Abstract
Tissue engineering aims to induce tissue self‐regeneration in vivo or to produce a functional tissue replacement in vitro to be then implanted in the body. To produce a viable and functional tendon, a uniaxially orientated collagen type I matrix has to be generated. Biochemical and physical factors can potentially alter both the production and the organisation of this matrix, and their combination in a dose‐ and time‐dependent manner is probably the key to in vitro engineered tendons. This review discusses the role of these different factors affecting tenocyte growth in a three‐dimensional environment in vivo and in vitro, and underlines the future challenge of tendon tissue engineering.
Artificial implants are relatively successful in reconstructive surgery, but there are still several drawbacks, including their finite lifetime and the associated degradation of their mechanical properties. Carbon fibres and Dacron grafts are the most commonly used artificial materials for tendon repair, but until now, no artificial materials have been able to achieve the adaptability and flexibility demonstrated by functional tissues that are in perpetual remodelling.
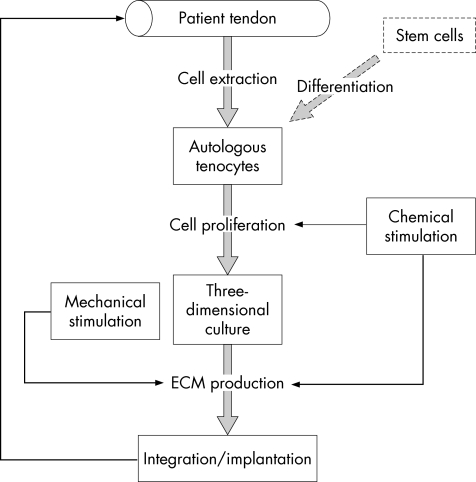
In this context, tissue engineering bridges the gap between materials science and cell biology to provide new engineered biomaterials that can mimic real tissues and induce in vivo regeneration, or can be developed in vitro as a functional tissue before their implantation (fig 1). Tendon tissue engineering faces a major challenge: the highly hierarchal organised collagen structure surrounded by proteoglycans allows tendons to face a wide range of non‐linear mechanical deformation.
Figure 1 Strategy for in vitro tendon tissue engineering. A replacement tissue is reconstructed in vitro from a patient's autologous cells.
The in vivo approach of tendon engineering deals with the repair of small defects and the induction of tissue self‐regeneration. In vitro tissue engineering aims to produce a functional replacement tissue, grown from a patient's autologous cells.
These two different approaches share common features, as they rely on cell–material interaction and the stimulation of pre‐tissue formation. Autologous tenocytes or non‐differentiated stem cells are loaded in a three‐dimensional construct, a scaffold of a particular shape, which promotes pre‐tissue formation instigated by chemical and mechanical stimuli. The combination of scaffold, cell and stimulation, or their stand‐alone applications is the essence of tissue engineering.
Scaffold
Collagen plays a central role in tendon engineering, as collagen type I is responsible for >70% of the dry weight of the tendon structure, and its hierarchical organisation in bundles contributes to most of the tendon's mechanical properties. Consequently, tendon engineering studies mainly deal with cell‐seeded collagen gels. Contraction of the gel is related to the cell‐seeding density, generally followed by alignment and reorganisation of the matrix.1,2 Collagen gels have been used as a model system for in vitro study,3,4 and could be used to assess the effects of different stimuli on cell proliferation. In vivo, these collagen gels have been used as a vehicle to deliver mesenchymal stem cells in defects of the Achilles tendon in rabbits.5 Preliminary alignment of the cells in the gel increases the efficiency of the method.6 Until now, no tenocyte–collagen constructs have been able to achieve sufficient mechanical properties, and the complex architecture of the tendon is never fully reproduced. Also, collagen type I does not account for all the tissue properties. The role of proteoglycans and small leucine‐rich protein in the organisation and mechanical properties of the tissue structure has been outlined recently.7
To enhance early mechanical properties, cross‐linking and hybridisation strategies have been proposed. Collagen hybridisation with poly(l)‐lactic acid showed higher mechanical properties and promotion of tendon cell migration,8 and a promising scaffold has been developed by cross‐linking collagen fibres with di‐catechol nordihydroguaiaretic acid. With an elastic modulus of 580 Pa and a tensile strength of 90 MPa, it has better mechanical properties than carbodiimide and glutaraldehyde for tendon repair.9
Chitin‐based scaffolds have been used in the rotator cuff with relative success. Unwoven chitin fabric was used as an acellular matrix to increase tenocyte proliferation and collagen deposition in 10 mm by 10 mm defects in a rabbit model after 4 weeks. After 8 weeks, the scaffold was absorbed in the tissue and induced better regeneration than control groups.10 Similarly, chitosan‐based hyaluronan hybrid fibre scaffolds have been used in rabbit infraspinatus tendon defects. Fibroblast‐seeded scaffolds achieved better collagen type I production and mechanical properties after 12 weeks compared with acellular scaffolds and controls.11
A different strategy is to improve allografts employed in routine surgery. For example, patellar tendon allografts have been emptied from intrinsic cells to reduce immune response, and could conveniently act as a delivery vehicle for cells or other therapeutic agents, once seeding efficiency is improved.12
An atypical kind of scaffold is treated tissue. An example is the acellular porcine small intestinal submucosa used in ligament replacement in an animal study.13 The graft was compared with patellar‐tendon autograft, and comparable failure forces were found after 12 months. However, the submucosa undergoes a dramatic decrease of its biomechanical properties 3 months after the implantation.
This natural tissue has also been used as tendon sheath and to enfold polyglycolic acid fibres seeded with tenocytes in an in vivo experiment on hen tendon to bridge 4 cm defects.14 After 14 weeks, both histological and biomechanical properties were found to be close to those of native tendon. They showed cell and collagen alignment, total degradation of the polyglycolic acid fibres and a steady increase in mechanical properties (83% at the end of the experiment). In vitro, the feasibility of this technique has been explored, and demonstrated pre‐tissue formation under tension, but without appropriate biomechanical properties.15
The contact guidance effect, which aligns cells along a microstructured surface, has been used to design a three‐dimensional scaffold for tendon repair.16 A microgrooved polydioxanone sheet forms a tubular scaffold in which the injured tendon can be inserted or wrapped. The scaffold provides early mechanical strength, and initiates cell migration and alignment. After 6 weeks, in vivo tests on Sprague–Dawley rats showed regeneration of the defect‐induced tendon.
Cell
Tendon cells are obtained from animal tissue by tissue dissociation or explant technique. After proliferation, they are used at cell culture passage two or three before losing their phenotype.17 Tendon fibroblasts are then seeded in collagen gels or into scaffolds at an appropriate cell‐seeding density (around 106 cells/ml). In the case of autologous cells from patients, the number of cells retrieved from biopsy can be problematic, as extended proliferation is not recommendable for both cell phenotype degradation and patient waiting time. Particular attention should be paid for the expanded cells to express type and levels of protein in a healthy window before injecting them back into the patient.
Two different tendon cells coexist in the tendon, the elongated tenocytes and the ovoid‐shaped tenoblasts, the latter being more involved in intrinsic healing, even though their differences are still not well established.
Several in vivo and in vitro experiments have demonstrated the potential of mesenchymal stem cells (MSCs) for tendon engineering retrieved from the bone marrow or from newer alternative sources (adipose tissue, Wharton's jelly). Under mechanical stimulation, MSCs differentiated in fibroblasts, aligned themselves in collagen gel and helped in vivo tendon regeneration. Typically, a surgically induced defect is filled with a collagen gel seeded with MSCs, with improvement of the natural healing process. However, biomechanical properties were only 33% higher than in control gels.18 Interestingly, these results can be improved by a preliminary organisation of the cell gel in vitro. Similarly, a ligament‐like extracellular matrix (ECM) was observed by histochemical analysis after 14 days in a collagen type I matrix filled with 5×105 MSCs/ml under cyclic stretching.4 The contraction of MSC‐seeded collagen gel is valuable for early mechanical support and cell alignment. Optimisation of cell‐seeding density is then critical.19,20 Although it is clear that this approach leads to a better healing of tendon defects,19,20 ectopic formation of bone has been reported, demonstrating that control of the variables affecting MSC differentiation in vitro is still challenging.
A specific signalling molecule, Smad8, enhances the differentiation of MSCs by inhibiting osteogenic pathways. Genetically modified MSCs, overexpressing Smad8, seeded in a collagen sponge were able to induce tendon repair in Achilles tendon defects after 4 weeks. The capacity to induce the ectopic formation of tendon in other tissues was also demonstrated.21
The distinctiveness of the tendon‐engineering field is the ability of the cells to self‐assemble to form a neotissue with characteristics close to those of a neotendon without any scaffold. Under optimal condition, a confluent cell layer of mature rat tenocytes was allowed to detach itself and to form, between two anchors, a macroscopic cylindrical structure identical to neonatal tendon.22 Its mechanical properties are still far from those of a mature tendon, but the stress–strain response displayed the typical non‐linear properties of soft tissues and was comparable to embryonic tendon strength. Improvement of the technique and the proper combination of chemical and mechanical stimulation should produce an in vitro rat tendon.
Likewise, Fish and Ralphs23 have reported the formation and organisation of the collageneous ECM from a high concentration (∼107) of tenocytes in suspension. The structure was grossly similar to the tendon, but the mechanical and biochemical properties were inferior. These studies are encouraging for the in vitro approach to tendon tissue engineering, and allow to discriminate the intrinsic role of tenocytes in tendon tissue organisation and that of the physical or chemical stimuli.
Stimulation
Chemical stimulation
In vitro cell culture relies on intake of ions and nutrients from the cell medium and serum. However, to induce specific phenotype expression and cell differentiation, various chemicals, such as cytokines, can be added to the culture medium. Most of these growth factors are produced endogenously in vivo, although an artificial increase in their concentration can be at times beneficial. However, most of the studies on the impact of growth factors deal with two‐dimensional cell cultures to limit the impact of other external variables. Fibroblasts could respond differently in a three‐dimensional environment.2 These growth factors play a distinct role when used in combination with MSCs, as they trigger the differentiation in a particular cell lineage. The understanding of their activity is not straightforward, as their effect can be dose‐dependent and they can act in combination with other chemicals.
Ectopic formation of tendon‐like tissue in rat has been observed following injection of growth and differentiation factor 5/6 and 7.24 Consequently, the role of the human homologue of growth and differentiation factor–7 (bone morphogenetic protein (BMP) 12) on human tenocytes has been assessed.25 The addition of 25 and 50 ng/ml BMP 12 induces stimulation of fibroblasts in culture, increases the production of procollagen type I and type III, and decreases decorin expression. This study advocates a predominant role of BMP 12 in the early stage of tendon regeneration. The ability to induce the ectopic formation of tissue is of critical importance in the tissue‐engineering field, as it is the ultimate goal for the in vitro approach. Similar conclusions have been drawn recently for the impact of BMP 13 on cultured human tendon fibroblasts.26
Basic fibroblast growth factor (bFGF) and platelet‐derived growth factor (PDGF) are the major mitogenic agents for fibroblasts, and increase cell proliferation in rat tenocytes.27 Injection of bFGF results in a dose‐dependent increase of cell proliferation and collagen type III production.28 PDGF reversed the effects of glucocorticoid injections, which led to tendon rupture in some points, as dexamethasone reduces significantly, in a dose‐dependent manner, cell tendon viability, cell proliferation and collagen synthesis.29
Low‐dose fibroblast growth factor 2 (3 ng/ml) stimulates both proliferation of bone marrow stromal cells and transcription of typical ECM proteins of tendons and ligaments. After 1 week, proliferation is significantly higher than with high concentration (30 ng/ml) or without growth factor. This first phase is then followed by an increase, relative to the other groups, of the transcription of collagen type I and other key ECM proteins at the second and third weeks of culture.30 Prostaglandin E2 is an inhibitor of cell proliferation and collagen synthesis. Excessive mechanical loading of the tendon is associated with the production of high levels of prostaglandin E2 affecting the matrix organisation and acellularity.31 However, from a tissue engineering point of view, it is likely that the high organisation of the tendon tissue, and the associated matrix turnover, results in the presence of inhibitor/regulator factors.32
Relying on the right cocktail of growth factor and other chemicals will necessitate an indepth understanding of the molecular aspect of the different stages of tendon formation, as they can play different roles in a dose‐ and time‐dependent manner, together with the synergetic combination of their effects. Apart from their use to trigger differentiation and kick‐start proliferation, it seems more realistic to rely on their endogeneous production and the factors that directly affect it.
In vivo, these events could be orchestrated by temporary nerve fibres that sprout at the site of injury. At the early stage of tendon healing, during the inflammation stage, innervation is observed in the tendon proper normally devoid of nerves.33,34 After the regenerative phase of the tissue, the nerves vanish. These studies point out that nerve sprouting and the associated release of neuropeptides could be responsible for the regulation of tissue healing. The production of scaffolds encouraging nerve ingrowth at the injury site should be considered as it will facilitate the integration of the implants and optimise their performance.
Gene therapy
Even if the potency of cytokines to improve tendon healing be well established, their in vivo delivery will still be problematic, as a single injection in situ will not be efficient.35 Recent advances in gene therapy have allowed the manipulation of cells to make them express specific cytokines. Hence, the gene coding for PDGF has been transfected directly by liposomal vector to an injured ligament.36 Enhanced production of the growth factor has been demonstrated up to 4 weeks.
Tissue engineering is more involved with the indirect approach where the cells are retrieved from the patient before being genetically modified via a viral or a non‐viral vector. These enhanced cells can then be used for in vitro tissue formation, or injected back into the patient with or without a scaffold. MSCs modified by BMP 12 gene transfection can be used as an alternative source for tenocytes.37
The main limitations are the same as in chemical stimulation, as the dose and the combinational effects are difficult to separate from each other. Since in vitro reconstructions are, by definition, not subjected to the influence of body fluids, the perspective of gene therapy for tissue engineering relies probably in the expression of naturally circulating growth factors, which cannot be produced naturally in the tendon itself.
Mechanical stimulation
The inherent and fundamental function of tendons has led to speculation about the role of mechanical stimulation in tendon cell proliferation and gene expression. Hence, an increase in cell proliferation, migration and collagen synthesis has been observed in lacerated chicken tendon cultured in vitro undergoing cyclic tension after 14 days.38
First demonstrated for the dermal fibroblasts by Kessler et al,39 collagen fibres and tendon cells can be orientated along the direction of the stress and can upregulate tissue inhibitor matrix metalloproteinase‐1, tissue inhibitor matrix metalloproteinase‐3 and collagen type I. Production of transforming growth factor‐β, bFGF and PDGF by human tendon fibroblast is increased by cyclic strain (5%, 1 Hz, for 15 or 60 min),40 in the same way as cyclic biaxial mechanical strains affect the proliferation of tendon cells in a duration‐dependent way.41 In a serum‐free medium, cyclic mechanical stretching (8%, 0.5 Hz, for 4 h) slightly but significantly increases tendon proliferation on a microgrooved substrate, and significantly increases collagen type‐1 and transforming growth factor‐β1 expression.42 Cyclic stretching (1 Hz) of collagen type I matrix seeded with MSCs for 14 days (8 h/day) resulted in the formation of a tendon‐like matrix. Expression of collagen types I and III, fibronectin and elastin gene was increased compared with the non‐stretched controls, where no ligament‐like matrix was found.4
Duration, frequencies and amplitude of loading seem to affect cellular response in many other tissues. The determination of a physiological window for these parameters is critical for the success of a pertinent mechanical stimulation, as a lack of stress is responsible for a loss of both biochemical and biomechanical properties.43 Conversely, overuse, associated with many sport injuries, has deleterious effects.44
In vivo shear stress induced by water content and fibres sliding inside the tendon is reported.45 Shear stress produced by increased fluid flow increases the expression of matrix metalloproteinase (MMP)‐1 and MMP‐3 by rabbit tendon fibroblasts46 in the absence of any change in the intracellular calcium. This release of matrixin, which initiates collagen degradation, has been linked to in vivo tendon degeneration. As physiological shear stresses occur naturally in the tendon, because sliding of the fascicle is in part responsible for the non‐linear mechanical properties, the upregulation of MMP‐1 and MMP‐3 could probably be related to collagen turnover and healing process. Tendons are highly dynamic tissues, and thus collagen‐degrading enzymes are critical for the ability of the tissue to respond and adapt to different stimuli.32
Interestingly, the type of mechanical stimulation affects cell response. Hence, the compression loading of tendon cells leads to a more fibrocartilaginous tissue,47 underlining the fact that each tissue is specialised and that the structure is dependent on function.
Conclusion
The main aspects of tendon tissue engineering are close to those involved in tissue repair and tissue‐healing mechanism, and they all make use of the most recent insight of molecular biology, genetics and mechanobiology. From a practical point of view, small defects will be treated by in vivo techniques involving the in situ delivery of genetically modified cells on a scaffold providing immediate mechanical support and boosting the regeneration and healing process. Uncontrolled release of high levels of cytokines in the vicinity of the injured tendon could be a major issue, as, although we begin to understand their direct effect, we are far from controlling their cascading consequence at the cellular level in different environments. The success of in vitro tissue engineering is then mainly linked to our understanding of chemical stimulation. The situation will be different in patients with tendinopathy, in whom the tendon lesion could result from non‐healthy cells. Tissue regeneration from autologous cells will not address the underlying problem.
One of the main current challenges for tendon tissue engineering is to export the experimental techniques developed in animal models to the operating theatre, and to apply these methods to repair human tendons in daily practice.
In vitro tissue engineering is more concerned with larger defects or tissue replacement. Its challenges are numerous. It relies mainly on mechanical stimulation, as healthy tendon cells or differentiated MSCs should be able to endogenously produce all the cytokines necessary for tissue generation. For each different tissue, the structure is intimately related to the function, and non‐stimulated cultures are unlikely to produce functional tissue. As with chemical stimulation, there is probably a dose and timing dependency. At different stages of tissue formation, tension and its frequency should be carefully adjusted within physiologically relevant windows. Control of the stimulation and the environment will be achieved through specially designed bioreactors, and techniques for tendon cell culture under mechanical stimulation have been developed.48 However, as these tissues will be grown on their own, they need the presence of some circulating growth factor not produced in situ and which will be able to induce tissue formation.
The drawback of this method is that, if we carefully reproduce a suitable environment, it seems unlikely that this environment will be better than the womb, the human bioreactor, and, consequently, in vitro human tissue engineering will have to face long‐term culture in bioreactors. Consequently, the rare successes of in vitro tissue engineering are produced with rat cells and other species with low gestation time. Hence, the future of tissue engineering for tendon regeneration will not be necessary to do it better than nature, but probably to do it faster.
What is already known on this topic
So far, the main successes of tissue engineering are in skin, bone and bladder repair.
Although some mechanisms are similar, tendon tissue engineering offers peculiar challenges of its own.
Some chemical and mechanical stimuli affecting tendon tissue growth have been identified.
What this study adds
This study reviews the field of tissue engineering, specifically for tendon tissue engineering.
It examines some of the variables to consider to plan a tissue engineering approach either to facilitate in vivo regeneration of damaged tissue or to reconstitute tendon tissue in vitro from autologous cells.
Acknowledgements
This work was supported by BBSRC grants BBS/B/04277 and BBS/B/04242.
Abbreviations
bFGF - basic fibroblast growth factor
BMP - bone morphogenetic protein
ECM - extracellular matrix
MMP - matrix metalloproteinase
MSC - mesenchymal stem cell
PDGF - platelet‐derived growth factor
Footnotes
Competing interests: None declared.
References
- 1.Takakuda K, Miyairi H. Tensile behaviour of fibroblasts cultured in collagen gel. Biomaterials 1996171393–1397. [DOI] [PubMed] [Google Scholar]
- 2.Grinnell F. Fibroblast biology in three‐dimensional collagen matrices. Trends Cell Biol 200313264–269. [DOI] [PubMed] [Google Scholar]
- 3.Lamberti P M, Wezeman F H. Biologic behavior of an in vitro hydrated collagen gel‐human tenocyte tendon model. Clin Orthop Relat Res 2002397414–423. [DOI] [PubMed] [Google Scholar]
- 4.Noth U, Schupp K, Heymer A.et al Anterior cruciate ligament constructs fabricated from human mesenchymal stem cells in a collagen type I hydrogel. Cytotherapy 20057447–455. [DOI] [PubMed] [Google Scholar]
- 5.Young R G, Butler D L, Weber W.et al Use of mesenchymal stem cells in a collagen matrix for Achilles tendon repair. J Orthop Res 199816406–413. [DOI] [PubMed] [Google Scholar]
- 6.Butler D L, Awad H A. Perspectives on cell and collagen composites for tendon repair. Clin Orthop Relat Res 1999367(Suppl)S324–S332. [DOI] [PubMed] [Google Scholar]
- 7.Canty E G, Kadler K E. Collagen fibril biosynthesis in tendon: a review and recent insights. Comp Biochem Physiol A Mol Integr Physiol 2002133979–985. [DOI] [PubMed] [Google Scholar]
- 8.Ide A, Sakane M, Chen G P.et al Collagen hybridization with poly(L‐lactic acid) braid promotes ligament cell migration. Mater Sci Eng C Biomimetic Supramol Syst 20011795–99. [Google Scholar]
- 9.Koob T J, Hernandez D J. Material properties of polymerized NDGA‐collagen composite fibers: development of biologically based tendon constructs. Biomaterials 200223203–212. [DOI] [PubMed] [Google Scholar]
- 10.Funakoshi T, Majima T, Suenaga N.et al Rotator cuff regeneration using chitin fabric as an acellular matrix. J Shoulder Elbow Surg 200615112–118. [DOI] [PubMed] [Google Scholar]
- 11.Funakoshi T, Majima T, Iwasaki N.et al Application of tissue engineering techniques for rotator cuff regeneration using a chitosan‐based hyaluronan hybrid fiber scaffold. Am J Sports Med 2005331193–1201. [DOI] [PubMed] [Google Scholar]
- 12.Cartmell J S, Dunn M G. Development of cell‐seeded patellar tendon allografts for anterior cruciate ligament reconstruction. Tissue Eng 2004101065–1075. [DOI] [PubMed] [Google Scholar]
- 13.Badylak S, Arnoczky S, Plouhar P.et al Naturally occurring extracellular matrix as a scaffold for musculoskeletal repair. Clin Orthop Relat Res 1999367S333–S343. [DOI] [PubMed] [Google Scholar]
- 14.Cao Y L, Liu Y T, Liu W.et al Bridging tendon defects using autologous tenocyte engineered tendon in a hen model. Plast Reconstr Surg 20021101280–1289. [DOI] [PubMed] [Google Scholar]
- 15.Cao D, Liu W, Wei X.et al In vitro tendon engineering with avian tenocytes and polyglycolic acids: a preliminary report. Tissue Eng 2006121369–1377. [DOI] [PubMed] [Google Scholar]
- 16.Curtis A S, Wilkinson C D, Crossan J.et al An in vivo microfabricated scaffold for tendon repair. Eur Cell Mater 2005950–57. [DOI] [PubMed] [Google Scholar]
- 17.Yao L, Bestwick C S, Bestwick L A.et al Phenotypic drift in human tenocyte culture. Tissue Eng 2006121843–1849. [DOI] [PubMed] [Google Scholar]
- 18.Awad H A, Butler D L, Boivin G P.et al Autologous mesenchymal stem cell‐mediated repair of tendon. Tissue Eng 19995267–277. [DOI] [PubMed] [Google Scholar]
- 19.Awad H A, Butler D L, Harris M T.et al In vitro characterization of mesenchymal stem cell‐seeded collagen scaffolds for tendon repair: effects of initial seeding density on contraction kinetics. J Biomed Mater Res 200051233–240. [DOI] [PubMed] [Google Scholar]
- 20.Awad H A, Boivin G P, Dressler M R.et al Repair of patellar tendon injuries using a cell‐collagen composite. J Orthop Res 200321420–431. [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann A, Pelled G, Turgeman G.et al Neotendon formation induced by manipulation of the Smad8 signalling pathway in mesenchymal stem cells. J Clin Invest 2006116940–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calve S, Dennis R G, Kosnik P E.et al Engineering of functional tendon. Tissue Eng 200410755–761. [DOI] [PubMed] [Google Scholar]
- 23.Fish R S, Ralphs J R. Matrix deposition by tendon cells in suspension culture. Eur Cells Mater 2003636–37. [Google Scholar]
- 24.Wolfman N M, Hattersley G, Cox K.et al Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF‐beta gene family. J Clin Invest 1997100321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fu S C, Wong Y P, Chan B P.et al The roles of bone morphogenetic protein (BMP) 12 in stimulating the proliferation and matrix production of human patellar tendon fibroblasts. Life Sci 2003722965–2974. [DOI] [PubMed] [Google Scholar]
- 26.Wong Y P, Fu S C, Cheuk Y C.et al Bone morphogenetic protein 13 stimulates cell proliferation and production of collagen in human patellar tendon fibroblasts. Acta Orthop 200576421–427. [PubMed] [Google Scholar]
- 27.Stein L E. Effects of serum, fibroblast growth factor, and platelet‐derived growth factor on explants of rat tail tendon: a morphological study. Acta Anat (Basel) 1985123247–252. [DOI] [PubMed] [Google Scholar]
- 28.Chan B P, Fu S C, Qin L.et al Effects of basic fibroblast growth factor (bFGF) on early stages of tendon healing ‐ A rat patellar tendon model. Acta Orthop Scand 200071513–518. [DOI] [PubMed] [Google Scholar]
- 29.Wong M W, Tang Y Y, Lee S K.et al Effect of dexamethasone on cultured human tenocytes and its reversibility by platelet‐derived growth factor. J Bone Joint Surg Am 200385‐A1914–1920. [DOI] [PubMed] [Google Scholar]
- 30.Hankemeier S, Keus M, Zeichen J.et al Modulation of proliferation and differentiation of human bone marrow stromal cells by fibroblast growth factor 2: potential implications for tissue engineering of tendons and ligaments. Tissue Eng 20051141–49. [DOI] [PubMed] [Google Scholar]
- 31.Cilli F, Khan M, Fu F.et al Prostaglandin E2 affects proliferation and collagen synthesis by human patellar tendon fibroblasts. Clin J Sport Med 200414232–236. [DOI] [PubMed] [Google Scholar]
- 32.Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev 200484649–698. [DOI] [PubMed] [Google Scholar]
- 33.Ackermann P W, Li J, Lundeberg T.et al Neuronal plasticity in relation to nociception and healing of rat achilles tendon. J Orthop Res 200321432–441. [DOI] [PubMed] [Google Scholar]
- 34.Lian O, Dahl J, Ackermann P W.et al Pronociceptive and antinociceptive neuromediators in patellar tendinopathy. Am J Sports Med 2006341801–1808. [DOI] [PubMed] [Google Scholar]
- 35.Maffulli N, Moller H D, Evans C H. Tendon healing: can it be optimised? Br J Sports Med 200236315–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakamura N, Shino K, Natsuume T.et al Early biological effect of in vivo gene transfer of platelet‐derived growth factor (PDGF)‐B into healing patellar ligament. Gene Ther 199851165–1170. [DOI] [PubMed] [Google Scholar]
- 37.Wang Q W, Chen Z L, Piao Y J. Mesenchymal stem cells differentiate into tenocytes by bone morphogenetic protein (BMP) 12 gene transfer. J Biosci Bioeng 2005100418–422. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka H, Manske P R, Pruitt D L.et al Effect of cyclic tension on lacerated flexor tendons in vitro. J Hand Surg [Am] 199520467–473. [DOI] [PubMed] [Google Scholar]
- 39.Kessler D, Dethlefsen S, Haase I.et al Fibroblasts in mechanically stressed collagen lattices assume a “synthetic” phenotype. J Biol Chem 200127636575–36585. [DOI] [PubMed] [Google Scholar]
- 40.Skutek M, Van G M, Zeichen J.et al Cyclic mechanical stretching modulates secretion pattern of growth factors in human tendon fibroblasts. Eur J Appl Physiol 20018648–52. [DOI] [PubMed] [Google Scholar]
- 41.Zeichen J, Van G M, Bosch U. The proliferative response of isolated human tendon fibroblasts to cyclic biaxial mechanical strain. Am J Sports Med 200028888–892. [DOI] [PubMed] [Google Scholar]
- 42.Yang G, Crawford R C, Wang J H. Proliferation and collagen production of human patellar tendon fibroblasts in response to cyclic uniaxial stretching in serum‐free conditions. J Biomech 2004371543–1550. [DOI] [PubMed] [Google Scholar]
- 43.Berry C C, Cacou C, Lee D A.et al Dermal fibroblasts respond to mechanical conditioning in a strain profile dependent manner. Biorheology 200340337–345. [PubMed] [Google Scholar]
- 44.Renstrom P, Johnson R J. Overuse injuries in sports. A review. Sports Med 19852316–333. [DOI] [PubMed] [Google Scholar]
- 45.Benjamin M, Ralphs J R. Tendons and ligaments‐‐an overview. Histol Histopathol 1997121135–1144. [PubMed] [Google Scholar]
- 46.Archambault J M, Elfervig‐Wall M K, Tsuzaki M.et al Rabbit tendon cells produce MMP‐3 in response to fluid flow without significant calcium transients. J Biomech 200235303–309. [DOI] [PubMed] [Google Scholar]
- 47.Benjamin M, Ralphs J R. The cell and developmental biology of tendons and ligaments. Int Rev Cytol 200019685–130. [DOI] [PubMed] [Google Scholar]
- 48.Garvin J, Qi J, Maloney M.et al Novel system for engineering bioartificial tendons and application of mechanical load. Tissue Eng 20039967–979. [DOI] [PubMed] [Google Scholar]