* | 2 (2, 2)* |
| 3wk1D+HE | 1 (0, 1)† | 1 (0.25, 1)† | 1 (0, 1)† | 0 (0, 1)† |
3wk1D, 1 day after 3‐week exercise training; HE, heat exposure; NT, normothermia; SED, sedentary.
Neuronal damage scores were evaluated by Wilcoxon's signed‐rank test. The data are presented as median values, with the first and third quartiles for the eight rats per group in parentheses.
*p<0.05 compared with SED+NT rats.
†p<0.05 compared with the SED+HE group.
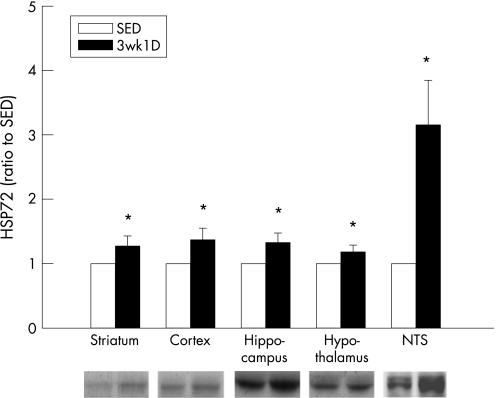
Figure 3 Heat‐shock protein (HSP)72 expression in various rat brain structures comparing SED and 3wk1D animals. Bottom, western blot analysis of HSP72 in different brain structures. Top, band intensities quantified by densitometry. *p<0.05, compared with control (Student's t test). Data are expressed as a ratio to the SED group as the mean (SEM) from eight rats per group. 3wk1D, 1 day after 3‐week exercise training; SED, sedentary; NTS, nucleus tractus solitarii.
Results
The physiological variables of 3wk1D+NT rats were not distinguishable from those of SED+NT rats (fig 1). After 70 min of heat exposure, SED rats had lower MAP and CBF than NT rats. In contrast, TCO, HR and extracellular concentrations of glutamate, glycerol and NO and lactate/pyruvate ratio in the striatum were significantly higher in the SED+HE group after HS onset than in the SED+NT group. However, MAP and CBF of the 3wk1D+HE were significantly higher than those of the SED+HE rats after HS onset. In other words, CBF, MAP and HR fell dramatically in the SED animals with HS compared with very modest changes in their conditioned counterparts. Furthermore, complete cardiac arrest was noted about 20 min after HS onset in the SED+HE group, whereas HR remained stable in the 3wk1D+HE rats. In addition, HS‐induced cerebral ischaemia and related damage were greatly attenuated by 3 weeks of exercise preconditioning. The concentrations of NOx−, glutamate and glycerol, and the lactate/pyruvate ratio remained normal without fluctuation throughout the HS period in the exercise group. Survival after HS (interval between HS onset and cardiac arrest) was significantly prolonged for the 3wk1D+HE group compared with the SED+HE group (185 (18) and 20 (3) min, respectively; p<0.05).
In separate studies, normothermic controls and rats in the HE groups were killed 15 min after HS onset for pathology studies. Table 1 summarises neuronal damage scores for the striatum, cortex, hippocampus and hypothalamus from the normothermic and HS animals in the SED and 3wk1D groups. Compared with NT rats, the SED+HE animals had higher neuronal damage scores in the striatum, cortex, hippocampus and hypothalamus 15 min after HS onset. However, the severity of HS‐induced cerebral neuronal injury was significantly (p<0.05) lower in the 3wk1D+HE groups than in those without the preconditioning.
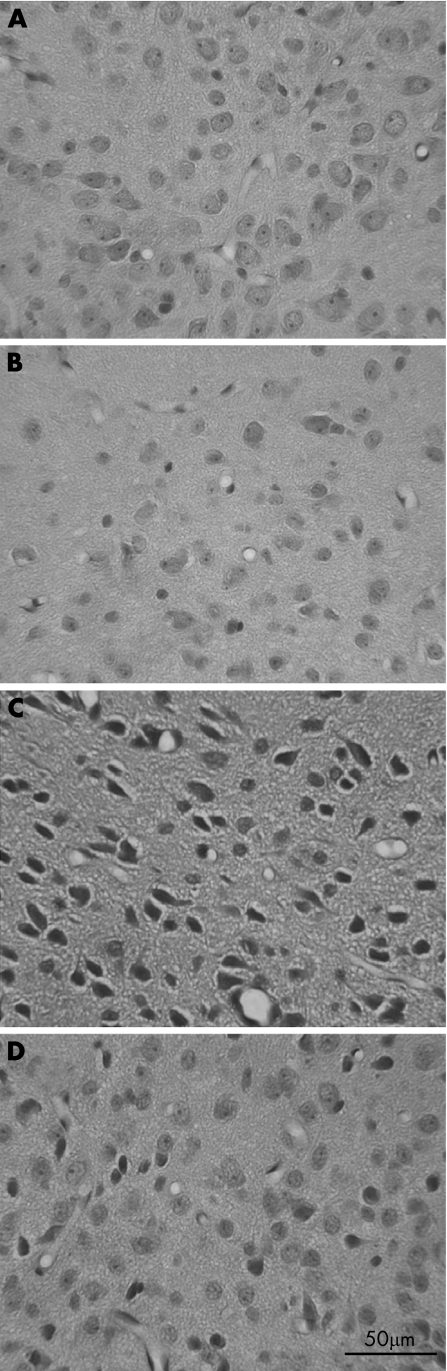
Hyperthermia‐induced cell‐body shrinkage and nuclear pyknosis were attenuated in various brain regions as a result of the 3 weeks of exercise pretraining (fig 2).
Figure 2 Histological examination of neuronal damage in hypothalamus for the SED+NT (A), 3wk1D+NT (B), SED+HE (C) and 3wk1D+HE (D) groups 70 min after the start of heat exposure. Pyknotic degeneration of the nucleus and cell shrinkage are evident in all brain regions of the SED+HE rats 15 min after HS onset (C). However, neuronal damage was reduced in the 3wk1D+HE group (D). 3wk1D, 1 day after 3‐week exercise training; HE, heat exposure; HS, heat stroke; NT, normothermic control; SED, sedentary. Scale bar, 50 μm.
Figure 3 shows HSP72 expression in the frontal cortex, striatum, hippocampus, hypothalamus and NTS after exercise training. Significantly greater HSP72 expression is evident in these brain regions in the 3wk1D rats compared with the SED controls; in particular, much greater expression was noted in the NTS after conditioning.
Discussion
In this study, we show that 3 weeks of exercise preconditioning diminishes HS‐induced cerebral ischaemia and injury in rats. The exercise apparently induces excess HSP72 expression in the brains of trained rats compared with sedentary controls, especially in the NTS. The protective benefits of heat‐shock protein18 are suggested by two established facts: (a) correct folding of many proteins in a cell requires protein‐folding machinery, the molecular chaperones19; (b) heat‐shock‐protein chaperones repair denatured proteins or promote their degradation.20 Further, our results are consistent with several previous investigations of different models. For instance, a number of studies have shown that raised HSP72 concentrations, brought about by exercise, confer cardioprotection against ischaemia/reperfusion injury.21,22 It has also been shown that treadmill pretraining protects against ischaemic brain damage caused by occlusion of the middle cerebral artery.23 Also, in addition to increasing HSP72 concentrations in the striatum and NTS, heat‐shock pretreatment before initiation of HS confers significant protection against HS‐induced syndromes.11,12,13 Transgenic mice overexpressing HSP72 in various organs increased their thermal tolerance by reducing arterial hypotension and cerebral ischaemia.10 Therefore, HSP72 expression in the brain after 3 weeks of progressive exercise may be the factor that both improved heat tolerance and maintained CBF at an adequate level throughout the HS period.
What is already known on this topic
Exercise preconditioning can diminish circulatory collapse induced by heat stroke.
What this study adds
Exercise protects rats against cerebral ischaemia and cerebral neuronal damage induced by heat stroke.
The physiological mechanisms underlying cerebral ischaemic insults resulting from induction of circulatory collapse by heat stress have been extensively studied. After the onset of HS, arterial hypotension results from both depressed ventricular depolarisation and decreased cardiac stroke volume.14 Therefore, cerebral perfusion pressure is lessened and causes cerebral ischaemia, ultimately due to both arterial hypotension and intracranial hypertension.7 A period of cerebral ischaemia is the major cause of severe damage to the cerebral neurons. Cerebral ischaemia induced by HS is associated with increased production of glycerol and glutamate and a raised lactate/pyruvate ratio in the rat brain.16 Glutamate concentration and lactate/pyruvate ratio are well‐known markers of cellular ischaemia, and glycerol concentration reflects how severely cells are affected by the ongoing pathology. Accordingly, survival prolongation in rats with preconditioning exercise is probably associated with augmentation of both arterial blood pressure and CBF as well as reduction of cerebral ischaemia and neuronal damage during HS.
Numerous transmitters and second‐messenger pathways are inappropriately activated after the initial ischaemic events. Both cerebral glutamate overload and glutamate receptor activation are included in the major pathway leading to cerebral neuronal injury in animals with HS.24 Furthermore, glutamate‐stimulated N‐methyl‐d‐aspartate receptors increase calcium flux and activate a variety of intracellular calcium‐dependent enzymes and processes, of which activation of nitric oxide synthase (NOS) plays a prominent role in brain neuronal damage.25,26 In fact, increased concentrations of NO have been associated with several models of circulatory shock, including focal ischaemia and sepsis.27,28 Enhanced NO concentrations have also been observed in the cortex and peripheral circulation of both animals and humans with HS.29,30 It has been found that hyperthermic brain injury is accompanied by distinct upregulation of a constitutive NOS isoform in various regions of the cerebral cortex and hippocampus that normally do not exhibit NOS activity.31 As administration of an inhibitor of inducible NOS proved beneficial in preventing HS‐induced cerebral ischaemia, it has been suggested that NO contributes to cerebral neuronal damage after HS.7
HS is characterised by a reduced baroreceptor reflex response. As the NTS in the medulla oblongata is the major integrative relay centre in the control of cardiovascular function, it is not surprising that it has been shown that severe HS‐induced hypotension is alleviated by HSP72 induction in the NTS of the rat brain.12 Previous studies have found evidence that HSP72 plays a critical role in the development of thermal tolerance and protection from the cellular damage associated with stress such as ischaemia.32,33 Moreover, it has been shown that an endotoxin administered systemically can elicit an increase in NO production in the NTS and induce arterial hypotension.34 Therefore, reducing NO formation in the brain, through pretreated progressive exercise that induces HSP72 overexpression in cerebral regions, alleviates arterial hypotension as well as the cerebral ischaemia exhibited during the onset of HS. In addition, it has been found that alterations in receptor systems and neurotransmitter regulation of NTS neurons are associated with chronic exercise training.35,36 As we found a more than threefold overexpression of HSP72 in the NTS compared with other rat brain regions after exercise training for 3 weeks, it appears reasonable to suggest that HSP72 in the NTS may play an important role in protecting against haemodynamic dysfunction during HS onset. This protective effect may be the result of potentiation of the baroreceptor reflex response both in terms of its sensitivity and capacity.12 Nonetheless, further investigation of the baroreceptor reflex response, neuropathology and ischaemia level in the NTS during HS after exercise is needed in future work.
There are some limitations to this study. Although we verified that brain damage from classic HS that results from exposure to a high environmental temperature can be prevented by moderate exercise training, it remains unclear whether moderate exercise preconditioning protects against exertional HS such as that due to strenuous exercise. Also, a direct relationship between brain HSP72 content and neuronal damage cannot be inferred because this study assessed the three consequences separately. That question could be addressed by microinjecting HSP72 antisense or antibody into the brain of exercising rats to further clarify the effect of exercise‐induced HSP72 expression on HS.
Our results show that preconditioning of rats with physical exercise is effective in improving heat tolerance and attenuating HS‐induced symptoms by maintaining appropriate concentrations of MAP and CBF. This protective effect may be related to overexpression of HSP72 in the brain, especially in the NTS.
Acknowledgements
We gratefully acknowledge the financial support provided by the National Science Council (NSC 94‐2314‐B‐ 006‐030) and Chi‐Mei Foundation Hospital (CMFHR9466) of Taiwan.
Abbreviations
3wk1D - 1 day after 3‐week exercise training
CBF - cerebral blood flow
HE - heat exposure
HR - heart rate
HS - heat stroke
HSP72 - heat‐shock protein 72
MAP - mean arterial pressure
NO - nitric oxide
NOS - nitric oxide synthase
NT - normothermic control
NTS - nucleus tractus solitarii
SED - sedentary
TB - blanket temperature
TCO - colonic temperature
Footnotes
Competing interests: None.
References
- 1.Jones T S, Liang A P, Kilbourne E M.et al Morbidity and mortality associated with the July 1980 heat wave in St Louis and Kansas City, Mo. JAMA 19822473327–3331. [PubMed] [Google Scholar]
- 2.Kwok J S, Chan T Y. Recurrent heat‐related illnesses during antipsychotic treatment. Ann Pharmacother 2005391940–1942. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong L E, Crago A E, Adams R.et al Whole‐body cooling of hyperthermic runners: comparison of two field therapies. Am J Emerg Med 199614355–358. [DOI] [PubMed] [Google Scholar]
- 4.Chou Y T, Chen J C, Liu R S.et al Dopamine overload visualized in the basal ganglia of rabbit brain during heatstroke can be suppressed by hypothermia. Neurosci Lett 200537587–90. [DOI] [PubMed] [Google Scholar]
- 5.Chen S H, Huang K F, Lin M T.et al Human umbilical cord blood cells or estrogen may be beneficial in treating heatstroke. Taiwan J Obstet Gynecol 20074615–25. [DOI] [PubMed] [Google Scholar]
- 6.Tsai H M, Gao C J, Li W X.et al Resuscitation from experimental heatstroke by hyperbaric oxygen therapy. Crit Care Med 200533813–818. [DOI] [PubMed] [Google Scholar]
- 7.Chang C P, Lee C C, Chen S H.et al Aminoguanidine protects against intracranial hypertension and cerebral ischemic injury in experimental heatstroke. J Pharmacol Sci 20049556–64. [DOI] [PubMed] [Google Scholar]
- 8.Niu C S, Lin M T, Liu I M.et al Role of striatal glutamate in heatstroke‐induced damage in streptozotocin‐induced diabetic rats. Neurosci Lett 200334877–80. [DOI] [PubMed] [Google Scholar]
- 9.Chen S H, Niu K C, Lin M T. Cerebrovascular dysfunction is an attractive target for therapy in heat stroke. Clin Exp Pharmacol Physiol 200633663–672. [DOI] [PubMed] [Google Scholar]
- 10.Lee W C, Wen H C, Chang C P.et al Heat shock protein 72 overexpression protects against hyperthermia, circulatory shock, and cerebral ischemia during heatstroke. J Appl Physiol 20061002073–2082. [DOI] [PubMed] [Google Scholar]
- 11.Wang J L, Ke D S, Lin M T. Heat shock pretreatment may protect against heatstroke‐induced circulatory shock and cerebral ischemia by reducing oxidative stress and energy depletion. Shock 200523161–167. [DOI] [PubMed] [Google Scholar]
- 12.Li P L, Chao Y M, Chan S H.et al Potentiation of baroreceptor reflex response by heat shock protein 70 in nucleus tractus solitarii confers cardiovascular protection during heatstroke. Circulation 20011032114–2119. [DOI] [PubMed] [Google Scholar]
- 13.Yang Y L, Lin M T. Heat shock protein expression protects against cerebral ischemia and monoamine overload in rat heatstroke. Am J Physiol 1999276H1961–H1967. [DOI] [PubMed] [Google Scholar]
- 14.Hung C H, Chang N C, Cheng B C.et al Progressive exercise preconditioning protects against circulatory shock during experimental heatstroke. Shock 200523426–433. [DOI] [PubMed] [Google Scholar]
- 15.Demirel H A, Powers S K, Caillaud C.et al Exercise training reduces myocardial lipid peroxidation following short‐term ischemia‐reperfusion. Med Sci Sports Exerc 1998301211–1216. [DOI] [PubMed] [Google Scholar]
- 16.Chen S H, Chang F M, Tsai Y C.et al Resuscitation from experimental heatstroke by transplantation of human umbilical cord blood cells. Crit Care Med 2005331377–1383. [DOI] [PubMed] [Google Scholar]
- 17.Paxinos G, Watson C.The rat brain in stereotaxic coordinates. New York, Academic Press 1996
- 18.Snoeckx L H, Cornelussen R N, Van Nieuwenhoven F A.et al Heat shock proteins and cardiovascular pathophysiology. Physiol Rev 2001811461–1497. [DOI] [PubMed] [Google Scholar]
- 19.Hartl F U. Molecular chaperones in cellular protein folding. Nature 1996381571–579. [DOI] [PubMed] [Google Scholar]
- 20.Hightower L E. Heat shock, stress proteins, chaperones, and proteotoxicity. Cell 199166191–197. [DOI] [PubMed] [Google Scholar]
- 21.Reger P O, Barbe M F, Amin M.et al Myocardial hypoperfusion/reperfusion tolerance with exercise training in hypertension. J Appl Physiol 2006100541–547. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton K L, Staib J L, Phillips T.et al Exercise, antioxidants, and HSP72: protection against myocardial ischemia/reperfusion. Free Radic Biol Med 200334800–809. [DOI] [PubMed] [Google Scholar]
- 23.Wang R Y, Yang Y R, Yu S M. Protective effects of treadmill training on infarction in rats. Brain Res 2001922140–143. [DOI] [PubMed] [Google Scholar]
- 24.Yang Y L, Pan W H, Chiu T H.et al Striatal glutamate release is important for development of ischemic damage to striatal neurons during rat heatstroke. Brain Res 1998795121–127. [DOI] [PubMed] [Google Scholar]
- 25.Liu P K. Ischemia‐reperfusion‐related repair deficit after oxidative stress: implications of faulty transcripts in neuronal sensitivity after brain injury. J Biomed Sci 2003104–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dawson V L, Dawson T M, London E D.et al Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci USA 1991886368–6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szabo C, Mitchell J A, Thiemermann C.et al Nitric oxide‐mediated hyporeactivity to noradrenaline precedes the induction of nitric oxide synthase in endotoxin shock. Br J Pharmacol 1993108786–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samdani A F, Dawson T M, Dawson V L. Nitric oxide synthase in models of focal ischemia. Stroke 1997281283–1288. [DOI] [PubMed] [Google Scholar]
- 29.Alzeer A H, Al Arifi A, Warsy A S.et al Nitric oxide production is enhanced in patients with heat stroke. Intensive Care Med 19992558–62. [DOI] [PubMed] [Google Scholar]
- 30.Canini F, Bourdon L, Cespuglio R.et al Voltametric assessment of brain nitric oxide during heatstroke in rats. Neurosci Lett 199723167–70. [DOI] [PubMed] [Google Scholar]
- 31.Sharma H S, Westman J, Alm P.et al Involvement of nitric oxide in the pathophysiology of acute heat stress in the rat. Influence of a new antioxidant compound H‐290/51. Ann N Y Acad Sci 1997813581–590. [DOI] [PubMed] [Google Scholar]
- 32.Bukau B, Horwich A L. The Hsp70 and Hsp60 chaperone machines. Cell 199892351–366. [DOI] [PubMed] [Google Scholar]
- 33.Lepock J R. Cellular effects of hyperthermia: relevance to the minimum dose for thermal damage. Int J Hyperthermia 200319252–266. [DOI] [PubMed] [Google Scholar]
- 34.Lin H C, Wan F J, Kang B H.et al Systemic administration of lipopolysaccharide induces release of nitric oxide and glutamate and c‐fos expression in the nucleus tractus solitarii of rats. Hypertension 1999331218–1224. [DOI] [PubMed] [Google Scholar]
- 35.De Souza C G, Michelini L C, Fior‐Chadi D R. Receptor changes in the nucleus tractus solitarii of the rat after exercise training. Med Sci Sports Exerc 2001331471–1476. [DOI] [PubMed] [Google Scholar]
- 36.Mueller P J, Hasser E M. Putative role of the NTS in alterations in neural control of the circulation following exercise training in rats. Am J Physiol Regul Integr Comp Physiol 2006290R383–R392. [DOI] [PubMed] [Google Scholar]