Abstract
Study objective
To assess prevalence of arsenic exposure through drinking water and skin lesions, and their variation by geographical area, age, sex, and socioeconomic conditions.
Design, setting, and participants
Skin lesion cases were identified by screening the entire population above 4 years of age (n = 166 934) living in Matlab, a rural area in Bangladesh, during January 2002 and August 2003. The process of case identification involved initial skin examinations in the field, followed by verification by physicians in a clinic, and final confirmation by two independent experts reviewing photographs. The tubewell water arsenic concentrations (n = 13 286) were analysed by atomic absorption spectrometry. Drinking water history since 1970 was obtained for each person. Exposure information was constructed using drinking water histories and data on water arsenic concentrations.
Main results
The arsenic concentrations ranged from <1 to 3644 μg/l, and more than 70% of functioning tubewells exceeded the World Health Organisation guideline of 10 μg/l. Arsenic exposure had increased steadily from 1970s to the late 1990s, afterwards a decrease could be noted. In total, 504 skin lesions cases were identified, and the overall crude prevalence was 3/1000. Women had significantly higher cumulative exposure to arsenic, while men had significantly higher prevalence of skin lesions (SMR 158, 95% CI 133 to 188). The highest prevalence occurred in 35–44 age groups for both sexes. Arsenic exposure and skin lesions had a positive association with socioeconomic groups and achieved educational level.
Conclusions
The result showed sex, age, and socioeconomic differentials in both exposure and skin lesions. Findings clearly showed the urgency of effective arsenic mitigation activities.
Keywords: drinking water, ecology, environmental health
Arsenic is a naturally occurring element and the use of drinking water with increased arsenic concentrations is primarily from natural contamination.1,2 A large number of countries have areas with increased arsenic concentrations in ground water causing exposure of millions of people. The arsenic problem in Bangladesh is perhaps the most devastating by its magnitude of exposure and number of people affected.3,4 About half of the country's 6–11 million tubewells yield drinking water containing arsenic above 10 µg/l—the WHO guideline value.5 It is estimated that 57 of the country's 130 million inhabitants are exposed to concentrations exceeding 10 µg/l, and 35 million to concentrations above 50 µg/l, the Bangladesh standard.
Inorganic arsenic is classified as a human carcinogen,1 and the scientific and regulatory focus has been on cancer risks. The earliest symptoms of exposure appear in skin,1,6 which has a strong tendency to accumulate arsenic bound to the sulfhydryl groups in keratin.7,8 The skin effects include pigmentation changes, especially on the trunk and extremities, and thickening of the outer horny layer of skin (keratosis), of the palms and soles. Generally, pigmentation changes appear first, often progressing to palmar‐plantar hyperkeratosis if exposure continues. The pigmentation change typically appears in a finely freckled, “raindrop” pattern of hyperpigmentation and hypopigmentation, but may also be more diffuse darkening of the skin.6,9 Although skin changes have served the purpose to signal arsenic toxicity in various populations, the lesions may often cause severe health and social consequences.10 Reportedly, skin effects appear after 5–10 years of exposure,6 but the knowledge base is still weak on the occurrence of skin lesions in relation to level of exposure, duration, age, sex, and other potential susceptibility factors.
There are several studies from Bangladesh, mainly from small geographical areas, on arsenic exposure and prevalence of skin lesions.11,12,13,14,15,16 However, still a decade after the first report in Bangladesh in 1993,4 reliable estimates of the magnitude of exposure and related health problems are not available.17 Published data show a considerably variation in prevalence of arsenic related skin lesions. Thus, there is a need for a more complete and systematic assessment of exposure and health effects in the population. We report from a large population based survey in Matlab—one of the areas in Bangladesh with the most pronounced arsenic contamination of tubewell water.5 The aims of this paper are to assess the prevalence of arsenic exposure and skin lesions, as well as their variation by geographical area, age, sex, and socioeconomic conditions.
Methods
Study base
This population based survey benefited from the ongoing Matlab Health and Demographic Surveillance System. Matlab is located 53 km south east of Dhaka, where ICDDR,B since 1963 has maintained a Health and Demographic Surveillance System (HDSS) in 142 villages, encompassing a population of 220 000, residing on 18 386 hectares of land. From the population database all inhabitants above 4 years of age were listed for inclusion in the survey. People who were not living and drinking water of a source in Matlab at least once per week were excluded. A total of 180 811 people were eligible and their homes visited during the study period (January 2002–August 2003). In total 13 877 peoples were not found despite repeated attempts. Thus, 166 934 people (92% of eligible) were interviewed and examined for skin lesions (fig 1).
Figure 1 Study profile. Screening for people with arsenic related skin lesions in Matlab, Bangladesh.
Socioeconomic data were excerpted from the HDSS databases. Household asset scores divided into quintiles were used to classify the participants' socioeconomic conditions. Asset scores and achieved level of formal schooling were used as stratifying variables for arsenic exposure and the occurrence of skin lesions.
Skin lesions
Two types of arsenic associated skin lesions were screened for; hyperpigmentation and keratosis. Hyperpigmention was characterised as raindrop‐like spots of pigmentation, diffuse dark brown spots, or diffuse darkening of the skin on the limbs or trunk. Keratosis was characterised as either bilateral thickening of the skin of the palms of hands or the soles of feet, or by nodular keratosis—that is, small protrusions that had emerged on palms and soles or occasionally on the dorsum of hands, feet or limbs.
Field procedures
A stepwise procedure was applied for identifying skin lesions. Firstly, each participant was carefully examined in the field for arsenic associated skin lesions according to a structured protocol after extensive piloting, training, and re‐training of field staff. Twenty four field teams, each consisting of one man and one woman, moved from village to village and house to house examining each person for skin lesions. Suspected cases were referred to a central clinic.
After performing the skin examinations the field staff obtained drinking water histories (types and location of all water sources used since 1970) from all people. We chose 1970 as starting point as the databases did not permit reliable tracking of people's residence before that year. We also asked for the year they started using tubewell water, even if before 1970, as that event was remembered by most people. The information was validated using results from previous household surveys in 1974, 1982, and 1996, which contained information on the source of drinking water. The information on water source that was excerpted from the previous household surveys was printed on the forms used in this study to permit crosschecking during the interview.
Confirmation of skin lesions
At the central clinic arsenic induced skin lesions were confirmed or rejected by the study physicians. Location and appearance of any visible or palpable dermal lesions were recorded and digitally photographed. Two independent dermatologists inspected all photos. Both physicians and dermatologists were blinded for the person's arsenic exposure. If one or both disagreed with the physicians' judgment, the patient was once again physically examined by physician and expert to permit a consensus on the diagnosis. There was some initial disagreement between physicians and dermatologists, mainly related to expert diagnosis of occupational keratosis, hereditary keratosis, familial melanosis, xeroderma pigmentosa, etc. However, consensus was reached in all cases.
Water sampling and exposure assessment
A total of 16 430 tubewells were identified in the Matlab area. Of those, 13 286 tubewells were functional and were screened for arsenic content by use of field kits (Merck, Darmstadt, Germany). This screening was done after the screening for skin lesions to avoid potential bias. In accordance with national standard all tubewells with water arsenic concentration ⩾50 µg/l were painted red and the others were painted green. A second water sample was collected from all tube wells for laboratory analysis. To obtain the water sample the tubewell was pumped 30 strokes and two 20 ml polyethylene vials were filled. The vials contained acid to prevent precipitation of iron and coprecipitation of arsenic. They were marked with the ID number of the tubewell and transported and kept at –20°C in Matlab laboratory until analysis. The concentrations of arsenic were determined in duplicate by hydride generation atomic absorption spectrophotometer (HG‐AAS, Shimadzu Model AA‐6800) at the ICDDR, B laboratory in Dhaka. The limit of detection (LOD) was 1 μg/l. In the calculations, results below LOD were assigned a value of 0.5 µg/l. Analytical performance was controlled by analysis of standard reference material, internal water quality control samples, and interlaboratory comparison (Karolinska Institutet).
Arsenic exposure assessment was based on information on the participants' drinking water history and the arsenic concentration of all water sources used. For tubewell water we used the AAS measurements, while surface water was assigned an arsenic concentration of zero µg/l. According to our experience and the experience of other groups in Bangladesh, we have allocated the value zero to these surface water sources. The drinking water histories were constructed from individual data on water sources for each calendar year from 1970 and onwards.
Several of the previously used tubewells were found to be destroyed (n = 2727). To reconstruct the historical exposure we used the average tubewell arsenic concentration of the village as a proxy for that particular period (a total of 113 946 periods of water use). For people who had migrated into the study area and previously consumed tubewell water the arsenic concentration for those periods (n = 8429 periods) were imputed by use of data from the British Geological Survey of the relevant district.5 The validity of the imputed data was evaluated. For 15 randomly selected functional tubewells, the assessed arsenic concentrations were replaced with imputed values for comparisons with true values. The replacements using village mean values were found to be a good proxy for tubewell arsenic concentration (r2 = 0.57). Use of village median (r2 = 0.31), bari (cluster of related households) mean (r2 = 0.26), or bari median (r2 = 0.10) showed lower correlations with true values.
Data analyses
Descriptive analysis included calculations of central tendency (means/medians) and variation (centiles). Arsenic concentrations in tubewell water are presented as frequency distributions. Cumulative exposures and prevalence of skin lesions are presented by sex, age, and socioeconomic categories. Age adjusted prevalence of skin lesions are given for sex groups as standardised morbidity ratios, using women as reference. The historical variation in arsenic exposure was calculated as mean or median of individual linked water arsenic concentrations for time periods. All analyses were done by use of SPSS software (SPSS 11.5).
Ethics
The main ethical problem of the study was that we identified increased arsenic levels in the drinking water of the study participants. However, a mitigation programme was part of the study and started in collaboration with Bangladesh Rural Advancement Committee (BRAC), Bangladesh.18 High priority was given to households with identified skin lesions and/or pregnant women exposed to arsenic. People with skin lesions and other diseases were given treatment and referral. A series of village information meetings were held before the start of study. All people were informed and asked for consent to participate. An institutional review committee and the ICDDR, B Ethical Review Committee approved the study.
Results
Tubewell water concentration
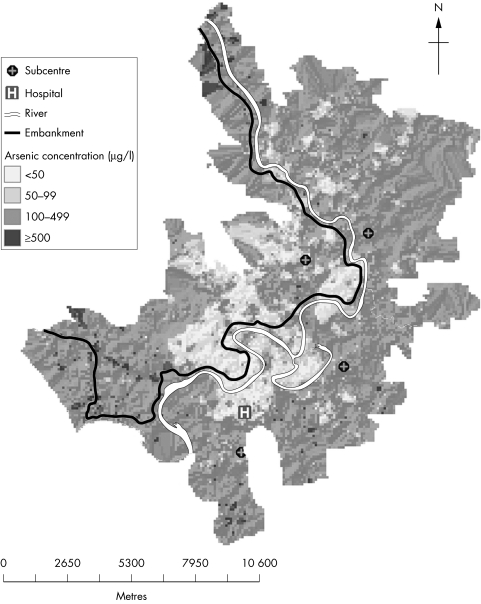
Arsenic concentrations ranged from <1 to 3644 μg/l. More than 70% of the tubewells exceeded 10 µg As/l, and more than 60% exceeded 50 µg/l (table 1). The distribution of arsenic concentrations was skewed, with a mean of 203 µg/l and median of 167 µg/l. There was a bimodal distribution of the water arsenic concentrations. Besides the frequency peak below 1 µg/l, there was a second lower peak around 200–300 µg/l. There was a pronounced geographical variation (fig 2, a colour version of the figure is available on the journal web site http://www.jech.com/supplemental). While some high and low concentration larger areas may be identified, the local variation was considerable.
Table 1 Concentrations (µg/l) of arsenic in all functioning tubewells in Matlab, Bangladesh.
| As concentration (μg/l) | Number | Percentage | Cumulative percentage |
|---|---|---|---|
| <1 | 2235 | 17 | 17 |
| 1–9 | 1559 | 12 | 29 |
| 10–49 | 1099 | 8.3 | 37 |
| 50–149 | 1471 | 11 | 48 |
| 150–299 | 3021 | 23 | 71 |
| 300–499 | 2651 | 20 | 91 |
| 500–999 | 1192 | 9.0 | 99.6 |
| ⩾1000 | 58 | 0.4 | 100 |
| Total | 13286 |
Figure 2 Geographical variation in tubewell arsenic concentrations in Matlab, Bangladesh.
Individual exposure history
People who despite repeated attempts were not available for interview and examination (7.7% of eligible) were especially young (15–34 years) male adults (58% as compared with 46% in study population). They had more often no or primary education and a tendency of lower asset scores than the participants.
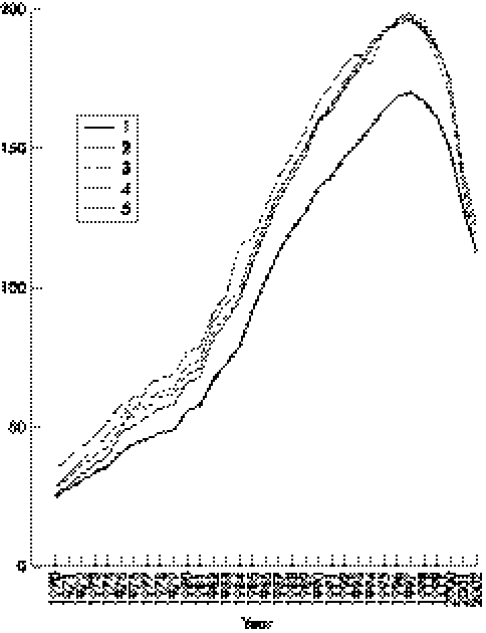
In total 166 934 people, 74 408 men and 92 526 women were screened for skin lesions and interviewed about lifetime water sources. The median age at the time of the study was 28 years for women and 24 years for men. Arsenic exposure increased noticeably from 1970 to 1997, after which it started to decrease (table 2 and fig 3).
Table 2 Variation in arsenic exposure (period means and medians) 1970–2003 in Matlab, Bangladesh.
| Time periods | Number of participants | Arsenic concentration in drinking water (µg/l) | |||||
|---|---|---|---|---|---|---|---|
| Mean | Median | 25th centile | 75th centile | 90th centile | |||
| 1970–74 | 79420 | 34 | 0 | 0 | 0 | 225 | |
| 1975–79 | 91810 | 52 | 0 | 0 | 54 | 279 | |
| 1980–84 | 108150 | 74 | 0 | 0 | 119 | 331 | |
| 1985–89 | 132005 | 117 | 65 | 0 | 188 | 395 | |
| 1990–94 | 155819 | 154 | 118 | 3 | 245 | 462 | |
| 1995–99 | 166921 | 181 | 154 | 25 | 276 | 502 | |
| 2000–03 | 166934 | 141 | 90 | 2 | 229 | 452 | |
Figure 3 Temporal variation in arsenic concentrations (µg/l) in drinking water used by 166 934 people screened for skin lesions in Matlab, Bangladesh. Data are based on interviews about historical water sources and measurements of tubewell water concentrations and stratified for all subjects' household asset score quintiles, where 1 is the poorest and 5 the richest quintile.
Table 3 summarises the cumulative arsenic exposure since 1970 by sex and age as well as the median age at start of tubewell water consumption. In the lower middle aged group (25–34 years), women had higher median age at first tubewell water use than men (p<0.01). Above about 35 years of age, the median cumulative arsenic exposure was significantly higher for women than for men (p<0.01).
Table 3 Distribution of cumulative arsenic exposure by sex and age, and median age at first use of tube well water. Results from Matlab, Bangladesh.
| Age | Number of participants | Median age at first use of tubewell | Cumulative arsenic exposure (µg/l × years) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| <100 | 100–499 | 500–999 | 1000–4999 | 5000–9999 | ⩾10000 | Median | |||
| Women | |||||||||
| 5–14 | 21594 | 0 | 3247 | 2117 | 2356 | 12630 | 1206 | 38 | 1619 |
| 15–24 | 19603 | 0 | 2547 | 1432 | 1582 | 9683 | 4066 | 293 | 2616 |
| 25–34 | 15102 | 14 | 1867 | 1056 | 1293 | 7455 | 2933 | 498 | 2427 |
| 35–44 | 14600 | 19 | 1861 | 922 | 950 | 7026 | 3174 | 667 | 2921 |
| 45–54 | 8778 | 26 | 1130 | 568 | 559 | 3844 | 2131 | 546 | 3116 |
| ⩾55 | 12849 | 41 | 2232 | 762 | 788 | 5295 | 2929 | 843 | 2863 |
| Total | 92526 | 3 | 12884 | 6857 | 7528 | 45933 | 16439 | 2885 | 2335 |
| Men | |||||||||
| 5–14 | 21434 | 0 | 3387 | 2119 | 2305 | 12357 | 1212 | 54 | 1582 |
| 15–24 | 16320 | 0 | 1948 | 1099 | 1138 | 8755 | 3075 | 305 | 2725 |
| 25–34 | 8874 | 6 | 1356 | 750 | 667 | 3808 | 1878 | 415 | 2450 |
| 35–44 | 9767 | 18 | 1625 | 765 | 730 | 4247 | 1926 | 474 | 2306 |
| 45–54 | 7023 | 28 | 1254 | 545 | 538 | 3033 | 1297 | 356 | 2212 |
| ⩾55 | 10990 | 45 | 1793 | 820 | 829 | 4620 | 2315 | 613 | 2503 |
| Total | 74408 | 0 | 11363 | 6098 | 6207 | 36820 | 11703 | 2217 | 2132 |
Exposure to arsenic in drinking water had a socioeconomic gradient with higher exposure in higher socioeconomic groups defined by household asset score (fig 3). This was especially so from 1970s to mid‐1990s, when annual exposure to arsenic through drinking water still was increasing. During the last years of the study period the socioeconomic differential in exposure had decreased considerably. The cumulative arsenic exposure showed a linear trend from lower to higher socioeconomic quintiles (2670, 3103, 3144, 3200, and 3264 µg/l × years, respectively, p<0.01, results adjusted for age). The association between cumulative arsenic exposure and level of education was U shaped; 2760, 3257, 3157, and 2723 µg/l × years for no formal education, primary, secondary, and higher secondary education, respectively (p<0.01, results adjusted for age).
Prevalence of skin lesions
A total of 504 people with arsenic induced skin lesions were identified, corresponding to a prevalence of 3/1000 (table 4). Generally, people had hyperpigmentation only or both hyperpigmentation and keratosis. Of the 504 identified cases 195 (39%) had pigmentation changes only, 26 (5.1%) had keratosis only, and 283 (56%) had both types of lesions. Men had higher prevalence than women (standardised morbidity ratio for men 158, 95% CI 133 to 188, with women as reference). There were about equal number of women with hyperpigmentation or both lesions, while only about one third of men had hyperpigmentation only and two thirds both lesions. The highest prevalence of skin lesions occurred in the age group 35–44 years for both men and women (table 4). There were only two cases below 15 years of age.
Table 4 Distribution of arsenic related skin lesions by sex and age groups. Age adjusted prevalence measure given as standardised morbidity ratio (SMR) with women as reference. Results from Matlab, Bangladesh.
| Age | Population | Hyperpigmentation | Keratosis | Hyperpigmentation and keratosis | Total cases | Prevalence per 1000 | SMR (95% CI) |
|---|---|---|---|---|---|---|---|
| Women | |||||||
| 5–14 | 21594 | 2 | 0 | 0 | 2 | 0.09 | |
| 15–24 | 19603 | 10 | 0 | 17 | 27 | 1.4 | |
| 25–34 | 15102 | 24 | 0 | 25 | 49 | 3.2 | |
| 35–44 | 14600 | 41 | 0 | 48 | 89 | 6.1 | |
| 45–54 | 8778 | 25 | 2 | 16 | 43 | 4.9 | |
| ⩾55 | 12849 | 8 | 3 | 11 | 22 | 1.7 | |
| Total | 92526 | 110 | 5 | 117 | 232 | 2.5 | 100 |
| Men | |||||||
| 5–14 | 21434 | 0 | 0 | 0 | 0 | 0 | |
| 15–24 | 16320 | 11 | 0 | 23 | 34 | 2.1 | |
| 25–34 | 8874 | 17 | 0 | 40 | 57 | 6.4 | |
| 35–44 | 9767 | 29 | 4 | 46 | 79 | 8.1 | |
| 45–54 | 7023 | 16 | 3 | 32 | 51 | 7.3 | |
| ⩾55 | 10990 | 12 | 14 | 25 | 51 | 4.6 | |
| Total | 74408 | 85 | 21 | 166 | 272 | 3.7 | 158 (133 to 188) |
Cases of arsenic related skin lesions were more common in the higher socioeconomic groups; 37% of cases belonged to the highest asset score quintile (20% in population, p<0.01). Similarly, 40% of cases had secondary school education or higher, while this was the case for 17% in the study population (p<0.01).
Discussion
The results of this study clearly show that arsenic in drinking water poses a serious public health problem in Bangladesh. More than 60% of the tubewells in Matlab were found to have arsenic concentrations exceeding 50 μg/l, the Bangladesh drinking water standard, and more than 70% had more than 10 µg/l, the WHO guideline value.19 The arsenic concentrations were highly skewed with 9.4% exceeding 500 g/l. This implies that the water arsenic concentrations in Matlab are among the highest in Bangladesh,5 most probably because the study area is located where the Meghna river joins the confluent streams of the Brahmaputra and Ganges rivers, and the ground is highly affected by the historical sedimentation of arsenic laden soils.
This is the first truly population based study on arsenic exposure and related health effects in Bangladesh. Frequency of non‐participation was low, and is not likely to have influenced the found prevalence of skin lesions, or the associations to age, sex, and socioeconomic factors. We mapped individual arsenic exposure histories for the entire population in Matlab based on arsenic concentrations in water sources used from 1970 to 2003 (fig 3). There had been a steady increase in exposure from the 1970s to the late 1990s, in parallel with the installation of tubewells. Most tubewells were installed during the 1980s and 1990s.4,12,18 In the latter decade, more than 95% of the population used tubewell water.4 The arsenic exposure seems to have decreased after 1997, showing an increasing awareness and a start of shift to drinking water with less arsenic. Higher socioeconomic groups took the lead in shifting to tubewell water sources in the 1970s and 1980s, and seem to have taken the lead in turning to low or arsenic free water during recent years. During the screening of arsenic related skin lesions and arsenic concentrations in drinking water, mitigation activities were started in collaboration with the non‐governmental organisation BRAC.18 Further advice and practical assistance concerning alternative water sources has thereafter been provided by BRAC.
We identified 504 people with arsenic induced skin lesions in a three step screening procedure. The overall prevalence of skin lesions was 0.3%. This is low compared with previous studies, which have shown prevalence values of several per cent.11,14,15,16 The main reason for the discrepancies is most probably that the previous studies involved small, often selected, study populations. Furthermore, this study shows the necessity to have the skin lesions confirmed by experts. Experts confirmed only 30% of the suspected cases identified by field research workers. There are substantial differences in opinions concerning the diagnosis of skin lesion in early or mild cases. Such cases need special attention for confirmation. Thus, an important strength of this study is that it is truly population based with a large study base, good individual level exposure data, and careful case ascertainment.
Drinking water histories were based on recall of water sources used in the past. Estimating duration of use of tubewell water at each residence was complicated because calendar years are not widely recognised in Bangladesh and years may be recalled inaccurately. To minimise such errors, we asked exposure histories in relation to life events, for example, general election and major flooding, to construct the lifetime exposure. Furthermore, we validated the obtained information using individual survey results from 1974, 1982, and 1996.
We assume that the arsenic concentrations in the tubewell water, as determined in 2002–2003, have remained on a similar level since the installation of the tubewells. Information on temporal variation of arsenic concentrations in the ground water is limited. Repeated water analyses in Bangladesh by British Geological Survey found no significant variation over 1.5 years,5 and two other studies from Bangladesh showed no indication of significant temporal variations in water arsenic concentrations during one year,20 or over a three year period.21 Also, in an Andean village in northern Argentina, the water arsenic concentration remained about the same over a period of about 10 years.22,23 Thus, we believe that the estimated historical exposure represent the true (or actual) exposure. We are presently following up the water arsenic concentrations in a subset of the tubewells for assessment of seasonal and temporal variations in Matlab.
The results showed that men started to use tubewell water earlier in life than women. This may be related to the fact that some women moved into Matlab from other areas for marriage. Probably, installation of tubewells started somewhat earlier in Matlab than in surrounding areas. Still, women had in general somewhat higher cumulative exposure than men, except for in young ages. This may be attributable to the more frequent use of surface water by the men working in the field, particularly some years ago when tubewells were less common. Women spend more time at home where the tubewells were first installed. Despite the differences in exposure, the prevalence of skin lesions was higher among men than women, which is consistent with results of other studies,6,14,16,24,25 although none was designed to evaluate sex differences. However, higher susceptibility among women has also been reported.26 We are currently analysing the prevalence of skin lesions using a case‐referent approach, which will enable us evaluating sex specific dose‐response patterns. Sex differences will also be evaluated by urinary arsenic measurements.
In this study, the highest prevalence of skin lesions was found among people 25–54 years of age, with a peak at 35–44 years. On average, those people had been using tubewell water for about 20 years. Both younger and older age groups had lower prevalence values. As children often are particularly susceptible to chemical toxicity,27 the susceptibility to arsenic toxicity will be further evaluated in a case‐referent study. For the risk assessment and risk management it is essential to identify susceptible population groups. Our further studies will also provide information on the role of nutrition and arsenic metabolism in the development of skin effects.
What this paper adds
The global water arsenic contamination has been reported in several articles, but this is the first time the effects are evaluated in a large population by sex, age, and socioeconomic differentials. The result showed sex, age, and socioeconomic differentials in exposure and skin lesions.
Policy implication
Our findings showed differentials in exposure and occurrence of skin lesions. This is the first time the effects are evaluated in a large population by sex, age, and socioeconomic differentials. There is an urgent necessity for reinforced arsenic mitigation activities in Bangladesh. The problem must be tackled in an integrated, comprehensive approach to minimise the risk to the affected population. Prudent public health decisions should not wait.
In summary, findings from this population based study show that a large part of the population in a rural area of Bangladesh has been drinking arsenic contaminated water for many years. The prevalence of arsenic related skin lesions was about 0.3%. The study shows that mitigation activities have started to have an effect on current exposure. However, further interventions are urgently required.
A colour version of figure 2 is available on line (http://www.jech.com/supplemental).
Supplementary Material
Footnotes
Funding: This study was conducted at the ICDDR, B: Centre for Health and Population Research with the support of Swedish International Development Agency (Sida), WHO, and United States of Agency for International Development (USAID). ICDDR, B acknowledges with gratitude the commitment of Sida, WHO, and USAID.
Conflicts of interest: none.
A colour version of figure 2 is available on line (http://www.jech.com/supplemental).
References
- 1.IARC Some drinking‐water disinfectants and contaminants, including arsenic. Lyon, France: International Agency for Research on Cancer, 2004 [PMC free article] [PubMed]
- 2.NRC Arsenic in drinking water: 2001 update. Washington, DC: National Academy Press, 2001 [PubMed]
- 3.WHO Arsenic in drinking water and resulting arsenic toxicity in India and Bangladesh. Recommendations for actions. WHO Regional Office for South‐East Asia 2002
- 4.Chowdhury A M. Arsenic crisis in Bangladesh. Sci Am 200429186–91. [DOI] [PubMed] [Google Scholar]
- 5.BGS Arsenic contamination of groundwater in Bangladesh. In: Kinniburgh DG, Smedley PL, eds. British Geological Survey technical report WC/00/19. Keyworth: British Geological Survey, Natural Environment Research Council, Department for International Development, Government of the People's Republic of Bangladesh, 2001
- 6.Guha Mazumder D N, Haque R, Ghosh N.et al Arsenic levels in drinking water and the prevalence of skin lesions in West Bengal, India. Int J Epidemiol 199827871–877. [DOI] [PubMed] [Google Scholar]
- 7.Yamauchi H, Yamamura Y. Concentration and chemical species of arsenic in human tissue. Bull Environ Contam Toxicol 198331267–270. [DOI] [PubMed] [Google Scholar]
- 8.Lindgren A, Vahter M, Dencker L. Autoradiographic studies on the distribution of arsenic in mice and hamsters administered 74As‐arsenite or ‐arsenate. Acta Pharmacol Toxicol (Copenh) 198251253–265. [DOI] [PubMed] [Google Scholar]
- 9.Guha Mazumder D N. Criteria for case definition of arsenicosis. In: Chappell WR, Calderon RL, Thomas DJ, eds. Arsenic exposure and health effects V. Amsterdam: Elsevier, 2003117–133.
- 10.Hassan M M, Atkins P J, Dunn C E. Social implications of arsenic poisoning in Bangladesh. Soc Sci Med 2005612201–2211. [DOI] [PubMed] [Google Scholar]
- 11.Tondel M, Rahman M, Magnuson A.et al The relationship of arsenic levels in drinking water and the prevalence rate of skin lesions in Bangladesh. Environ Health Perspect 1999107727–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith A H, Lingas E O, Rahman M. Contamination of drinking‐water by arsenic in Bangladesh: a public health emergency. Bull World Health Organ 2000781093–1103. [PMC free article] [PubMed] [Google Scholar]
- 13.Mitra A K, Bose B K, Kabir H.et al Arsenic‐related health problems among hospital patients in southern Bangladesh. J Health Popul Nutr 200220198–204. [PubMed] [Google Scholar]
- 14.Kadono T, Inaoka T, Murayama N.et al Skin manifestations of arsenicosis in two villages in Bangladesh. Int J Dermatol 200241841–846. [DOI] [PubMed] [Google Scholar]
- 15.Ahsan H, Perrin M, Rahman A.et al Associations between drinking water and urinary arsenic levels and skin lesions in Bangladesh. J Occup Environ Med 2000421195–1201. [DOI] [PubMed] [Google Scholar]
- 16.Hadi A, Parveen R. Arsenicosis in Bangladesh: prevalence and socio‐economic correlates. Public Health 2004118559–564. [DOI] [PubMed] [Google Scholar]
- 17.Caussy D. Normative role of WHO in mitigating health impacts of chronic arsenic exposure in the South‐east Asia region. In: Chappell WR, Calderon RL, Thomas DJ, eds. Arsenic exposure and health effects V. Amsterdam: Elsevier, 2003439–447.
- 18.Jakariya M, Rahman M, Chowdhury A M R.et al Sustainable safe water options in Bangladesh. Experiences in Bangladesh. In: Bundschuh J, Bhattacharya P, Chandrashekharam D, eds. Natural arsenic in groundwater: occurrence, remediation and management. London: A A Balkema Publishers, 2005319–330.
- 19.WHO Guidelines for drinking‐water quality. 2nd ed. Geneva: World Health Organisation, 1993
- 20.Van Geen A, Ahsan H, Horneman A H.et al Promotion of well‐switching to mitigate the current arsenic crisis in Bangladesh. Bull World Health Organ 200280732–737. [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng Z, Van Geen A, Seddique A A.et al Limited temporal variability of arsenic concentrations in 20 wells monitored for 3 years in Araihazar, Bangladesh. Environ Sci Technol 2005394759–4766. [DOI] [PubMed] [Google Scholar]
- 22.Concha G. Metabolism of inorganic arsenic and biomarkers of exposure. (PhD Thesis). Stockholm: Karolinska Institutet, 2001
- 23.Concha G N B, Nermell B, Vahter M. Spatial and temporal variation in arsenic exposure via drinking water in northern Argentina. J Health Popul Nutr. (in press) [PMC free article] [PubMed]
- 24.Smith A H, Arroyo A P, Mazumder D N.et al Arsenic‐induced skin lesions among Atacameno people in Northern Chile despite good nutrition and centuries of exposure. Environ Health Perspect 2000108617–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watanabe C, Inaoka T, Kadono T.et al Males in rural Bangladeshi communities are more susceptible to chronic arsenic poisoning than females: analyses based on urinary arsenic. Environ Health Perspect 20011091265–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmad S A, Sayed M H, Faruquee M H.et al Arsenicosis: sex differentials. J Prev Soc Med 19991835–40. [PubMed] [Google Scholar]
- 27.WHO Children's health and the environment: a review of evidence. Copenhagen: World Health Organisation, Regional Office for Europe, 2002
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.