Abstract
Background and purpose:
Previously we demonstrated that the spinal sigma-1 receptor (Sig-1 R) plays an important role in pain transmission, although the exact mechanism is still unclear. It has been suggested that Sig-1 R agonists increase glutamate-induced calcium influx through N-methyl-D-aspartate (NMDA) receptors. Despite data suggesting a link between Sig-1 Rs and NMDA receptors, there are no studies addressing whether Sig-1 R activation directly affects NMDA receptor sensitivity.
Experimental approach:
We studied the effect of intrathecal (i.t.) administration of Sig-1 R agonists on protein kinase C (PKC) and protein kinase A (PKA) dependent phosphorylation of the NMDA receptor subunit NR1 (pNR1) as a marker of NMDA receptor sensitization. In addition, we examined whether this Sig-1 R mediated phosphorylation of NR1 plays an important role in sensory function using a model of NMDA-induced pain.
Key results:
Both Western blot assays and image analysis of pNR1 immunohistochemical staining in the spinal cord indicated that i.t. injection of the Sig-1 R agonists, PRE-084 or carbetapentane dose dependently enhanced pNR1 expression in the murine dorsal horn. This increased pNR1 expression was significantly reduced by pretreatment with the specific Sig-1 R antagonist, BD-1047. In another set of experiments Sig-1 R agonists further potentiated NMDA-induced pain behaviour and pNR1 immunoreactivity and this was also reversed with BD-1047.
Conclusions and implications:
The results of this study suggest that the activation of spinal Sig-1 R enhances NMDA-induced pain via PKC- and PKA-dependent phosphorylation of the NMDA receptor NR 1 subunit.
Keywords: sigma-1 receptor, NMDA receptor, NR1 subunit, phosphorylation, pain, mice
Introduction
Recently it has been shown using sigma-1 receptor (Sig-1 R)-knockout mice that Sig-1 R plays an important role in formalin-induced pain (Cendan et al., 2005b). Further support for a pro-nociceptive role for Sig-1 R comes from a study demonstrating that systemic administration of the putative Sig-1 R antagonist, haloperidol, markedly reduces nociceptive behaviours in both phases of the formalin test (Cendan et al., 2005a). Sig-1 R is found in high concentrations in both the midbrain and the dorsal horn of the spinal cord, which serve as intermediary areas for nociceptive signal processing (Alonso et al., 2000; Phan et al., 2005). A recent study from our laboratories further revealed that intrathecal (i.t.) pretreatment with selective Sig-1 R antagonists, N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(dimethylamino) ethylamine dihydrobromide (BD-1047) and BMY-14802, reduces nociceptive behaviours and spinal cord Fos expression induced by hind-paw formalin injection (Kim et al., 2006b). On the basis of these findings, it seems likely that Sig-1 Rs in the spinal cord play a role in formalin-induced nociception. While evidence is accumulating to support a role for these receptors in formalin-induced pain, from a mechanistic standpoint it remains unclear how activation of Sig-1 Rs in the spinal cord facilitates pain transmission.
In rat hippocampal neurons, Sig-1 R agonists have been shown to potentiate glutamate-induced intracellular Ca2+ influx through the N-methyl-D-aspartate (NMDA) ionotropic glutamate receptor (Monnet et al., 2003). Since phosphorylation of the NMDA receptor increases synaptic strength, this event may act as a critical factor in the process of central sensitization. The NMDA receptor is composed of NMDA-receptor subunit 1 (NR1), NR2 and NR3 subunits; (Hollmann and Heinemann, 1994; Alexander et al., 2008) and phosphorylation of the NR1 subunit (pNR1) at protein kinase C (PKC) and protein kinase A (PKA)-dependent sites has been demonstrated to play a key role in enhancement of NMDA receptor activity related to pain transmission in the spinal cord (South et al., 2003; Ultenius et al., 2006). In support of this hypothesis, it has been shown that intradermal injection of capsaicin dramatically increases pNR1 in the spinal dorsal horn and produces central sensitization (Zou et al., 2000). Similarly, phosphorylation of the NR1 subunit is correlated with the presence of behavioural signs of neuropathy and also with persistent pain that follows nerve injury (Ultenius et al., 2006). On the basis of these findings, we postulated that the NMDA-receptor NR1 subunit was one of the major phosphorylation targets of the Sig-1 R in the spinal cord dorsal horn and further hypothesized that this phosphorylation mechanism might underlie the Sig-1 R-mediated effects on nociception.
To investigate the potential role of Sig-1 Rs in nociceptive processing in the present study, we evaluated (1) whether i.t. treatment with the Sig-1 R agonists, PRE-084 or carbetapentane, produced an increase in PKC- and PKA-dependent pNR1 in the spinal dorsal horn, using western blot analysis of pNR-1 and image analysis of pNR-1 immunohistochemical staining, and (2) whether activation of spinal Sig-1 Rs was also capable of producing spontaneous pain behaviour or enhancing i.t. NMDA-induced pain behaviours via PKC- and PKA-dependent phosphorylation of the NR1 subunit.
Methods
Animals
All animal procedures and the experimental protocols for animal usage were reviewed and approved by the SNU Animal Care and Use Committee and conform to NIH guidelines (NIH publication no. 86–23, revised 1985). This study was carried out in accordance with the ethical guidelines for investigations of experimental pain in conscious animals (Zimmermann, 1983).
Male ICR mice (24–30 g body weight) were purchased from the Laboratory Animal Center of Seoul National University (SNU) and were maintained under the following conditions: 12 h light/dark cycle, room temperature (20–25 °C) and 40–60% humidity. Food and water were available ad libitum.
Intrathecal injection
Drugs were dissolved in 5 μl of vehicle and an i.t. injection was given using a 50-μl Hamilton syringe with a 30-gauge needle based on the method of Hylden and Wilcox (1980). Flick of the tail was considered indicative of a successful i.t. administration.
Western blot analysis for pNR1 (phosphorylated at Ser896 or Ser897) in the dorsal horn of the spinal cord
Mice were deeply anaesthetized by intraperitoneal injection of chloral hydrate (400 mg kg−1) at one time point before and at several time points (10, 30, 60 and 120 min) after i.t. injection of PRE-084(3 nmol) or carbetapentane (10 nmol) to determine the time-dependent effect of Sig-1 R activation on spinal pNR1 (Ser896 and Ser897) using immunoblot analysis. In a separate set of experiments, we also measured the dose-dependent effect of PRE-084(0.3, 1 and 3 nmol) or carbetapentane (1, 3 and 10 nmol) administration at the 30 min time point post injection. In addition, a separate group of mice were pretreated with the Sig-1 R antagonist, BD-1047 (100 nmol), 10 min prior to i.t. injection of PRE-084(3 nmol) or carbetapentane (10 nmol) in order to determine whether PRE-084 or carbetapentane-induced phosphorylation of NR1 is specifically mediated by the Sig-1 R (n=5 in each group, total n=80).
After i.t. injection of PRE-084or CAR, mice were immediately placed in the Plexiglass box that was filled with 5% isoflurane and oxygen. Then the spinal cord was extracted by pressure expulsion with air into an ice-cooled, saline-filled glass dish and snap-frozen in liquid nitrogen. To verify the location of the L4−6 spinal cord segments for western blotting, we identified the attachment site of each spinal nerve in anaesthetized mice. In addition, spinal segments were separated into left and right halves under a neuro-surgical microscope. The spinal cord was subsequently further subdivided into dorsal and ventral halves by cutting straight across from the central canal laterally to a midpoint in the white matter. The right and left spinal cord dorsal horns were then used for western blot analysis. This method allowed us to analyse the changes in Sig-1 R agonist-induced pNR1 selectively in the dorsal horn of the spinal cord.
The L4−6 spinal dorsal segments were homogenized in buffer containing 1 M Tris (pH 7.5), 1% NP-40, 0.5 M EDTA (pH 7.5), 50 mM EGTA, 1 M dithiothreitol, 1 M benzidine and 0.1 M phenyl methyl sulphonyl fluoride. The total amount of protein in each sample was determined using Bradford dye assay prior to loading on polyacrylamide gels. Spinal cord homogenates (20 μg protein) were separated by electrophoresis using 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes. After blots had been washed with TBST (10 mM Tris-HCl (pH 7.6), 150 mM NaCl, 0.05% Tween-20), the membranes were blocked with 5% skim milk for 1 h and incubated with the appropriate primary antibody for β-actin (loading control; Sigma, St Louis, MO, USA) or protein kinase C (PKC)-dependent pNR1 (1:500, cat no. 06–640; Upstate Biotechnology, Lake Placid, NY, USA; this antibody is specific for NR1 phosphorylated on Ser896) or protein kinase A (PKA)-dependent pNR1 (1:500, cat no. 06–641; Upstate Biotechnology; this antibody is specific for NR1 phosphorylated on Ser897). The membranes were washed and primary antibodies were detected using goat anti-rabbit IgG conjugated to horseradish peroxidase. The bands were visualized with enhanced chemiluminescence (Amersham Pharmacia Biotech, England, UK).
Immunohistochemistry for pNR1 (phosphorylated at Ser896 or Ser897) in spinal dorsal horn
The immunohistochemical procedures used in the present study have been described in detail previously (Lee and Beitz, 1993; Kim et al., 2006b). Thirty minutes after i.t. injection of Sig-1 R agonists, mice were deeply anaesthetized with 5% isoflurane and perfused transcardially with calcium-free Tyrode′s solution, followed by treatment with a fixative containing 4% paraformaldehyde and 0.2% picric acid in 0.1 M phosphate buffer (pH 6.9). The spinal cord (L4−6) was removed immediately after perfusion, post-fixed in the same fixative for 4 h and then cryoprotected in 30% sucrose in phosphate-buffered saline (pH 7.4).
A series of frozen sections (40 μm thickness) were cut through the L4−6 segments of the lumbar spinal cord using a cryostat (Microm, Walldorf, Germany). After elimination of endogenous peroxidase activity with 3% hydrogen peroxide in phosphate-buffered saline and preblocking with 3% normal goat serum and 0.3% Triton X-100 in phosphate-buffered saline, the sections were incubated in pNR1 antisera (Ser896 or Ser897, 1:1000; Upstate Biotechnology) overnight. The sections were rinsed in phosphate-buffered saline and processed with the avidin–biotin–peroxidase technique as previously described (Lee and Beitz, 1993). Finally, visualization was performed using 3,3′-diaminobenzidine (Sigma) and the 3,3′-diaminobenzidine reaction was intensified with 0.2% nickel chloride. The sections were mounted on gelatin-subbed slides and slides were dried, dehydrated in ethanol (70–100% gradually), cleared in xylene and coverslipped.
Image analysis
Tissue sections were first examined by dark-field microscopy (Zeiss Axioscope, Hallbergmoos, Germany) to determine the segmental level, according to the procedure described by Abbadie and Besson (1994), and to identify specific grey-matter landmarks in order to define individual spinal cord laminae. For quantitative analysis, sections were scanned and the five sections with the greatest number of labelled cells at the L4−6 level were selected from each animal. Individual sections were digitized with 4096 grey levels using a cooled CCD camera (Micromax Kodak 1317; Princeton Instruments, Trenton, NJ, USA) connected to a computer-assisted image analysis system (Metamorph version 6.3r2; Molecular Devices Corporation, Downingtown, PA, USA). To maintain a constant threshold for each image and to compensate for subtle variability of the immunostaining, we only counted immunoreactive pNR1s that were at least 50% darker than the average grey level of each image after background subtraction and shading correction were performed in the three areas of dorsal horn that were carefully analysed. These three areas included the superficial dorsal horn (laminae I–II), the nucleus proprius (laminae III–IV) and the neck region of the dorsal horn (laminae V–VI). Microscope illumination and data acquisition settings were fixed throughout the entire analysis procedure. The average number of immunoreactive pNR1 per section from each animal was obtained and these values from at least five animals in each group were averaged and presented as group data. All analytical procedures described above were performed without the knowledge of the experimental conditions.
Spontaneous pain behaviour mediated by Sig-1 R ligands
After acclimation, mice were intrathecally injected with NMDA (0.4 nmol, positive control) or Sig-1 R agonists (PRE-084, 3 nmol or carbetapentane, 10 nmol) or Sig-1 R antagonist (BD-1047, 100 nmol). Following injection, animals were immediately placed in an observation chamber and nociceptive behaviours (licking, scratching and biting directed toward the hind limb, gluteal region and base of the tail) were recorded for 60 min after injection. Time spent by pain behaviour was measured and analysed in 5-min time blocks.
NMDA-induced pain behaviour and pNR1 expression in the dorsal horn
Each mouse was acclimated to an acrylic observation chamber for at least 30 min before this experiment. NMDA (0.4 nmol in 5 μl sterile saline) was injected intrathecally in order to produce spinally mediated NMDA-induced pain behaviours (Yajima et al., 2000). Following injection, animals were immediately placed in an observation chamber and nociceptive behaviours were recorded for a 10-min period. Cumulative response time (s) of these behaviours was measured. To evaluate a possible role of the Sig-1 R in NMDA-induced nociception and in NMDA-induced NR1 phosphorylation, PRE-084(3 nmol) or carbetapentane (10 nmol) was intrathecally injected 30 min prior to NMDA injection. One group of animals was pretreated with BD-1047 (100 nmol) 10 min prior to i.t. injection of Sig-1 R agonists. At the termination of the NMDA test (40 min after i.t. injection of Sig-1 R agonists), mice were immediately killed and perfused transcardially prior to pNR1 immunohistochemistry.
Statistical analysis
All experimental results are shown as the mean±s.e.mean. The levels of statistical significance were determined by an unpaired Student's t-test for comparisons between two means, and by analysis of variance followed by a Newman–Keuls test for multiple comparisons. P-values of less than 0.05 were considered statistically significant. For western blot analysis, positive pixel area of specific bands were subsequently measured with a computer-assisted image analysis system and normalized against corresponding loading control bands. Differences were compared using repeated-measure one-way analysis of variance followed by post hoc Newman–Keuls tests.
Reagents
All Sig-1 R ligands, including PRE-084 (agonist), carbetapentane (agonist) and BD-1047 (antagonist), were purchased from Tocris (Avonmouth, UK), while NMDA was purchased from Sigma . All drugs were freshly prepared just prior to each experiment by dissolving them in physiological saline solution.
Results
Effect of i.t. Sig-1 R agonists on PKC-dependent pNR1 (Ser896) expression in spinal dorsal horn
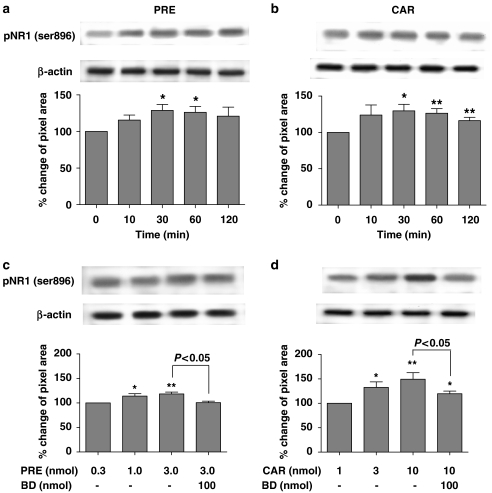
The expression of PKC-dependent pNR1 protein in the dorsal half of the spinal cord was found to increase significantly as early as 30 min after i.t. injection of PRE-084(3 nmol; Figure 1a) or carbetapentane (10 nmol; Figure 1b), and peaked at 60 min post injection (*P<0.05 and **P<0.01 as compared with those of non-treated mice). In addition, i.t. PRE-084(0.3, 1 and 3 nmol; Figure 1c) or carbetapentane (1, 3 and 10 nmol; Figure 1d) dose dependently enhanced the protein expression of spinal cord pNR1 (Ser896) at the 30-min post-injection time point (*P<0.05 and **P<0.01 effect of middle and high doses of Sig-1 R agonists, respectively, as compared with the lowest dose of Sig-1 R agonists). On the other hand, i.t. PRE-084- or carbetapentane-induced pNR1 protein expression was significantly reduced by i.t. pretreatment with the selective Sig-1 R antagonist, BD-1047, at a dose of 100 nmol (Figures 1c and d, P<0.05).
Figure 1.
Western blots and graphs illustrating the effect of i.t. treatment with the Sig-1 R agonists, PRE-084 (PRE, a and c) or carbetapentane (CAR, b and d) on PKC-dependent pNR1 (approximately 122 kDa) in the spinal dorsal horn. Expression of pNR1 (phosphorylated at Ser896) in the spinal cord dorsal horn was found to increase as early as 30 min after i.t. injection of either PRE-084(3 nmol, a) or carbetapentane (10 nmol, b), and peaked between 30–60 min post injection. *P<0.05 and **P<0.01 compared with those of non-treated mice; indicated by the ‘0' time point in panels (a) and (b). Protein expression of pNR1 before treatment with Sig-1 R agonists was calculated as 100%. I.t. injection of PRE-084 (0.3, 1 and 3 nmol, c) or carbetapentane (1, 3 and 10 nmol, d) dose dependently increased pNR1 (Ser896) subunit in the spinal cord dorsal horn. I.t. pretreatment with 100 nmol BD-1047 (BD, a selective Sig-1 R antagonist) significantly reduced the increase in pNR1 (Ser896) expression induced by i.t. injection of PRE-084 or CAR. Protein expression of pNR1 in response to the lowest dose of Sig-1 R agonists was calculated as 100%. *P<0.05 and **P<0.01 as compared with pNR1 (Ser896) expression in response to the lowest dose of PRE-084 (0.3 nmol) or carbetapentane (3 nmol). All immunoblots were normalized for analysis using β-actin (as a loading control) in each group (see text). The number of mice was five in each group. I.t., intrathecal; PKC, protein kinase C; pNR1, phosphorylation of the NMDA receptor subunit NR1; Sig-1 R, sigma-1 receptor.
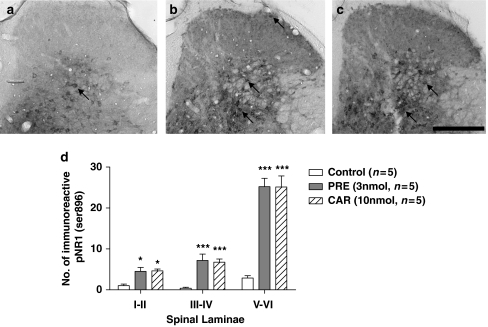
Thirty minutes after an i.t. injection of saline, a relatively small number of immunoreactive pNR1s (Ser896) were observed in the spinal cord dorsal horn of control mice, as depicted in Figure 2a (mean number of immunoreactive pNR1s=1.0±0.4 in laminae I–II; 0.4±0.3 in laminae III–IV; 2.9±0.6 in laminae V–VI). In contrast, 30 min after an i.t. injection of PRE-084(3 nmol) or carbetapentane (10 nmol), there was a significant increase in the number of immunoreactive pNR1 in the dorsal horn (*P<0.05 for laminae I & II and ***P<0.001 for laminae III–VI as compared with those of control mice; see Figures 2b–d).
Figure 2.
Representative photomicrographs (a–c) and graph (d) illustrating the anatomical distribution pattern of PKC-dependent pNR1 (Ser896) in the spinal cord dorsal horn induced by i.t. injection of saline (control, a) PRE-084 (PRE, 3 nmol, b) or carbetapentane (CAR, 10 nmol, c). (a) Only small numbers of immunoreactive pNR1 were observed in the dorsal horn of saline-treated control mice. In contrast, i.t. injection of PRE-084 (b) or carbetapentane (c) significantly increased pNR1 expression (arrows) in the dorsal horn. Arrows indicate immunoreactive PKC-dependent pNR1 (Ser896). (d) The number of immunoreactive pNR1 in the laminae I–II, III–IV and V–VI was significantly increased 30 min after injection of the Sig-1 R agonists. *P<0.05 and ***P<0.001 as compared with those of the control group. Scale bar, 200 μm. I.t., intrathecal; PKC, protein kinase C; pNR1, phosphorylation of the NMDA receptor subunit NR1; Sig-1 R, sigma-1 receptor.
Effect of i.t. Sig-1 R agonists on PKA-dependent pNR1 (Ser897) expression in spinal dorsal horn
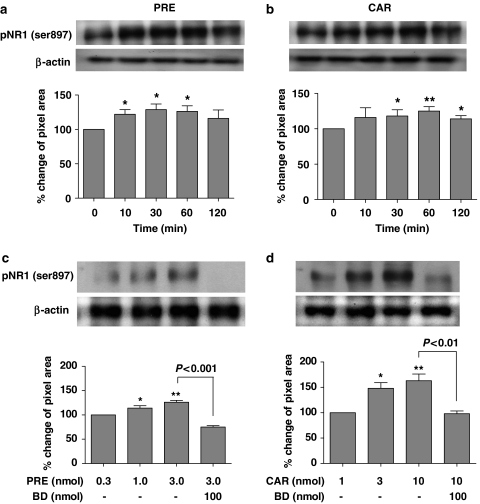
Spinal delivery of the Sig-1 R agonists PRE-084 (3 nmol) or carbetapentane (10 nmol) significantly increased the expression of PKA-dependent pNR1 protein in the dorsal half of the spinal cord as shown in Figures 3a and b. The amount of Sig-1 R-mediated pNR1 (Ser897) peaked at 30–60 min post injection (*P<0.05 and **P<0.01 as compared with those of non-treated mice). In addition, i.t. PRE-084 (0.3, 1 and 3 nmol; Figure 3c) or carbetapentane (1, 3 and 10 nmol; Figure 3d) dose dependently enhanced the protein expression of spinal cord pNR1 (Ser897) at the 30 min post-injection time point (*P<0.05 and **P<0.01 effect of middle and high doses of Sig-1 R agonists, respectively, as compared with the lowest dose of Sig-1 R agonists). These Sig-1 R-mediated effects were confirmed by demonstrating that the i.t. PRE- or CAR-induced pNR1 protein expression was significantly reduced by i.t. pretreatment with the selective Sig-1 R antagonist, BD-1047 at a dose of 100 nmol (Figures 3c and d, P<0.001 and P<0.01).
Figure 3.
Western blots and graphs illustrating the effect of i.t. treatment with Sig-1 R agonists, PRE-084 (PRE, a and c) or carbetapentane (CAR, b and d) on PKA-dependent pNR1 (122 kDa) in the spinal dorsal horn. Expression of pNR1 (Ser897) in the spinal cord dorsal horn was found to increase as early as 10 min after i.t. injection of either PRE-084(3 nmol, a) or carbetapentane (10 nmol, b), and peaked between 30–60 min post-injection. *P<0.05 and **P<0.01 as compared with those of non-treated mice; indicated by the ‘0' time point in panels (a) and (b). Protein expression of pNR1 before treatment with Sig-1 R agonists was calculated as 100%. I.t. injection of PRE-084 (0.3, 1 and 3 nmol, c) or carbetapentane (1, 3 and 10 nmol, d) dose dependently increased the expression of the pNR1 (Ser897) subunit in the spinal cord dorsal horn. I.t. pretreatment with 100 nmol BD-1047 (BD, a selective Sig-1 R antagonist) significantly reduced the increase in pNR1 (Ser897) expression induced by i.t. injection of PRE-084or CAR. Protein expression of pNR1 in response to the lowest dose of Sig-1 R agonists was calculated as 100%. *P<0.05 and **P<0.01 as compared with pNR1 (Ser897) expression in response to the lowest dose of PRE-084(0.3 nmol) or carbetapentane (3 nmol). All immunoblots were normalized for analysis using β-actin (as a loading control) in each group (see text). The number of mice was five in each group. I.t., intrathecal; PKA, protein kinase A; pNR1, phosphorylation of the NMDA receptor subunit NR1; Sig-1 R, sigma-1 receptor.
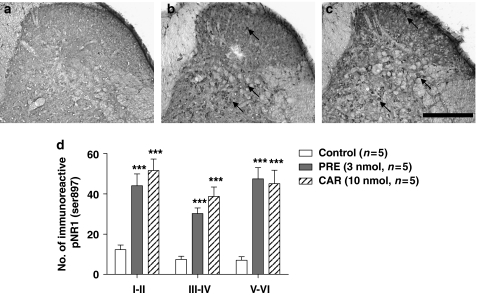
Thirty minutes after an i.t. injection of saline, a relatively small number of immunoreactive pNR1s (Ser897) were observed in the dorsal horn of control mice, as illustrated in Figure 4a (number of immunoreactive pNR1s=12.3±2.3 (mean±s.e.mean) in laminae I–II; 7.4±1.6 in laminae III–IV; 7.1±1.7 in laminae V–VI). In contrast, 30 min after an i.t. injection of PRE-084 (3 nmol) or carbetapentane (10 nmol), there was a significant increase in the number of immunoreactive pNR1 in the spinal dorsal horn (***P<0.001 for all laminae as compared with those of control mice; Figures 4b–d).
Figure 4.
Representative photomicrographs (a–c) and graph (d) illustrating the anatomical distribution pattern of PKA-dependent pNR1 (Ser897) in the spinal cord dorsal horn induced by i.t. injection of saline (control, a) PRE-084 (PRE, 3 nmol, b) or carbetapentane (CAR, 10 nmol, c). (a) Only a small number of immunoreactive pNR1 were observed in the dorsal horn of saline-treated control mice. In contrast, i.t. injection of PRE-084 (b) or carbetapentane (c) significantly increased pNR1 expression (arrows) in the dorsal horn. Arrows represent PKA-dependent pNR1 (Ser897). (d) The number of immunoreactive pNR1 in the laminae I–II, III–IV and V–VI was significantly increased at 30 min after injection of Sig-1 R agonists. ***P<0.001 as compared with those of the control group. Scale bar, 200 μm. I.t., intrathecal; PKA, protein kinase A; pNR1, phosphorylation of the NMDA receptor subunit NR1; Sig-1 R, sigma-1 receptor.
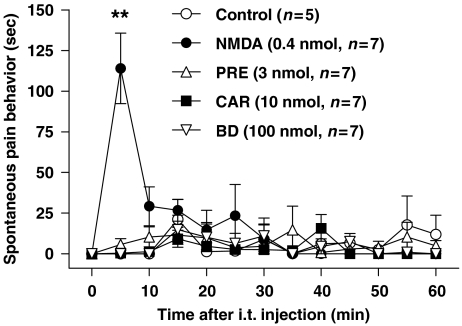
Effect of i.t. Sig-1 R ligands administration on spontaneous pain behaviour
Intrathecal treatment with Sig-1 R agonists (PRE-084 or carbetapentane) or the antagonist (BD-1047) did not produce any noticeable pain behaviour for 60 min after injection (Figure 5). In the positive control group, i.t. NMDA (0.4 nmol) administration elicited a prominent spontaneous pain behaviour (**P<0.01 as compared with that of the saline-treated vehicle control group) and this pain sensation was reduced to normal level within 10 min after injection.
Figure 5.
Effect of i.t. treatment with NMDA or Sig-1 R ligands on spontaneous pain behavior. Injection of the Sig-1 R agonists PRE-084 (PRE) or carbetapentane (CAR) or the Sig-1 R antagonist, BD-1047 did not produce any noticeable pain behavior during the 60 min observation period following injection. On the other hand, i.t. injection of NMDA (0.4 nmol) elicited a prominent pain behavior. **P<0.01 as compared with that of the control group. I.t., intrathecal; NMDA, N-methyl-D-aspartate; Sig-1 R, sigma-1 receptor.
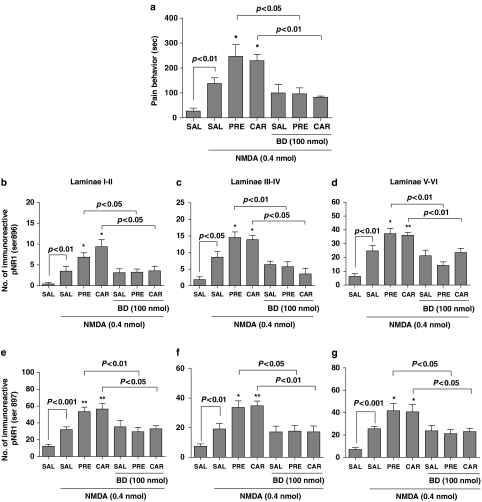
Effect of i.t. Sig-1 R agonists on NMDA-induced pain behaviour and spinal pNR1 expression
Intrathecal administration of NMDA (0.4 nmol) elicited significant pain-related behaviours that included licking, scratching and biting that were directed toward the hind limb, gluteal region and base of the tail, and lasted for the entire 10 min observation period as illustrated in Figure 6a. I.t. PRE-084 (3 nmol) or carbetapentane (10 nmol) was found to significantly potentiate this i.t. NMDA-induced nociceptive behaviour (*P<0.05 as compared with that of saline–NMDA-treated mice). This enhanced nociceptive behaviour induced by PRE-084or carbetapentane was completely abolished by i.t. pretreatment with the selective Sig-1 R antagonist, BD-1047 (100 nmol).
Figure 6.
Effect of i.t. treatment with PRE-084 (PRE) or carbetapentane (CAR) on NMDA-induced pain-associated behaviors and NMDA-induced PKC and PKA-dependent spinal pNR1. I.t. treatment with NMDA (0.4 nmol) elicited significant nociceptive behaviors, which included caudally directed licking, scratching and biting (a), as well as a significant increase in the numbers of immunoreactive phosphorylated NR1 subunits in laminae I–II (b and e), III–IV (c and f) and V–VI (d and g) of the dorsal horn. (b–d) Sig-1 R agonist-induced PKC-dependent pNR1 (Ser896). (e–g) Sig-1 R agonist-induced PKA-dependent pNR1 (Ser897), respectively. I.t. pretreatment with PRE-084(3 nmol) or carbetapentane (10 nmol) potently enhanced i.t. NMDA-induced pain behaviors and pNR1 expression, and this enhancement was blocked by BD-1047 (BD, a selective Sig-1 R antagonist, 100 nmol) administration. Mice in the control group received i.t. saline treatment (SAL). *P<0.05 and **P<0.01 as compared with those of SAL-NMDA-treated mice. The number of mice was seven in each group. I.t., intrathecal; PKA, protein kinase A; PKC, protein kinase C; pNR1, phosphorylation of the NMDA receptor subunit NR1.
In addition to eliciting profound nociceptive behaviours, i.t. NMDA markedly increased the number of PKC-dependent pNR1 in laminae I–II (3.5±1.2), laminae III–IV (8.6±1.7) and laminae V–VI (24.8±3.9), and PKA-dependent pNR1 in laminae I–II (32.0±3.4), laminae III–IV (19.1±3.7) and laminae V–VI (25.6±2.1) when compared to saline-injected mice as shown in Figures 6b–g. I.t. administration of PRE-084 (3 nmol) or carbetapentane (10 nmol) was found to significantly increase the number of NMDA-induced PKC and PKA-dependent pNR1 in laminae I–VI (*P<0.05 and **P<0.01 as compared with those of saline-NMDA treated mice). This Sig-1 R agonist-induced increase in the number of pNR1 was significantly reduced by i.t. pretreatment with the Sig-1 R antagonist, BD-1047 (100 nmol, P<0.05 and P<0.01). The graphs illustrated in Figures 6b–d represent the number of Sig-1 R-mediated PKC-dependent pNR1 (Ser896), while those in Figures 6e–f represent the number of Sig-1 R-mediated PKA-dependent pNR1 (Ser897).
Discussion
The present data show that i.t. administration of the Sig-1 R agonists, PRE-084 or CAR, results in significant upregulation of dorsal horn pNR1 (at both the PKC-dependent Ser896 and the PKA-dependent Ser897 sites) in a dose-dependent manner. However, the lowest doses of Sig-1 R agonists (0.3 nmol for PRE-084 and 1 nmol for CAR) did not significantly alter the pNR1 immunoblots as compared with that of the saline-treated control group (data not shown). This increased pNR1 was significantly reduced by pretreatment with the highly selective Sig-1 R antagonist, BD-1047. This finding indicates that Sig-1 R activation in the spinal cord leads directly or indirectly to phosphorylation of the NR1 subunit and suggests that Sig-1 R agonists may alter NMDA functional characteristics in the lumbar spinal cord. As shown in Figures 3c and d, the effect of carbetapentane on pNR1 ser897 expression in the western blots seemed to be greater than that produced by PRE-084. One interpretation of this result is that carbetapentane has a higher intrinsic efficacy than PRE-084 as an agonist of the Sig-1 R, since a previous report suggested that the affinities of several Sig-1 R agonists can be altered by pharmacological approaches such as phenytoin treatment (Cobos et al., 2005). Furthermore, the mean value of pNR1 at Ser896 also seemed to be different between the PRE-084 and carbetapentane treated groups, as illustrated in Figures 1c and d. However, these differences were not statistically significant. Because pharmacological profiles of PRE-084 and carbetapentane have not been fully investigated yet, it is not entirely clear whether the different effects of these Sig-1 R ligands on pNR1 are due to differences in intrinsic efficacies of PRE-084 and carbetapentane in this study.
Since peak level of spinal cord pNR1 protein expression was found to occur at 30 min post Sig-1 R agonist administration, we examined the anatomical distribution pattern of pNR1 immunostaining in the spinal dorsal horn at the same time point. I.t. injection of PRE-084 or carbetapentane significantly increased the number of immunoreactive pNR1 in laminae I–VI of the dorsal horn, which is an important area for pain processing (Wu et al., 2000). In this regard, our immunohistochemical data further show that the Sig-1 R agonist-induced upregulation of dorsal horn pNR1 expression is greater in laminae V–VI than in laminae I–IV. This is perhaps not surprising, since there are almost a sevenfold greater number of pNR1-positive neurons in laminae V–VI of naive mice as compared to laminae I–II (Brenner et al., 2004). On the other hand, these authors have also shown that noxious heat stimulation causes significant induction of pNR1 in laminae I–II, as well as in laminae V–VI. As neurons located within superficial laminae I and II are critical for mediating nociceptive information induced by peripherally applied noxious stimuli, our results together with those of Brenner et al. (2004) raise the possibility that the Sig-1 R-induced upregulation of pNR1 expression in these superficial laminae plays an important role in nociception.
N-methyl-D-aspartate receptors are composed of three related families of subunits, NR1, NR2 and NR3 (Mori and Mishina, 1995). NR1 and NR2 subunits are known to be essential for forming a functional receptor (Monyer et al., 1994). The NR3 subunit co-assembles with NR1 and NR2 subunits to form a receptor complex with distinct channel properties, but recent evidence indicates that the NR3 subunits are unlikely to be involved in the formation of channel-blocking sites in NR1/NR2/NR3 channels (Yamakura et al., 2005). The NR1 subunit is localized throughout the dorsal horn of the spinal cord, including the laminae that play an important role in pain transmission (Nagy et al., 2004). On the other hand, the NR2 isoforms are less abundant in the dorsal horn (Yukhananov et al., 2002), with NR2A concentrated in laminae III–IV and NR2B in laminae I–II (Nagy et al., 2004). The NR1 subunit undergoes a PKC-mediated phosphorylation at Ser896, as well as a cyclic AMP-dependent protein kinase (PKA)-mediated phosphorylation at Ser897 (Gao et al., 2005). As both PRE-084 and carbetapentane significantly increased the number of PKC- and PKA-dependent pNR1s in the dorsal horn, this suggests that one of the mechanisms by which Sig-1 ligands affect spinally mediated nociception is via phosphorylation of the NR1 subunit.
Of interest is the observation that i.t. injection of Sig-1 R agonists alone (PRE or CAR) did not produce any noticeable pain behaviour for 60 min after injection, although pNR1, both at Ser896 and Ser897, was upregulated by Sig-1 R agonist treatments. This result suggested that Sig-1 R may have a modulatory role on NMDA receptors, and thus activation of spinal Sig-1 R alone did not produce prominent behavioural effect. NMDA receptors in the dorsal horn have been implicated in central sensitization, a process in which responsiveness of central nociceptive neurons is amplified in response to peripheral stimuli following peripheral injury. In a separate set of experiments, we also evaluated whether central Sig-1 R agonist administration facilitates NMDA-induced pain. I.t. NMDA administration has been used previously to study spine-associated pain mechanisms and central injection elicits spontaneous nociceptive behaviors that include licking, biting and scratching (Menendez et al., 1997). Here we demonstrate that in addition to producing pain behaviours, i.t. NMDA also significantly increases the number of PKC- and PKA-dependent pNR1s in the spinal cord dorsal horn (laminae I–VI). Interestingly, i.t. Sig-1 R agonists significantly increased the number of NMDA-induced spinal pNR1 expression as well as NMDA-induced nociceptive behaviors. Sig-1 R specificity of these stimulatory effects was verified, since they were significantly reduced by i.t. pretreatment with the selective Sig-1 R antagonist, BD-1047. Collectively, these data suggest that spinal Sig-1 Rs play a crucial role in modulating NMDA-induced pain.
Significant decreases in formalin-induced pain behaviors in both the first and second phases of the formalin test have been previously reported in Sig-1 R-knockout mice and following systemic administration of the putative Sig-1 R antagonist, haloperidol, in normal mice (Cendan et al., 2005a, 2005b). Unfortunately these approaches alone are unable to determine the mechanism of action by which Sig-1 Rs alter pain behaviours. On the basis of the present findings, we hypothesize that the spinal Sig-1 Rs may modify nociceptive behaviour by ultimately causing phosphorylation of spinal NMDA receptors at both PKC- and PKA-dependent sites. It is not clear at this point in time whether exertion of spinal Sig-1 R effects on NMDA phosphorylation is direct or indirect. In this regard, Martina et al. (2007) demonstrated that potentiation of NMDA-receptor currents following activation of the Sig-1 R by (+)pentazocine in hippocampal neurons is due to prevention of small conductance Ca2+-activated K+ (SK) channel activation. Ca2+ entering the cells through the NMDA receptor activates a Ca2+-activated K+ current, carried by SK channels, which in turn inhibits NMDA-receptor responses. Consequently, preventing SK channel opening by Sig-1 R activation would increase NMDA-receptor response and subsequent long-term potentiation. Whether a similar mechanism occurs in nociceptive spinal neurons remains to be determined.
Previously, we reported that i.t. injection of Sig-1 R antagonists suppressed formalin-induced pain behaviors and spinal cord pNR1 expression, indicating that the spinal Sig-1 R was involved in the formalin-induced pain sensation (Kim et al., 2006b). However, although this work reinforces the concept that the Sig-1 R is involved in formalin-induced pain behaviors, it does not shed any light on the neuronal mechanisms underlying the Sig-1 R antagonist-induced antinociceptive effect. Therefore, we designed the present study to determine the possible effect of Sig-1 R activation on spinal pNR1 expression. Generally, increased spinal pNR1 expression can be evoked by noxious stimulation, including inflammatory and/or neuropathic pain (Zou et al., 2000, 2004; Gao et al., 2005, 2007; Kim et al., 2006a). However, we found in the present study that activation of spinal Sig-1 receptors elevated spinal cord pNR1 expression, without inducing significant pain behaviour. Moreover, activation of spinal Sig-1 receptors also enhanced i.t. NMDA-induced pain behaviour and spinal pNR1 expression. On the basis of these results, we believe that elevated pNR1 expression may be a critical and necessary component that underlies the ability of spinal Sig-1 receptors to mediate pain.
In conclusion, we observed in the present study that i.t. injection of the Sig-1 R agonists, PRE-084 (3 nmol) or carbetapentane (10 nmol), significantly increased phosphorylation of the NMDA NR1 subunit both at PKC- and PKA-dependent sites. In addition, activation of spinal Sig-1 Rs enhanced nociceptive responses and pNR1 elicited by i.t. NMDA injection. These results suggest that spinal Sig-1 Rs may play an important pro-nociceptive modulatory role in spinally mediated pain sensation via PKC- and PKA-dependent phosphorylation of the NR1 subunit.
Acknowledgments
This work was supported by a grant (R01-2005-000-10580-0) from the Basic Research Program of the Korea Science & Engineering Foundation. In addition, it was also supported by a grant (M103KV010016-07K2201-01610) from Brain Research Center of the 21st Century Frontier Research Program funded by the Ministry of Science and Technology, the Republic of Korea.
Abbreviations
- BD-1047
N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(dimethylamino) ethylamine dihydrobromide
- NMDA
N-methyl-D-aspartate
- PKA
protein kinase A
- PKC
protein kinase C
- pNR1
phosphorylation of NMDA receptor subunit 1
- Sig-1 R
sigma-1 receptor
Conflict of interest
The authors state no conflict of interest.
References
- Abbadie C, Besson JM. Chronic treatments with aspirin or acetaminophen reduce both the development of polyarthritis and Fos-like immunoreactivity in rat lumbar spinal cord. Pain. 1994;57:45–54. doi: 10.1016/0304-3959(94)90106-6. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC) Br J Pharmacol. 2008;153 Suppl 2:S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso G, Phan V, Guillemain I, Saunier M, Legrand A, Anoal M, et al. Immunocytochemical localization of the sigma(1) receptor in the adult rat central nervous system. Neuroscience. 2000;97:155–170. doi: 10.1016/s0306-4522(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Brenner GJ, Ji RR, Shaffer S, Woolf CJ. Peripheral noxious stimulation induces phosphorylation of the NMDA receptor NR1 subunit at the PKC-dependent site, serine-896, in spinal cord dorsal horn neurons. Eur J Neurosci. 2004;20:375–384. doi: 10.1111/j.1460-9568.2004.03506.x. [DOI] [PubMed] [Google Scholar]
- Cendan CM, Pujalte JM, Portillo-Salido E, Montoliu L, Baeyens JM. Formalin-induced pain is reduced in sigma(1) receptor knockout mice. Eur J Pharmacol. 2005b;511:73–74. doi: 10.1016/j.ejphar.2005.01.036. [DOI] [PubMed] [Google Scholar]
- Cendan CM, Pujalte JM, Portillo-Salido E, Baeyens JM. Antinociceptive effects of haloperidol and its metabolites in the formalin test in mice. Psychopharmacology (Berlin) 2005a;182:485–493. doi: 10.1007/s00213-005-0127-z. [DOI] [PubMed] [Google Scholar]
- Cobos EJ, Baeyens JM, Del Pozo E. Phenytoin differentially modulates the affinity of agonist and antagonist ligands for sigma 1 receptors of guinea pig brain. Synapse. 2005;55:192–195. doi: 10.1002/syn.20103. [DOI] [PubMed] [Google Scholar]
- Gao X, Kim HK, Chung JM, Chung K. Enhancement of NMDA receptor phosphorylation of the spinal dorsal horn and nucleus gracilis neurons in neuropathic rats. Pain. 2005;116:62–72. doi: 10.1016/j.pain.2005.03.045. [DOI] [PubMed] [Google Scholar]
- Gao X, Kim HK, Chung JM, Chung K. Reactive oxygen species (ROS) are involved in enhancement of NMDA-receptor phosphorylation in animal models of pain. Pain. 2007;131:262–271. doi: 10.1016/j.pain.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- Kim HK, Kim JH, Gao X, Zhou JL, Lee I, Chung K, et al. Analgesic effect of vitamin E is mediated by reducing central sensitization in neuropathic pain. Pain. 2006a;122:53–62. doi: 10.1016/j.pain.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Kim HW, Kwon YB, Roh DH, Yoon SY, Han HJ, Kim KW, et al. Intrathecal treatment with sigma1 receptor antagonists reduces formalin-induced phosphorylation of NMDA receptor subunit 1 and the second phase of formalin test in mice. Br J Pharmacol. 2006b;148:490–498. doi: 10.1038/sj.bjp.0706764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Beitz AJ. The distribution of brain-stem and spinal cord nuclei associated with different frequencies of electroacupuncture analgesia. Pain. 1993;52:11–28. doi: 10.1016/0304-3959(93)90109-3. [DOI] [PubMed] [Google Scholar]
- Martina M, Turcotte ME, Halman S, Bergeron R. The sigma-1 receptor modulates NMDA receptor synaptic transmission and plasticity via SK channels in rat hippocampus. J Physiol. 2007;578:143–157. doi: 10.1113/jphysiol.2006.116178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez L, Hidalgo A, Baamonde A. Spinal calmodulin inhibitors reduce N-methyl-D-aspartate- and septide-induced nociceptive behavior. Eur J Pharmacol. 1997;335:9–14. doi: 10.1016/s0014-2999(97)01158-8. [DOI] [PubMed] [Google Scholar]
- Monnet FP, Morin-Surun MP, Leger J, Combettes L. Protein kinase C-dependent potentiation of intracellular calcium influx by sigma1 receptor agonists in rat hippocampal neurons. J Pharmacol Exp Ther. 2003;307:705–712. doi: 10.1124/jpet.103.053447. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Mori H, Mishina M. Structure and function of the NMDA receptor channel. Neuropharmacology. 1995;34:1219–1237. doi: 10.1016/0028-3908(95)00109-j. [DOI] [PubMed] [Google Scholar]
- Nagy GG, Watanabe M, Fukaya M, Todd AJ. Synaptic distribution of the NR1, NR2A and NR2B subunits of the N-methyl-d-aspartate receptor in the rat lumbar spinal cord revealed with an antigen-unmasking technique. Eur J Neurosci. 2004;20:3301–3312. doi: 10.1111/j.1460-9568.2004.03798.x. [DOI] [PubMed] [Google Scholar]
- Phan VL, Miyamoto Y, Nabeshima T, Maurice T. Age-related expression of sigma1 receptors and antidepressant efficacy of a selective agonist in the senescence-accelerated (SAM) mouse. J Neurosci Res. 2005;79:561–572. doi: 10.1002/jnr.20390. [DOI] [PubMed] [Google Scholar]
- South SM, Kohno T, Kaspar BK, Hegarty D, Vissel B, Drake CT, et al. A conditional deletion of the NR1 subunit of the NMDA receptor in adult spinal cord dorsal horn reduces NMDA currents and injury-induced pain. J Neurosci. 2003;23:5031–5040. doi: 10.1523/JNEUROSCI.23-12-05031.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ultenius C, Linderoth B, Meyerson BA, Wallin J. Spinal NMDA receptor phosphorylation correlates with the presence of neuropathic signs following peripheral nerve injury in the rat. Neurosci Lett. 2006;399:85–90. doi: 10.1016/j.neulet.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Wu J, Fang L, Lin Q, Willis WD. Fos expression is induced by increased nitric oxide release in rat spinal cord dorsal horn. Neuroscience. 2000;96:351–357. doi: 10.1016/s0306-4522(99)00534-5. [DOI] [PubMed] [Google Scholar]
- Yajima Y, Narita M, Tsuda M, Imai S, Kamei J, Nagase H, et al. Modulation of NMDA- and (+)TAN-67-induced nociception by GABA(B) receptors in the mouse spinal cord. Life Sci. 2000;68:719–725. doi: 10.1016/s0024-3205(00)00975-9. [DOI] [PubMed] [Google Scholar]
- Yamakura T, Askalany AR, Petrenko AB, Kohno T, Baba H, Sakimura K. The NR3B subunit does not alter the anesthetic sensitivities of recombinant N-methyl-D-aspartate receptors. Anesth Analg. 2005;100:1687–1692. doi: 10.1213/01.ANE.0000152324.30272.49. [DOI] [PubMed] [Google Scholar]
- Yukhananov R, Guan J, Crosby G. Antisense oligonucleotides to N-methyl-D-aspartate receptor subunits attenuate formalin-induced nociception in the rat. Brain Res. 2002;930:163–169. doi: 10.1016/s0006-8993(02)02243-6. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Zou X, Lin Q, Willis WD. Enhanced phosphorylation of NMDA receptor 1 subunits in spinal cord dorsal horn and spinothalamic tract neurons after intradermal injection of capsaicin in rats. J Neurosci. 2000;20:6989–6997. doi: 10.1523/JNEUROSCI.20-18-06989.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X, Lin Q, Willis WD. Effect of protein kinase C blockade on phosphorylation of NR1 in dorsal horn and spinothalamic tract cells caused by intradermal capsaicin injection in rats. Brain Res. 2004;1020:95–105. doi: 10.1016/j.brainres.2004.06.017. [DOI] [PubMed] [Google Scholar]