Abstract
Objective
To determine the interobserver and intraobserver reliability of the interpretation of MRIs for supraspinatus tendinosis.
Methods
In the interobserver trial, the MRIs of 52 athletes' shoulders were observed by 3 observers on one occasion within a 2‐month period. All 52 images were read by the most experienced musculoskeletal radiologist on 3 different occasions on separate days without access to the previous readings for the intraobserver trial. Supraspinatus tendinosis was graded using a modified 4‐point scale from grades 0 to grade 3.
Results
The grading of MRI‐determined supraspinatus tendinosis was reliable, having an intraclass correlation (ICC) of 0.85 when assessed by the single well‐trained observer. Interobserver reliability was only fair to good (ICC = 0.55).
Conclusions
Supraspinatus tendinosis can be accurately identified on MRI with little variation by a single well‐trained observer. Interobserver reliability was only fair to good. Our data indicated that the reliability of the assessment was much greater in more experienced radiologists than in those with less experience.
MRI has proven to be useful in the assessment of rotator cuff injuries. MRI is a non‐invasive method of imaging and is unique in that it allows the differentiation of soft tissue structures.1 Improvements in MRI techniques, including fast spin‐echo imaging and fat saturation, have facilitated demonstration of tendinous abnormalities of the rotator cuff.
The MRI findings of rotator cuff tendinopathy are characterised by thickened inhomogeneous rotator cuff tendon with increased signal intensity on all pulse sequences.2 Fluid intensity filling an incomplete gap in the tendon on fat‐suppressed T2‐weighted sequences changes are seen on MRI for partial‐thickness tears.3 On MRI, an area of high signal intensity on all pulse sequences outlines complete disruption of the tendon.4
MRI is a non‐invasive technique for investigating lesions of the rotator cuff.5,6,7,8,9,10,11 It is widely used in clinical practice to investigate shoulder problems in patients, particularly those relating to the rotator cuff and to glenohumeral joint instability. Advances in technology have greatly improved the quality of MRI.
Many studies have demonstrated acceptable levels of sensitivity, specificity and accuracy in the diagnosis of cuff and capsulo‐labral pathology.12,13,14,15,16,17 The reliability of assessing supraspinatus tendinopathy has not been determined.
Materials and methods
Subjects
Under ethical approval from the SouthEast Health Human Research Ethics Committee (Sydney, Australia), 52 elite swimmers, of club to international levels, participated in the MRI reliability study. The group consisted of 28 (54%) males and 24 (46%) females. They were aged between 13 and 25 years, with a mean (SD) age of 15.5 (2.7) years and a median age of 16 years. The swimmers underwent an MRI of a single shoulder: either the dominant shoulder if asymptomatic or the most affected shoulder if symptomatic. Each swimmer's evaluation included a shoulder pain and function examination and a swimming training profile before the MRI. Swimmers were excluded if they had any previous surgery, fracture of the shoulder, or inability or unwillingness to participate in the MRI and clinical shoulder examinations. Each participant of the study was given an information sheet outlining the MRI investigation and a consent form to complete.
Study design
One observer made three readings of the same MRIs (intraobserver trial) and three observers independently made readings on the same set of images (interobserver trial).
MRI unit
Oblique coronal proton density (PD) and fat‐suppressed T2, sagittal T2 and axial PD sequencing were performed on a Signa 1.5 T superconducting magnet, Hi Speed MRI unit (General Electric Medical Systems, Milwaukee, Wisconsin, USA), using system software V.9.1, slew rate 77 T/m/s, 33 mm T gradient amplitude, utilising a high resolution, non‐arthrographic technique with a four‐channel phased array shoulder coil (Medical Advances, Milwaukee, Wisconsin, USA). Table 1 outlines the MRI protocol used in this study.
Table 1 MRI protocol for reliability of the MRI‐determined supraspinatus tendinosis examination.
| Orientation | Oblique coronal suppressed proton density | Coronal fat suppressed saggital T2 | Saggital T2 | Axial proton density | Axial proton density fat suppressed |
|---|---|---|---|---|---|
| Swimmers | Adduction | Adduction | Adduction | Adduction | Adduction |
| Position | Neutral rotation | Neutral rotation | Neutral rotation | Neutral rotation | Neutral rotation |
| FOV (cm) | 13 | 13 | 13 | 15 | 15 |
| TR (ms) | 3500 | 3500 | 3500 | 3500 | 3500 |
| TE (eff) (ms) | 34 | 90 | 50 | 34 | 34 |
| Slice thickness | 3 | 3 | 3 | 3 | 3 |
| Matrix size | 512×256 | 256×192 | 512×256 | 384×320 | 384×256 |
| Echo train | 8 | 10 | 10 | 8 | 8 |
| Bandwidth (kHz) | 25 | 20 | 20 | 20 | 20 |
| NEX | 2 | 3 | 2 | 2 | 2 |
FOV, field of view; NEX, number of excitations; TE (eff), echo time (effective); TR, repetition time.
Supraspinatus tendinosis grading
Tendinopathy is characterised by an increased intrasubstance signal on short TE sequences that is not as bright as the fluid on T2‐weighted images.18 The involved tendons may be of a normal calibre or thickened. Differentiating tendinosis with a morphologically normal tendon from the magic angle phenomenon is facilitated by signal alteration that persists on long TE images in tendinopathy.16 In this study, supraspinatus tendinosis was graded using a modified 4‐point scale from 0 to 3 based on previous studies.4,13,19,20
Diagnosis was based on the appearance of the rotator cuff tendons (grading system) and the presence or absence of signs denoting involvement of the subacromial bursa and subacromial–subdeltoid plane.13,20
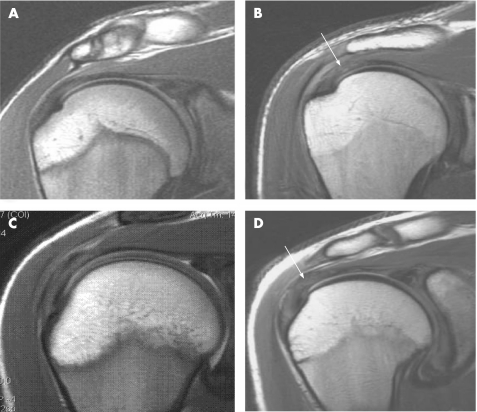
In our study, grade 0 (normal) was a tendon with complete homogeneous low intensity on all pulse sequences or minor intratendon signal hyperintensity consistent with magic angle artefact (fig 1 A), grade 1 (mild tendinosis) mild focal increase in tendon signal on PD and fat‐suppressed T2 sequencing not equal to that of fluid (fig 1 B), grade 2 (moderate tendinosis) moderate focal increase in tendon signal on PD and fat‐suppressed T2 sequencing not equal to that of fluid (fig 1 C), and grade 3 (marked tendinosis) marked a generalised increase in tendon signal without frank fluid signal intensity (fig 1 D).
Figure 1 MRIs of the shoulder illustrating tendinosis grading. (A) Grade 0 (normal). A tendon with complete homogeneous low intensity on all pulse sequences or minor intratendon signal hyperintensity consistent with magic angle artefact. (B) Grade 1 (mild tendinosis). Mild focal increase in tendon signal on PD and fat‐suppressed T2 sequencing not equal to that of fluid. (C) Grade 2 (moderate tendinosis). Moderate focal increase in tendon signal on PD and fat suppressed T2 sequencing not equal to that of fluid. (D) Grade 3 (marked tendinosis). Marked generalised increase in tendon signal without frank fluid signal intensity.
Interobserver reliability trial
Three musculoskeletal radiologists received for the interobserver reliability trial. The experience levels of the three musculoskeletal radiologists varied. The first radiologist had 9 years of experience in musculoskeletal MRI. The second radiologist had a 1‐year training fellowship and 1 year in the clinical practice of MRI reading. The third musculoskeletal radiologist had much less experience in MRI reading. For each swimmer, the three musculoskeletal radiologists (ie, observers) recorded their supraspinatus tendinosis grade using the standardised criteria (fig 1A–D).
All three observers were blinded to the identity of the swimmers, and each of them read all 52 images on one occasion. Before the reading, each observer was briefed on the study protocol by the most experienced radiologist and given the criteria for grading, together with the MRI forms for the supraspinatus tendinosis reliability test. The readings were performed within 2 months of each other.
Intraobserver reliability trial
The intraobserver reliability trial was based on the same 52 swimmers. The most experienced musculoskeletal radiologist read all 52 images on 3 different occasions without access to the previous readings.
Statistical analysis
Intraobserver reliability and intraobserver reliability trails were analysed for their intraclass correlation coefficients (ICCs) with SPSS, using a 2‐way random‐effects model with absolute agreement (2, 1). According to Fleiss21,22, an ICC value <0.4 represents poor reliability, values >0.75 represent excellent reliability and values between 0.4 and 0.75 represent fair to good reliability (194–196).
Results
Incidence of tendinosis
Of the 52 shoulders examined, 69% (25 male, 11 female) had MRI‐assessed tendinosis, 27 (52%) grade 1, 8 (15%) grade 2 and 1 (2%) grade 3.
Clinical relevance
Some authors have identified abnormal signal intensity of the supraspinatus tendon15,19,23,24,25,26,27,28,29,30,31,32,33,34 of asymptomatic individuals. Of the elite swimmers in our study, 36/52 (69%) had MRI‐determined supraspinatus tendinosis. Each of these swimmers had a positive impingement sign. The positive impingement sign correlated significantly with the MRI assessment of tendinosis (r = 0.49, p<0.001).
Reliability of the supraspinatus tendinosis grading
Interobserver reliability trial
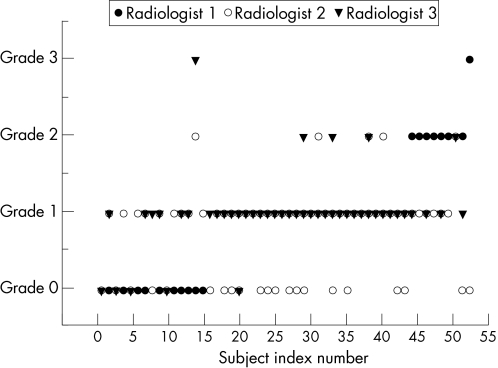
Figure 2 shows the interobserver ICC for the three observers' readings for the MRI‐determined supraspinatus tendinosis grading. Interobserver reliability of the readings for MRI‐determined supraspinatus tendinosis grading had an ICC = 0.55, with a 95% CI ranging from 0.27 to 0.72. According to Fleiss,21,22 this level of agreement between the three radiologists is rated as fair to good.
Figure 2 Interobserver reliability of supraspinatus tendinosis grading.
Intraobserver reliability trial
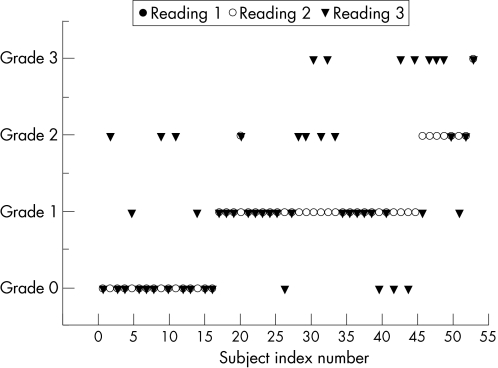
Figure 3 shows the intraobserver ICC for three readings. Intraobserver reliability of readings of the MRI‐determined supraspinatus tendinosis grading was ICC = 0.85, with the 95% CI between 0.72 and 0.9. On the basis of the criteria of Fleiss,21,22 this level of agreement between the three readings is rated as excellent.
Figure 3 Intraobserver reliability of supraspinatus tendinosis grading.
Discussion
In elite swimmers, supraspinatus tendinopathy is one of the main causes of shoulder pain. Improvements in MRI, including fast spin‐echo imaging and fat saturation, have facilitated demonstration of tendinous abnormalities of the rotator cuff. MRI changes in the rotator cuff and tendinitis have been correlated with findings from conventional double‐contrast arthrography,5,8,20,35,23 computed arthrotomography,5,8 arthroscopy11,23 and histological evaluation of the rotator cuff.18
Supraspinatus tendinopathy can be detected using MRI. Kjellin et al18 compared MRIs and histological analysis on cadaver shoulders and showed that increased signal intensity on PD‐weighted images (without further increased signal intensity on T2‐weighted images) and an indistinct margin at the articular side of the supraspinatus tendon corresponded to eosinophilic, fibrillar, and mucoid degeneration and scarring. Areas of increased signal intensity on T2‐weighted images were associated with severe degeneration and disruption of the supraspinatus tendon.18 A study by Gagey et al17 and Williams et al12 also found that gross anatomical and MRI abnormalities of the rotator cuff corresponded to histological changes consistent with tendon degeneration. We observed an excellent correlation between MRI‐determined tendinopathy and a positive impingement sign in swimmers.
The sensitivity and specificity of magnetic resonance scanning in the diagnosis of partial‐thickness and full‐thickness defects of the rotator cuff have been well documented.13,36,37 We found that the grading of MRI‐determined supraspinatus tendinosis grading was reliable (ICC = 0.85) when assessed by a single well‐trained observer. However, the interobserver reliability was only fair to good (ICC = 0.55). Our data indicated that the reliability of the assessment was much greater in more experienced radiologists and less in the junior radiologist.
What is already known on this topic
MRI is a useful, non‐invasive technique for the assessment of shoulder problems, particularly those relating to the rotator cuff and glenohumeral joint instability. The reliability of assessing supraspinatus tendinopathy using MRI, however, has not been determined.
What this study adds
In elite swimmers, supraspinatus tendinopathy is one of the main causes of shoulder pain.
This study found MRI assessment of tendinopathy to be reliable with a single well‐trained observer and less so with other observers.
There was an excellent correlation between MRI‐determined tendinopathy and a positive impingement sign in swimmers.
Acknowledgements
This study was supported by St George Hospital/South East Sydney and Illawarra Area Health Service. We thank the New South Wales Institute of Sport, its coaches, swimmers and their parents for their enthusiastic participation, and the Castlereagh Imaging group for their expertise in MRI interpretation.
Abbreviations
ICC - intraclass correlation
PD - proton density
Footnotes
Competing interests: None declared.
References
- 1.Lee S U, Lang P. MR and MR arthrography to identify degenerative and posttraumatic diseases in the shoulder joint. Eur J Radiol 200035126–135. [DOI] [PubMed] [Google Scholar]
- 2.Stoller D W, Tirman P, Bredella M A.et alDiagnostic imaging orthopaedics 1st edn. Utah:AMIRSYS 2004
- 3.Hawkins R J, Hobeika P E. Impingement syndrome in the athletic shoulder. Clin Sports Med 19832391–405. [PubMed] [Google Scholar]
- 4.Farley T E, Neumann C H, Steinbach L S.et al Full‐thickness tears of the rotator cuff of the shoulder: diagnosis with MR imaging. Am J Roentgenol 1992158347–351. [DOI] [PubMed] [Google Scholar]
- 5.Kieft G J, Bloem J L, Rozing P M.et al Rotator cuff impingement syndrome: MR imaging. Radiology 1988166211–214. [DOI] [PubMed] [Google Scholar]
- 6.Kieft G J, Bloem J L, Obermann W R.et al Normal shoulder: MR imaging. Radiology 1986159741–745. [DOI] [PubMed] [Google Scholar]
- 7.Kneeland B J, Middleton W D, Carrera G F.et al MR imaging of the shoulder: diagnosis of rotator cuff tears. Am J Roentgenol 1987149333–337. [DOI] [PubMed] [Google Scholar]
- 8.Reeder J D, Andelman S. The rotator cuffn tear: MR evaluation. Magn Reson Imaging 19875331–338. [DOI] [PubMed] [Google Scholar]
- 9.Reiser M, Erlemann R, Bongartz G.et al Possibilities of magnetic resonance tomography in diagnostic imaging of the shoulder joint. Radiologe 19882879–83. [PubMed] [Google Scholar]
- 10.Seeger L L, Gold R H, Bassett L W.et al Shoulder impingement syndrome: MR findings in 53 shoulders. AJR Am J Roentgenol 1988150343–347. [DOI] [PubMed] [Google Scholar]
- 11.Seeger L L, Ruszkowski J T, Bassett L W.et al MR imaging of the normal shoulder: anatomic correlation. AJR Am J Roentgenol 198714883–91. [DOI] [PubMed] [Google Scholar]
- 12.Williams G R, Jr, Iannotti J P, Rosenthal A.et al Anatomic, histologic, and magnetic resonance imaging abnormalities of the shoulder. Clin Orthop 1996(330)66–74. [DOI] [PubMed]
- 13.Iannotti J P, Zlatkin M B, Esterhai J L.et al Magnetic resonance imaging of the shoulder. Sensitivity, specificity, and predictive value. J Bone Joint Surg Am 19917317–29. [PubMed] [Google Scholar]
- 14.Kjellin I, Ho C, Cervilla V.et al Alterations in the supraspinatus tendon at MR imaging: correlation with histopathologic findings in cadavers. Radiology 1991181837–841. [DOI] [PubMed] [Google Scholar]
- 15.Robertson P L, Schweitzer M E, Mitchell D G.et al Rotator cuff disorders: interobserver and intraobserver variation in diagnosis with MR imaging. Radiology 1995194831–835. [DOI] [PubMed] [Google Scholar]
- 16.Rafii M, Firooznia H, Sherman O.et al Rotator cuff lesions: signal patterns at MR imaging. Radiology 1990177817–823. [DOI] [PubMed] [Google Scholar]
- 17.Gagey N, Quillard J, Gagey O.et al Tendon of the normal supraspinatus muscle: correlation between MR imaging and histology. Surg Radiol Anat 199517329–334. [DOI] [PubMed] [Google Scholar]
- 18.Kjellin I, Ho C P, Servilla V.et al Alterations in the supraspinatus tendon at MR imaging: correlation with histopathologic findings in cadavers. Radiology 1991181837–841. [DOI] [PubMed] [Google Scholar]
- 19.Neuman C H, Holt R G, Steinbach L S.et al MR imaging of the shoulder: appearance of the supraspinatus tendon in asymptomatic volunteers. Am J Roentgenol 19921581281–1287. [DOI] [PubMed] [Google Scholar]
- 20.Zlatkin M B, Iannotti J P, Roberts M C.et al Rotator cuff tears: diagnostic performance of MR imaging. Radiology 1989172223–229. [DOI] [PubMed] [Google Scholar]
- 21.Fleiss J L. Reliability of measurement. The design and analysis of clinical experiments. New York: John Wiley & Sons, 1986
- 22.Fleiss J L. Reliability in the clinical setting. Am Phys Ther Assoc Res Section Newsletter 1986242–8. [Google Scholar]
- 23.Evancho A M, Stiles R G, Fajman W A.et al MR imaging diagnosis of rotator cuff tears. Am J Roentgenol 1988151751–754. [DOI] [PubMed] [Google Scholar]
- 24.Mirowitz S A. Normal rotator cuff: MR imaging with conventional and fat‐suppression techniques. Radiology 1991180735–740. [DOI] [PubMed] [Google Scholar]
- 25.Miniaci A, Dowdy P A, Willits K R.et al Magnetic resonance imaging evaluation of the rotator cuff tendons in the asymptomatic shoulder. Am J Sports Med 199523142–145. [DOI] [PubMed] [Google Scholar]
- 26.Nelson M C, Leather G P, Nirschl R P.et al Evaluation of the painful shoulder. A prospective comparison of magnetic resonance imaging, computerized tomographic arthrography, ultrasonography, and operative findings. J Bone Joint Surg Am 199173707–716. [PubMed] [Google Scholar]
- 27.Torstensen E T, Hollinshead R M. Comparison of magnetic resonance imaging and arthroscopy in the evaluation of shoulder pathology. J Should Elbow Surg 1999842–45. [DOI] [PubMed] [Google Scholar]
- 28.Sahin‐Akyar G, Miller T T, Staron R B.et al Gradient‐echo versus fat‐suppressed fast spin‐echo MR imaging of rotator cuff tears. Am J Roentgenol 1998171223–227. [DOI] [PubMed] [Google Scholar]
- 29.Morrison D S, Ofstein R. The use of magnetic resonance imaging in the diagnosis of rotator cuff tears. Orthopedics 199013633–637. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y M, Shih T T, Jiang C C.et al Magnetic resonance imaging of rotator cuff lesions. J Formosan Med Assoc 199493234–239. [PubMed] [Google Scholar]
- 31.Wnorowski D C, Levinsohn E M, Chamberlain B C.et al Magnetic resonance imaging assessment of the rotator cuff: is it really accurate? Arthroscopy 199713710–719. [DOI] [PubMed] [Google Scholar]
- 32. Swen WA, Jacobs JW, Algra PR, et al. Sonography and magnetic resonance imaging equivalent for the assessment of full‐thickness rotator cuff tears. Arthritis Rheum 1999422231–2238. [DOI] [PubMed] [Google Scholar]
- 33.Patten R M, Spear R P, Richardson M L. Diagnostic performance of magnetic resonance imaging for the diagnosis of rotator cuff tears using supplemental images in the oblique sagittal plane. Invest Radiol 19942987–93. [DOI] [PubMed] [Google Scholar]
- 34.Blanchard T K, Bearcroft P W, Constant C R.et al Diagnostic and therapeutic impact of MRI and arthrography in the investigation of full‐thickness rotator cuff tears. Eur Radiol 19999638–642. [DOI] [PubMed] [Google Scholar]
- 35.Habibian A, Stauffer A, Resnick D.et al Comparison of conventional and computed arthrotomography with MR imaging in the evaluation of the shoulder. J Comput Assist Tomogr 198913 [DOI] [PubMed] [Google Scholar]
- 36.Burk D L, Karasick D, Kurtz A B.et al Rotator cuff tears: prospective comparison of MR imaging with arthrography, sonography, and surgery. Am J Roentgenol 198915387–92. [DOI] [PubMed] [Google Scholar]
- 37.Flannigan B, Kursunoglu‐Brahme S, Snyder S.et al MR arthrography of the shoulder: comparison with conventional MR imaging. Am J Roentgenol 1990155829–832. [DOI] [PubMed] [Google Scholar]