Abstract
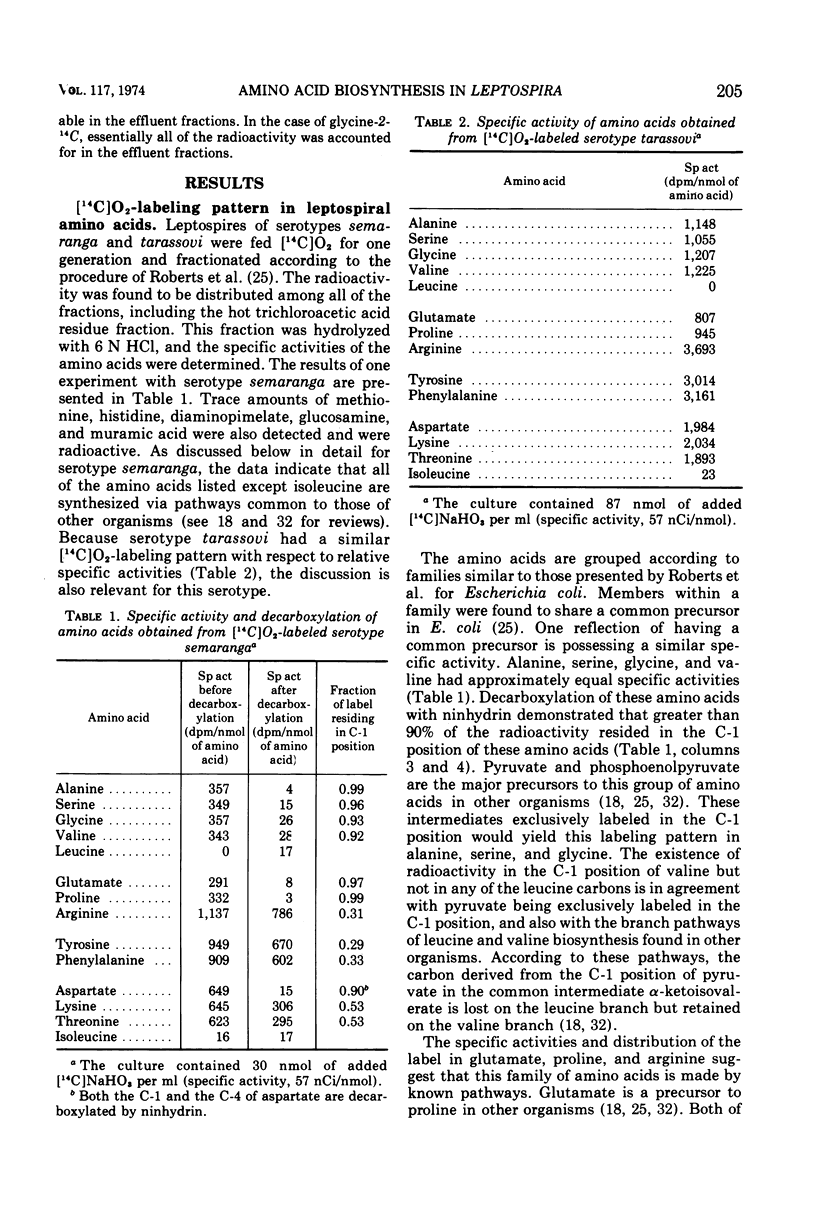
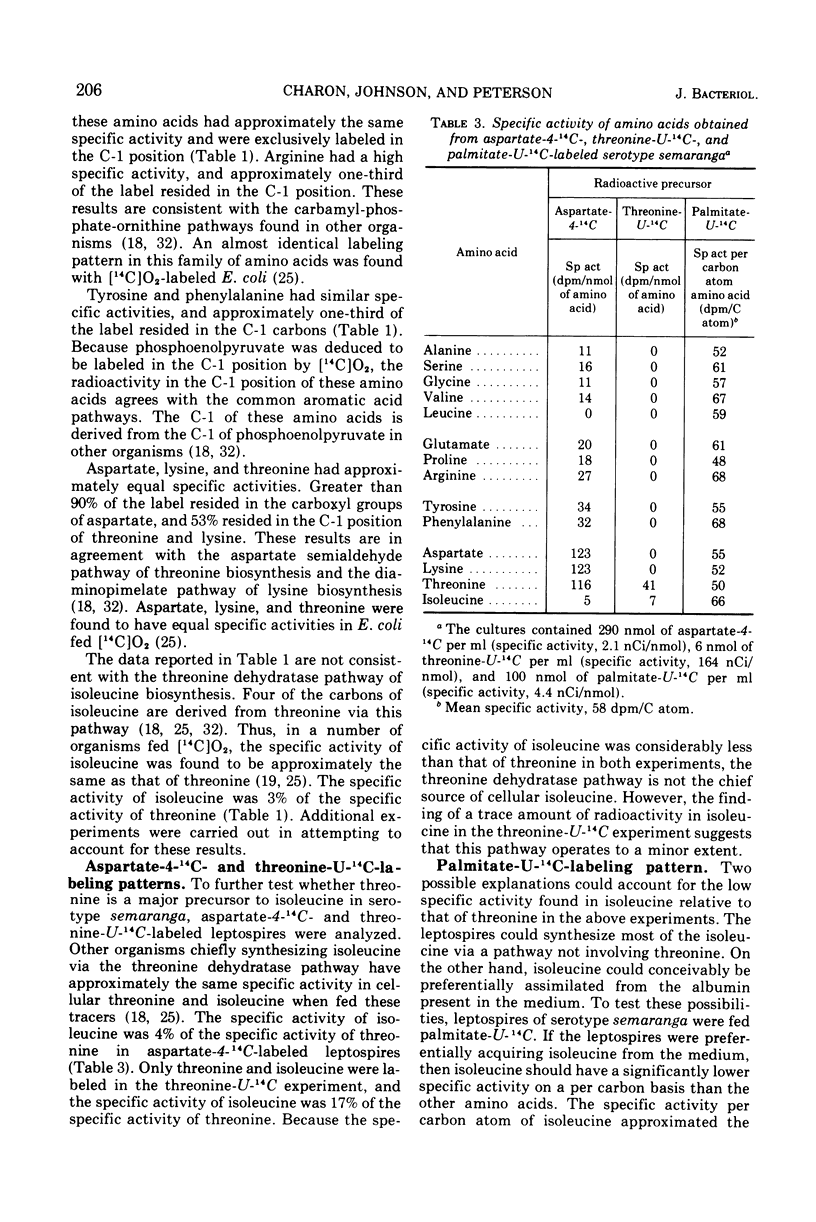
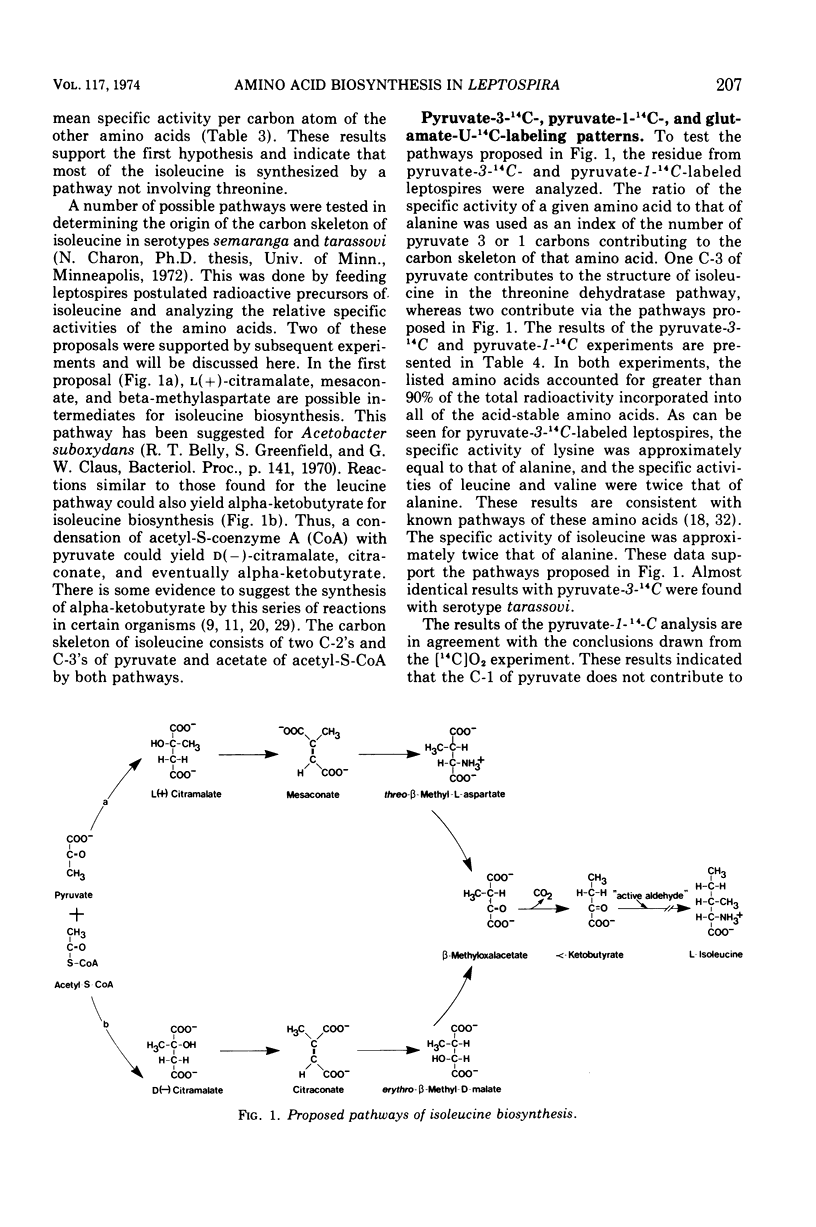
Radioactive carbon dioxide was incubated with growing cells of Leptospira interrogans serotypes semaranga and tarassovi, and the specific activities and distribution of the label within the cellular amino acids were determined. The origins of the carbon skeletons of all the acid-stable amino acids except isoleucine were found to be consistent with known biosynthetic pathways for these amino acids. Experiments using radioactive carbon dioxide and other tracers indicated that most of the isoleucine was synthesized by a pathway not involving threonine. The origin of the carbon skeleton of isoleucine consisted of two residues of pyruvate (carbons 2 and 3) and acetate of acetyl-coenzyme A by this pathway. Isotope competition studies indicated that the pathway was regulated by isoleucine. The results are discussed in relation to two proposed pathways of isoleucine biosynthesis involving citramalate as an intermediate.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAMSKY T., SHEMIN D. THE FORMATION OF ISOLEUCINE FROM BETA-METHYLASPARTIC ACID IN ESCHERICHIA COLI W. J Biol Chem. 1965 Jul;240:2971–2975. [PubMed] [Google Scholar]
- BARKER H. A., ROOZE V., SUZUKI F., IODICE A. A. THE GLUTAMATE MUTASE SYSTEM. ASSAYS AND PROPERTIES. J Biol Chem. 1964 Oct;239:3260–3266. [PubMed] [Google Scholar]
- BENSON J. V., Jr, PATTERSON J. A. ACCELERATED AUTOMATIC CHROMATOGRAPHIC ANALYSIS OF AMINO ACIDS ON A SPHERICAL RESIN. Anal Chem. 1965 Aug;37:1108–1110. doi: 10.1021/ac60228a008. [DOI] [PubMed] [Google Scholar]
- Baseman J. B., Cox C. D. Intermediate energy metabolism of Leptospira. J Bacteriol. 1969 Mar;97(3):992–1000. doi: 10.1128/jb.97.3.992-1000.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo J. M., Freundlich M., Umbarger H. E. Regulation of branched-chain amino acid biosynthesis in Salmonella typhimurium: isolation of regulatory mutants. J Bacteriol. 1969 Mar;97(3):1272–1282. doi: 10.1128/jb.97.3.1272-1282.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinco M., Petelin N. Serogroups and serotypes in Water-Leptospira strains. Trop Geogr Med. 1970 Jun;22(2):237–244. [PubMed] [Google Scholar]
- GUYMON J. F., INGRAHAM J. L., CROWELL E. A. The formation of n-propyl alcohol by Saccharomyces cerevisiae. Arch Biochem Biophys. 1961 Oct;95:163–168. doi: 10.1016/0003-9861(61)90122-9. [DOI] [PubMed] [Google Scholar]
- Henneberry R. C., Cox C. D. Beta-oxidation of fatty acids by Leptospira. Can J Microbiol. 1970 Jan;16(1):41–45. doi: 10.1139/m70-007. [DOI] [PubMed] [Google Scholar]
- JOHNSON R. C., GARY N. D. NUTRITION OF LEPTOSPIRA POMONA. II. FATTY ACID REQUIREMENTS. J Bacteriol. 1963 May;85:976–982. doi: 10.1128/jb.85.5.976-982.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON R. C., ROGERS P. METABOLISM OF LEPTOSPIRAE. I. UTILIZATION OF AMINO ACIDS AND PURINE, AND PYRIMIDINE BASES. Arch Biochem Biophys. 1964 Sep;107:459–470. doi: 10.1016/0003-9861(64)90302-9. [DOI] [PubMed] [Google Scholar]
- Johnson R. C., Harris V. G. Differentiation of pathogenic and saprophytic letospires. I. Growth at low temperatures. J Bacteriol. 1967 Jul;94(1):27–31. doi: 10.1128/jb.94.1.27-31.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Harris V. G., Walby J. K. Characterization of leptospires according to fatty acid requirements. J Gen Microbiol. 1969 Mar;55(3):399–407. doi: 10.1099/00221287-55-3-399. [DOI] [PubMed] [Google Scholar]
- Johnson R. C., Livermore B. P., Walby J. K., Jenkin H. M. Lipids of parasitic and saprophytic leptospires. Infect Immun. 1970 Sep;2(3):286–291. doi: 10.1128/iai.2.3.286-291.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlhaw G., Leary T. R., Umbarger H. E. Alpha-isopropylmalate synthase from Salmonella typhimurium. Purification and properties. J Biol Chem. 1969 Apr 25;244(8):2218–2225. [PubMed] [Google Scholar]
- MOSES V. The metabolic significance of the citric acid cycle in the growth of the fungus Zygorrhynchus moelleri. J Gen Microbiol. 1957 Jun;16(3):534–549. doi: 10.1099/00221287-16-3-534. [DOI] [PubMed] [Google Scholar]
- Nakano H., Sasaki K., Kurokawa Y., Katsuki H. Metabolism of -methylmalic acid by a soil bacterium. J Biochem. 1971 Sep;70(3):429–440. doi: 10.1093/oxfordjournals.jbchem.a129657. [DOI] [PubMed] [Google Scholar]
- Olson A. C., White L. M., Noma A. T. Scintillation counting of the ninhydrin-amino acid-C14 reaction products from an automatic amino acid analyzer. Anal Biochem. 1968 Jul;24(1):120–127. doi: 10.1016/0003-2697(68)90066-3. [DOI] [PubMed] [Google Scholar]
- PIEZ K. A. Continuous scintillation counting of carbon-14 and tritium in effluent of the automatic amino acid analyzer. Anal Biochem. 1962 Dec;4:444–458. doi: 10.1016/0003-2697(62)90126-4. [DOI] [PubMed] [Google Scholar]
- Phillips A. T., Nuss J. I., Moosic J., Foshay C. Alternate pathway for isoleucine biosynthesis in Escherichia coli. J Bacteriol. 1972 Feb;109(2):714–719. doi: 10.1128/jb.109.2.714-719.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin R., Salamon I. I., Bleiweis A. S., Carlin J., Ajl S. J. Metabolism of ethylmalic acids by Pseudomonas aeruginosa. Biochemistry. 1968 Jan;7(1):377–388. doi: 10.1021/bi00841a048. [DOI] [PubMed] [Google Scholar]
- Robinson I. M., Allison M. J. Isoleucine biosynthesis from 2-methylbutyric acid by anaerobic bacteria from the rumen. J Bacteriol. 1969 Mar;97(3):1220–1226. doi: 10.1128/jb.97.3.1220-1226.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K., Nakano H., Katsuki H. Enzymatic isomerization of -methylmate to (-)citramalate by a soil bacterium. J Biochem. 1971 Sep;70(3):441–449. doi: 10.1093/oxfordjournals.jbchem.a129658. [DOI] [PubMed] [Google Scholar]
- Stern N., Shenberg E., Tietz A. Studies on the metabolism of fatty acids in Leptospira: the biosynthesis of delta 9- and delta 11-monounsaturated acids. Eur J Biochem. 1969 Mar;8(1):101–108. doi: 10.1111/j.1432-1033.1969.tb00501.x. [DOI] [PubMed] [Google Scholar]
- UMBARGER H. E. Feedback control by endproduct inhibition. Cold Spring Harb Symp Quant Biol. 1961;26:301–312. doi: 10.1101/sqb.1961.026.01.036. [DOI] [PubMed] [Google Scholar]



