Abstract
Using parental FVB mice and their neu transgenic counterparts, FVBN202, we showed for the first time that dangerous hyperplasia of mammary epithelial cells coincided with breaking immunological tolerance to the neu “self” tumor antigen, though such immune responses failed to prevent formation of spontaneous neu-overexpressing mammary carcinoma (MMC) or reject transplanted MMC in FVBN202 mice. On the other hand, neu-specific immune responses appeared to be effective against MMC in parental FVB mice because of the fact that rat neu protein was seen as “nonself” antigen in these animals and the protein was dangerously overexpressed in MMC. Interestingly, low/intermediate expression of the neu “nonself” protein in tumors induced immune responses but such immune responses failed to reject the tumor in FVB mice. Our results showed that self–nonself (SNS) entity of a tumor antigen or danger signal alone, while may equally induce an antigen-specific immune response, will not warrant the efficacy of immune responses against tumors. On the other hand, entity of antigen in the context of dangerous conditions, i.e. abnormal/dangerous overexpression of the neu nonself protein, will warrant effective anti-tumor immune responses in FVB mice. This unified “danger-SNS” model suggests focusing on identification of naturally processed cryptic or mutated epitopes, which are considered semi-nonself by the host immune system, along with novel dangerous adjuvant in vaccine design.
Keywords: HER-2/neu, Breast cancer, Danger signal
Introduction
The marvelous work of Frank MacFarlane Burnett in the 1940s and 1950s introduced immunology as the “science of self-nonself (SNS) discrimination” [2–4]. According to this model, the immune system responds to foreign antigens and tolerates self antigens. The SNS model began to encounter problems when immunologists recognized the importance of antigen presenting cells (APC) in the induction of adaptive immune responses, even against self proteins. In 1989, Charles Janeway improved the SNS model by discovering pattern-recognition receptors on APC, which specifically recognize foreign antigens [11, 12]. In response to Janeway’s idea, Polly Matzinger laid out the idea that the immune system responds to “danger signals” and does not care about the “self” or “nonself” entity of antigens [7, 22, 23]. Subsequently, the danger model was supported by the discovery of endogenous, self products, including mammalian DNA [10], RNA, heat shock proteins (HSP), inflammation and IL-1 beta, and breakdown products of hyaluron following vessel damage [13, 22, 28, 30]. HSP, as self proteins, were then shown to bind toll like receptors (TLR) and induce the maturation of APC [13, 35]. These findings further challenged Janeway’s model by demonstrating that TLR were not specific for recognizing “nonself” proteins. Pro-inflammatory cytokines have also been shown to enhance T cell proliferation in vivo and in vitro [5] and overcome age-related deficiency of naive CD4+ T cells in producing IL-2 [9]. In naïve T cells, the NF-κB family member c-Rel is associated with an inhibitory molecule IκBβ. Exposure of naïve T cells to pro-inflammatory cytokines shifts the c-Rel from IκBβ to IκBα-associated complexes and results in the activation of T cells [1]. These reports consider pro-inflammatory cytokines as signal III (danger signal) for the induction of immune responses.
In order to determine whether the danger or SNS model can explain the mechanisms of immune-mediated tumor rejection or tolerance, we used parental FVB mice and their neu-transgenic counterpart, FVBN202 mice. These mice differ only in the expression of rat neu protein. While rat neu oncogenic product is “nonself” protein in parental FVB, it is seen as “self” protein in FVBN202 mouse. We determined that abnormal/dangerous increase in the number of mammary epithelial cells along with overexpression of neu coincided with breaking tolerance against the neu “self” protein but such immune responses fail to protect the mice against the tumor. While dangerous overexpression of the neu “nonself” protein did warrant tumor rejection, low/intermediate expression of the neu “nonself” protein induced neu-specific immune responses that failed to reject mammary tumors. Our results suggest that a unified “danger-SNS” model could better explain the induction as well as the efficacy of immune responses against breast tumors.
Materials and methods
Mice
Parental FVB (Jackson Laboratories) and female FVBN202 transgenic mice (Charles River Laboratories) were used throughout these studies. FVBN202 over-express a non-activated rat neu transgene under the regulation of the mouse mammary tumor virus (MMTV) promoter [8]. Four mice per group were used throughout the experiments unless stated otherwise. The studies have been reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at Virginia Commonwealth University.
Tumor cell lines
MMC was established from the spontaneous mammary tumor of the FVBN202 transgenic mice and ANV was established from a relapsed neu negative variant of MMC in parental FVB mice, as previously described [16, 20]. ANVpos was established from residual neu positive clones of ANV [14].
HSP110-neu vaccination
Recombinant proteins, HSP110, intracellular domain (ICD) and extracellular domain (ECD) of neu protein, are routinely produced in our laboratory using E. coli. The HSP110-neu complex was prepared as described by our group [21] and FVBN202 mice (n = 4) were vaccinated s.c. with the vaccine complex (50 μg/mouse) followed by two boosters 3 weeks apart.
ELISA
Protein ELISA was performed as previously described by our group [21]. Briefly, plates were coated with the extracellular domain (ECD) of rat neu recombinant protein (1 μg/well), sub-domain II (aa187–332) or sub-domain IV (aa503–642) of ECD (0.3 μg/well) followed by blocking with 2% skim milk and adding fivefold serial dilutions of the sera (100 μl/well) after washing the plates to remove unbound ECD. After 2 h incubation at 4°C, plates were washed and added with HRP-conjugated IgG1 Ab (1:2,000; Caltag, Burlingame, CA). The plates were then washed and the reactions were developed by adding 100 μl/well of the TMB Microwell peroxidase substrate (Kierkegaard & Perry, Gaithersburg, MD), stopping the reaction with 2 M H2SO4 and reading at 450 nm. Mean Ab titers were then calculated.
In vivo tumor challenge studies
Animals were inoculated s.c. with tumors (5 × 106 cells/mouse). Animals were inspected twice weekly for the development of tumors. Masses were measured with calipers along the two perpendicular diameters. Tumor volume was calculated by: V (volume) = [L (length) × W 2 (width)]/2. Mice were killed before tumor volume exceeded 2,000 mm3.
IFN-γELISA
Splenocytes were cultured in the presence of IL-2 (40 U/ml) for 5 days in order to enrich for effector T cells. Purity of CD3+ T cells was >94% after a 5-day culture. Splenocytes were cultured with complete medium (RPMI1640 supplemented with 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin) in the presence or absence of irradiated tumors (15,000 rad, in a E:T ratio of 10:1). Supernatants were collected after 20 h and IFN-γ was detected using a Mouse IFN-γELISA Set (BD Pharmingen, San Diego, CA) according to manufacture’s protocol. Results were reported as the mean values of duplicate wells.
Flow cytometry
Three-color flow cytometric analysis was performed as previously. All staining procedures were conducted on ice, and washing steps were performed with a PBS containing 1% FBS and 0.1% sodium azide to avoid internalization/recycling of the receptors. Anti-mouse CD16/CD32 Ab (0.5 μg/200 μl/2–5 × 105 cells; BD Pharmingen) was used prior to the specific Ab staining in order to block the cell surface Fc receptors. After washing, FITC-conjugated anti-mouse CD62L, PE-conjugated anti-mouse CD44, PE/Cy5-conjugated anti-mouse CD4 and CD8, and isotype control Ig were used at concentrations recommended by the manufacturer (Biolegend, San Diego, CA). Cells were finally washed and red blood cells were lyzed using FACS Lysing Solution (BD Bioscience, San Diego, CA). After washing, cells were fixed with 1% ultra pure formaldehyde and assayed on a Beckman Coulter Epics XL and data were analyzed using Expo 32 software. For detection of apoptosis, cells were added with anti-neu Ab (Ab-4, Calbiochem, San Diego, CA), washed and then added with PE-conjugated anti-mouse IgG (Biolegend). Cells were then stained using Annexin V-FITC Apoptosis Detection Kit (BD Pharmingen) and read within 1 h.
Statistical analysis
Results were analyzed using Student’s t test. A value of P < 0.05 was considered significant.
Results
Tolerance to the neu “self” protein was broken in the presence of dangerous hyperplasia of mammary epithelial cells
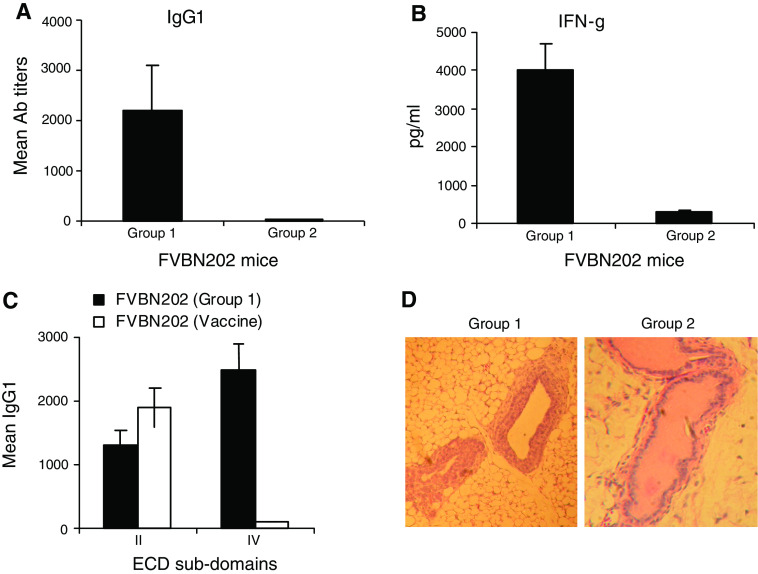
FVBN202 mice overexpress the rat neu protein in their mammary glands and develop hyperplasia in mammary epithelial cells prior to the formation of spontaneous mammary carcinoma [8]. According to the SNS model of immunity, these mice tolerate the rat neu “self” protein. However, it was reported that tolerance to the neu “self” protein was broken in these animals prior to the formation of spontaneous mammary tumors [34]. Since these mice develop hyperplasia of mammary glands prior to the formation of mammary tumors [8], we hypothesized that appearance of neu-specific humoral and cell-mediated immune responses coincided with dangerous increase in the number of mammary epithelial cells, hyperplasia. We examined FVBN202 mice at different ages (8–34 weeks of age) and age-matched animals were evaluated for the presence or absence of the neu-specific immune responses. Subsequently, animals were killed and their mammary glands were subjected to hematoxylin and eosin (H & E) staining for the detection of hyperplastic epithelial cells. We detected the neu-specific IgG1 Ab and IFN-γ producing T cell responses in a fraction of animals (group 1) prior to the formation of spontaneous mammary tumors (Fig. 1a, b). No immune responses were detected against neu-negative ANV. The immune response in group 1 was qualitatively different from those induced by vaccination so that sera from group 1 recognized both sub-domains of ECD while sera from the HSP110-neu vaccinated mice recognized sub-domain II of ECD only (Fig. 1c). In vitro T cell stimulation studies showed that the amounts of IFN-γ production by T cells of FVBN202 mice and FVB mice were comparable while their T cells were stimulated with different concentrations of the neu antigen (data not shown). However, it is not known whether these T cells may recognize different neu epitopes. Interestingly, hyperplastic mammary glands were abundant only in group 1 manifesting immune responses against the neu antigen (Fig. 1d). No marked hyperplastic epithelial cells were detected in mammary glands of FVBN202 mice carrying spontaneous mammary tumors, perhaps, because of developing pre-malignant hyperplastic cells into tumors (data not shown). It was previously reported that involution of mouse mammary glands was associated with the induction of immune responses [32]. Microarray analysis of mammary glands revealed the expression of acute-phase response genes in the mammary gland, particularly activation of genes involved in innate immune response. However, the mechanisms by which dangerous hyperplasia of mammary glands could induce immune responses against neu are poorly understood and require further investigation.
Fig. 1.
Immunological tolerance to the neu “self” protein is broken in the presence of dangerous increase in the number of epithelial cells. a FVBN202 mice (8–34 wk of age) were monitored for the presence or absence of anti-neu IgG1 Ab responses in their sera using ELISA. Animals were divided into two groups. Group 1 (n = 6) had pre-existing IgG1 Ab responses against the neu self protein and group 2 (n = 10) had no detectable neu-specific Ab responses. Assay was performed in triplicates; b Two groups of animals were killed and their splenocytes subjected to IFN-γELISA assay in the presence or absence of the neu-overexpressing MMC or neu-negative ANV as control. Assay was performed in duplicates; c Naïve FVBN202 mice (n = 4) were immunized with HSP110-HER-2/neu vaccine [21] and presence of IgG1 Abs against sub-domains of ECD (II and IV) were detected in their sera as well as in the sera of group 1; d Mammary tissues from groups 1 and 2 were fixed in formalin and subjected to H & E staining to determine normal and hyperplastic epithelial cells. Presence of more than three cell layers is considered hyperplasia. Panel C is representative of 16 mammary tissues examined
Pre-existing immune responses against the neu “self” protein failed to reject MMC
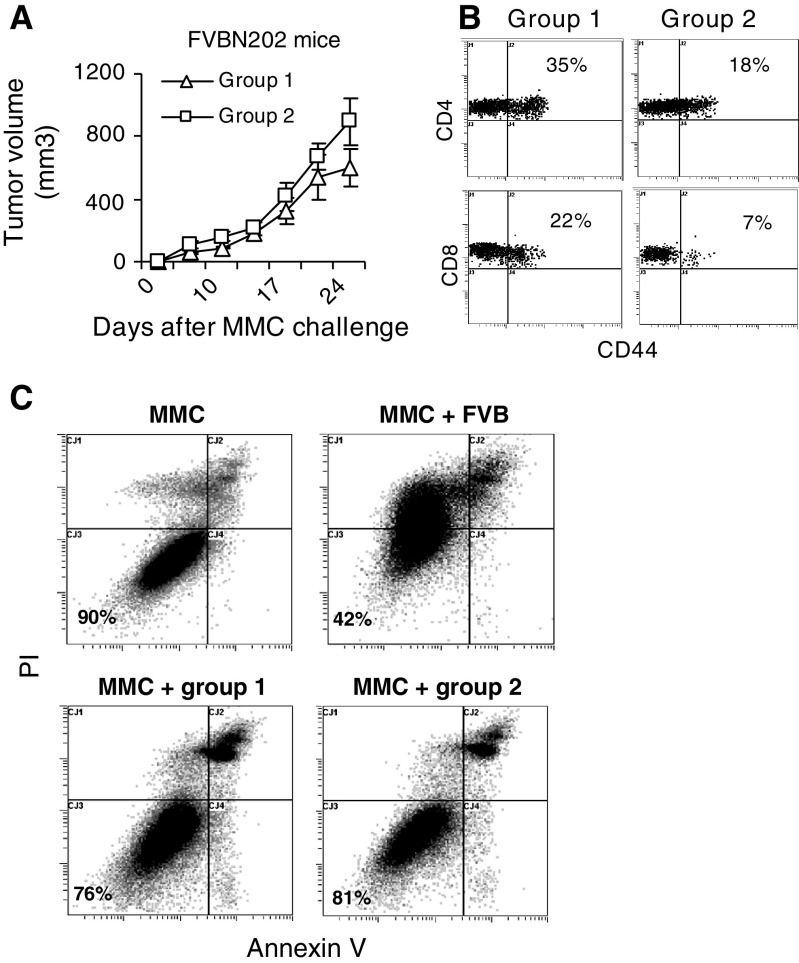
Because of the stochastic nature of spontaneous tumor formation in FVBN202 mice, it would be difficult to determine whether pre-existing immune responses may delay spontaneous tumor formation in these animals. However, tumor transplantation studies could answer this question. Therefore, we split FVBN202 mice into two groups: group 1 with an evidence of neu-specific Abs and group 2 with no evidence of anti-neu Abs. Animals were then challenged with MMC to determine whether group 1 with pre-existing immunity may inhibit MMC tumor growth. As shown in Fig 2a, no protection against tumor growth was observed in animals with pre-existing immune responses against the neu “self” protein (group 1) compared to those with no pre-existing immunity (group 2). These observations suggest that the neu-specific T cell and Ab responses in group 1 did not have effective anti-tumor function. In order to evaluate the presence of CD4+ and CD8+ effector T cells during tumor challenge in groups 1 and 2, blood samples were drawn 17 days following tumor challenge and subjected to three-color staining using FITC-CD62L, PE-CD44, and PE/CY5-CD4 or PE/CY5-CD8 Abs. Data were analyzed within gated lymphocyte regions. Percent effector T cells (CD44+) were then analyzed on gated CD4+CD62L- and CD8+CD62L-channels. As shown in Fig. 2b, compared to group 2, group 1 had marked increase in percent CD4+CD44+CD62L-effector T cells (35 vs. 18%) and CD8+CD44+CD62L-effector T cells (22 vs. 7%). These effector T cells were functional in secreting IFN-γ upon in vitro stimulation with MMC and CD8+ T cells were the main source of IFN-γ (data not shown).
Fig. 2.
Presence of pre-existing immune responses against the neu self protein cannot protect animals against challenge with the neu-overexpressing MMC. a Age-matched FVBN202 mice (3–8 months of age; n = 3) with (group 1) or without (group 2) pre-existing IgG1 Ab responses against the neu self protein were challenged s.c. with MMC (4 × 106 cells/mouse). Tumors were measured once every 3 days using digital caliper; b Blood were drawn 17 days post challenge and were subjected to flow cytometry analysis. Data were analyzed within gated lymphocyte regions. Percent effector T cells (CD44+) were then analyzed on gated CD4+CD62L- and CD8+CD62L-channels. Representative data are presented from three mice per group; c Above-mentioned groups were killed 25 days after MMC challenge and their splenocytes were cultured with MMC (E:T at 10:1) in the presence of 20 U/ml IL-2 for 48 h. Splenocytes from the parental FVB mice were used as positive control in an in vitro cytotoxicity assay. MMC in the absence of splenocytes was used as negative control. MMC target cells were then stained with annexin V, PI, and neu-specific Ab and subjected to flow cytometry analysis to detect T cell-induced apoptosis. Neu positive cells were gated and analyzed for early apoptotic (annexin V+), late apoptotic (annexin V+/PI+), and necrotic (PI+) cells. MMC was also cultured in the presence of medium to detect spontaneous apoptosis. Representative data from triplicate experiments are presented for each group
Pre-existing immune responses against the neu “self” but not “nonself” protein failed to induce apoptosis in MMC in vitro
In order to determine whether lymphocytes of group 1, which recognize the neu “self” antigen, were able to kill MMC in vitro as efficient as lymphocyes of FVB mice, which recognize the neu “nonself” antigen, their splenocytes were cultured in the presence or absence of MMC (E:T 10:1) and 20 U/ml IL-2 for 48 h. Lymphocytes of group 2 were used as negative control. Tumor cells were then stained with anti-neu Ab, annexin V, and PI to determine annexin V and/or PI negative viable MMC. As shown in Fig. 2C, splenocytes of FVB mice induced apoptosis in MMC so that numbers of viable MMC dropped from 90% in the absence of lymphocytes down to 42% in the presence of lymphocytes, whereas viable MMC appeared to be 76–80% in the presence of lymphocytes of group 1 or group 2. We never obtained 100% viability in MMC in vitro. We believe that experimental procedure would induce background apoptosis in MMC. We trusted the experiments where viability of control MMC was above 85%.
Low/intermediate expression of the neu “nonself” protein in tumors induced neu-specific immune responses that failed to reject the tumor
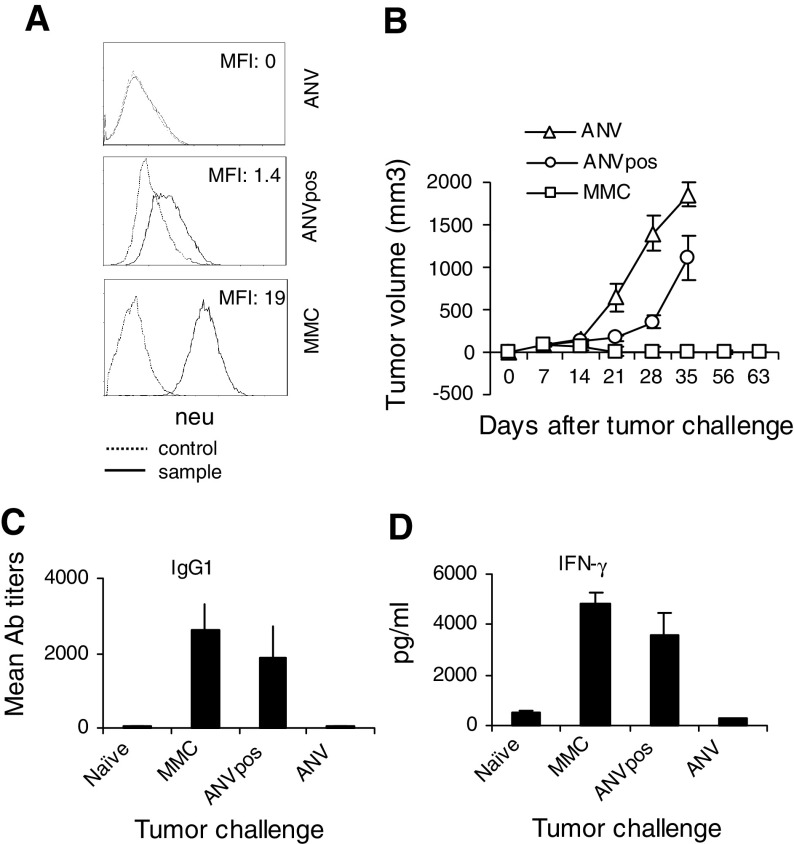
We as well as others have previously reported that parental FVB mice managed to reject MMC within 3–4 weeks because of the overexpression of neu “nonself” antigen in MMC [14–16]. In order to determine whether “nonself” entity of the neu antigen alone regardless of its overexpression would warrant rejection of neu positive tumors, we challenged parental FVB mice with ANVpos cells, which express low/intermediate levels of neu “nonself” protein (Fig. 3a). We established ANVpos from residual neu positive clones of ANV [14]. As shown in Fig. 3b, animals rejected MMC but they failed to reject ANV because of the overexpression or lack of expression of the neu “nonself” antigen, respectively. However, they failed to reject ANVpos despite the expression of neu “nonself” protein in this tumor, though at lower levels compared to MMC (log MFI: 1.4 vs. 19). Compared to ANV, there was significant delay in the growth rates of ANVpos on days 21 (P = 0.04) and 28 (P = 0.01) only. Evaluation of the neu-specific immune responses showed no significant differences between the animals challenged with MMC or ANVpos. Both groups showed comparable levels of IgG1 Ab (C) and IFN-γ producing T cell responses (D) against the neu antigen.
Fig. 3.
FVB mice failed to reject ANVpos despite the presence of neu-specific immune responses. a Levels of neu expression in three mammary tumor lines used in this study were determined by flow cytometry. Autofluorescence and control Abs showed similar background fluorescence. Data are representative of three independent experiments; b FVB mice (n = 4) were inoculated with ANV, ANVpos, or MMC (5 × 106 cells/mouse) and tumor growth was monitored once every 3 days; c Sera were collected from the FVB mice 3 weeks after the tumor challenge and subjected to ELISA using plates coated with the ECD of rat neu protein (1 μg/well). Sera from naïve mice were used as negative controls. Data are presented as mean Ab titers; d Above-mentioned groups were sacrificed 7 weeks after the tumor challenge and their splenocytes were subjected to IFN-γELISA
Discussion
Inclusion of adjuvant in the formulation of cancer vaccines has been justified by the danger model of immunity in order to provide the immune system with signal II (co-stimulation) and efficient delivery of tumor antigens (signal I). Although most of vaccine formulations induce tumor-specific immunity, they often fail to eradicate solid tumors and result in relapse-free survival. Our recent findings in the FVBN202 transgenic mouse model of mammary carcinoma necessitates revisiting self–nonself (SNS) and danger model of immunity in order to improve our approach in designing an effective cancer vaccine.
Although FVBN202 mice tolerate the neu “self” protein, we detected IgG1 Ab and IFN-γ producing T cell responses against the neu “self” protein, prior to the development of spontaneous mammary carcinoma in these mice. Interestingly, appearance of such immune responses was associated with dangerous increase in the number of breast epithelial cells (hyperplasia) as a consequence of overexpressing the neu protein in their mammary glands. Therefore, timing of immune monitoring when hyperplasia of mammary glands is present appears to be critical for the detection of pre-existing immune responses against neu tumor antigen. However, such immune responses failed to prevent spontaneous mammary tumor formation or reject transplanted MMC. On the other hand, parental FVB mice elicited immune responses against the neu “nonself” protein and rejected the neu-overexpressing MMC within 3–4 weeks but they failed to reject ANVpos, moderately expressing the neu “nonself” protein. These data suggest that danger signal alone (hyperplasia of mammary epithelial cells and overexpression of the neu protein) or “nonself” entity of the antigen (low/moderate expression of the neu nonself protein in ANVpos) by itself may break tolerance against the neu tumor antigen but combination of the two could improve the efficacy of such immune responses to reject MMC (Table 1). Our observations were consistent with the previous reports showing that suboptimal presentation of an antigen, regardless of being self or nonself, could not induce effective immune responses. Therefore, combination of danger signals (adjuvant) and semi-nonself tumor antigens such as cryptic epitopes [26] in vaccine formulations may improve anti-tumor efficacy of cancer immunotherapy, provided that tumors overexpress these target antigens. Since T cells were not exposed to the cryptic epitopes of self proteins during positive and negative selection in the thymus, they are predicted to see such cryptic epitopes as new or semi-nonself antigens and become fully activated to destroy the target cells that express these epitopes. Such cryptic epitopes resembling nonself epitopes are in fact more immunogenic than self dominant epitopes when, due to point mutation, are endogenously processed and presented to T cells. It was shown that the reactivity of cryptic epitops of myelin basic protein with T cells could induce autoimmunity [17, 18].
Table 1.
The unified “danger-SNS” model of immunity
| Danger | Nondanger | |
|---|---|---|
| Self | + | − |
| Nonself | ++ | + |
−Tolerance
+ Ineffective anti-tumor immune response
++ Effective anti-tumor immune response
Our results were generated in animal models where induction of endogenous tumor-specific immune responses occurred in the absence of vaccination. However, it is likely that cancer vaccines may induce distinct types of immune responses, which could be qualitatively different from the pre-existing immune responses in terms of recognizing different epitopes. In fact, sera from the HSP110-neu-immunized FVBN202 mice recognized different portion of the extracellular domain (ECD) of HER-2/neu compared to the sera from FVBN202 mice harboring pre-existing immunity. This would suggest that vaccination is not simply a booster of pre-existing immunity but induction of new immune responses. Our observation on detecting comparable levels of IFN-γ upon in vitro stimulation of T cells from FVBN202 or FVB mice with different concentrations of neu suggest (data not shown) that these T cells may recognize different epitopes rather than recognizing the same epitope with different affinities. Such differences in the quality of immune responses may be due to delivery of the neu antigen associated with different danger signals. Therefore, we need to focus the immune monitoring on the quality of immune responses more than on the quantity.
Based on our observation we suggest that a unified “danger-SNS” model can better explain induction and efficacy of immune responses against tumor antigens. According to this unified model, abnormal and harmful physiological conditions can be seen by the host immune system as danger. For instance, cellular stress may give rise to misfolded or mutated “self” proteins, which in turn result in the presentation of sequences being hidden from the immune system under normal healthy conditions. Such hidden cryptic epitopes of “self” proteins are considered as endogenous danger signal and also as “nonself” or new peptides by the host’s immune system because of the fact that T cells were not exposed to these hidden epitopes during positive and negative selection in the thymus. Such definition would unify two seemingly opposing schools of thought, i.e. danger and SNS models and explain many immunological phenomena when either model alone with classical definitions would fail to do so. An elegant hypothesis put out by Seong and Matzinger [31] supports this possibility and necessities transfer from SNS or danger model to unified “danger-SNS” model. This unified danger-SNS model suggests focusing on identification of naturally processed cryptic epitopes and antigen mimics along with novel dangerous adjuvants in vaccine design. Tumor-derived HSP vaccines are one example of combined danger signal and presentation of cryptic epitopes [19, 33]. Such HSPs are expected to carry a repertoire of tumor antigens, some of which would be subdominant or cryptic epitopes and seen by the host’s immune system as nonself peptides [19]. Endogenous processing of these peptides by tumors could then facilitate tumor regression. However, development of escape mechanisms in tumors such as HLA loss [15, 25, 29], loss of immunogenic antigens [14], defects in endogenous processing and presentation of cryptic epitopes [24], immune suppressive microenvironment supported by tumor-derived factors [6], concentration of cryptic epitopes in vaccine formulation and sustained immune responses supported by endogenous cytokines [27] would eventually determine the outcome. Therefore, inconclusive results may be obtained using HSP vaccines that may attribute to such escape mechanisms rather than to the efficacy of the vaccine by itself. These possibilities remain to be addressed.
Acknowledgments
This work was supported by the NIH R01 CA104757 grant (MHM) and flow cytometry shared resources facility, supported in part by the NIH grant P30CA16059. We gratefully acknowledge the support of VCU Massey Cancer Center and the Commonwealth Foundation for Cancer Research. We greatly appreciate Laura Graham in assisting us with adoptive T cell therapy. We thank Hooman Nikizad, Frances White and Julie S. Farnsworth for their assistance with flow cytometry. We also thank Dr. John Subjeck of Roswell Park Cancer Institute for providing us with the HSP110 expression vectors.
References
- 1.Banerjee D, Liou HC, Sen R. c-Rel-dependent priming of naive T cells by inflammatory cytokines. Immunity. 2005;23:445–458. doi: 10.1016/j.immuni.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 2.Bretscher P, Cohn M. A theory of self-nonself discrimination. Science. 1970;169:1042–1049. doi: 10.1126/science.169.3950.1042. [DOI] [PubMed] [Google Scholar]
- 3.Burnet FM. The clonal selection theory of acquired immunity. Nashville: Vanderbilt University Press; 1959. [Google Scholar]
- 4.Burnet FM, Fenner F. The production of antibodies. 2nd edn. Melbourne: Macmillan and Company Limited; 1949. [Google Scholar]
- 5.Curtsinger JM, Schmidt CS, Mondino A, Lins DC, Kedl RM, Jenkins MK, Mescher MF. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J Immunol. 1999;162:3256–3262. [PubMed] [Google Scholar]
- 6.Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, Castelli C, Mariani L, Parmiani G, Rivoltini L. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25:2546–2553. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 7.Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol. 2001;13:114–119. doi: 10.1016/S0952-7915(00)00191-6. [DOI] [PubMed] [Google Scholar]
- 8.Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD, Muller WJ. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci USA. 1992;89:10578–10582. doi: 10.1073/pnas.89.22.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haynes L, Eaton SM, Burns EM, Rincon M, Swain SL. Inflammatory cytokines overcome age-related defects in CD4 T cell responses in vivo. J Immunol. 2004;172:5194–5199. doi: 10.4049/jimmunol.172.9.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishii KJ, Suzuki K, Coban C, Takeshita F, Itoh Y, Matoba H, Kohn LD, Klinman DM. Genomic DNA released by dying cells induces the maturation of APCs. J Immunol. 2001;167:2602–2607. doi: 10.4049/jimmunol.167.5.2602. [DOI] [PubMed] [Google Scholar]
- 11.Janeway CA. The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol Today. 1992;13:11–16. doi: 10.1016/0167-5699(92)90198-G. [DOI] [PubMed] [Google Scholar]
- 12.Janeway CA, Medzhitov R. Introduction: the role of innate immunity in the adaptive immune response. Semin Immunol. 1998;10:349–350. doi: 10.1006/smim.1998.0142. [DOI] [PubMed] [Google Scholar]
- 13.Khoruts A, Osness RE, Jenkins MK. IL-1 acts on antigen-presenting cells to enhance the in vivo proliferation of antigen-stimulated naive CD4 T cells via a CD28-dependent mechanism that does not involve increased expression of CD28 ligands. Eur J Immunol. 2004;34:1085–1090. doi: 10.1002/eji.200324170. [DOI] [PubMed] [Google Scholar]
- 14.Kmieciak M, Knutson KL, Dumur CI, Manjili MH. HER-2/neu antigen loss and relapse of mammary carcinoma are actively induced by T cell-mediated anti-tumor immune responses. Eur J Immunol. 2007;37:675–685. doi: 10.1002/eji.200636639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knutson KL, Almand B, Dang Y, Disis ML. Neu antigen-negative variants can be generated after neu-specific antibody therapy in neu transgenic mice. Cancer Res. 2004;64:1146–1151. doi: 10.1158/0008-5472.CAN-03-0173. [DOI] [PubMed] [Google Scholar]
- 16.Knutson KL, Lu H, Stone B, Reiman JM, Behrens MD, Prosperi CM, Gad EA, Smorlesi A, Disis ML. Immunoediting of cancers may lead to epithelial to mesenchymal transition. J Immunol. 2006;177:1526–1533. doi: 10.4049/jimmunol.177.3.1526. [DOI] [PubMed] [Google Scholar]
- 17.Lehmann PV, Forsthuber T, Miller A, Sercarz EE. Spreading of T-cell autoimmunity to cryptic determinants of an autoantigen. Nature. 1992;558:155–157. doi: 10.1038/358155a0. [DOI] [PubMed] [Google Scholar]
- 18.Lehmann PV, Sercarz EE, Forsthuber T, Dayan CM, Gammon G. Determinant spreading and the dynamics of the autoimmune T-cell repertoire. Immunol Today. 1993;14:203–208. doi: 10.1016/0167-5699(93)90163-F. [DOI] [PubMed] [Google Scholar]
- 19.Makki A, Weidt G, Blachere NE, Lefrançois L, Srivastava PK. Immunization against a dominant tumor antigen abrogates immunogenicity of the tumor. Cancer Immun. 2002;16;2:4. [PubMed] [Google Scholar]
- 20.Manjili MH, Arnouk H, Knutson KL, Kmieciak M, Disis ML, Subjeck JR, Kazim AL. Emergence of immune escape variant of mammary tumors that has distinct proteomic profile and a reduced ability to induce “danger signals”. Breast Cancer Res Treat. 2006;96:233–241. doi: 10.1007/s10549-005-9044-4. [DOI] [PubMed] [Google Scholar]
- 21.Manjili MH, Wang XY, Chen X, Martin T, Repasky EA, Henderson R, Subjeck JR. HSP110-HER2/neu chaperone complex vaccine induces protective immunity against spontaneous mammary tumors in HER-2/neu transgenic mice. J Immunol. 2003;171:4054–4061. doi: 10.4049/jimmunol.171.8.4054. [DOI] [PubMed] [Google Scholar]
- 22.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 23.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 2004;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 24.Mehta AM, Jordanova ES, Kenter GG, Ferrone S, Fleuren G. Association of antigen processing machinery and HLA class I defects with clinicopathological outcome in cervical carcinoma. Cancer Immunol Immunother. 2008;57:197–206. doi: 10.1007/s00262-007-0362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Méndez R, Ruiz-Cabello F, Rodríguez T, Del Campo A, Paschen A, Schadendorf D, Garrido F. Identification of different tumor escape mechanisms in several metastases from a melanoma patient undergoing immunotherapy. Cancer Immunol Immunother. 2007;56:88–94. doi: 10.1007/s00262-006-0166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monzavi-Karbassi B, Artaud C, Jousheghany F, Hennings L, Carcel-Trullols J, Shaaf S, Korourian S, Kieber-Emmons T. Reduction of spontaneous metastases through induction of carbohydrate cross-reactive apoptotic antibodies. J Immunol. 2005;174:7057–7065. doi: 10.4049/jimmunol.174.11.7057. [DOI] [PubMed] [Google Scholar]
- 27.Moroz A, Eppolito C, Li Q, Tao J, Clegg CH, Shrikant PA. IL-21 enhances and sustains CD8+ T cell responses to achieve durable tumor immunity: comparative evaluation of IL-2, IL-15, and IL-21. J Immunol. 2004;173:900–909. doi: 10.4049/jimmunol.173.2.900. [DOI] [PubMed] [Google Scholar]
- 28.Ohashi K, Burkart V, Flohe S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000;164:558–561. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- 29.Rivoltini L, Carrabba M, Huber V, Castelli C, Novellino L, Dalerba P, Mortarini R, Arancia G, Anichini A, Fais S, Parmiani G. Immunity to cancer: attack and escape in T lymphocyte-tumor cell interaction. Immunol Rev. 2002;188:97–113. doi: 10.1034/j.1600-065X.2002.18809.x. [DOI] [PubMed] [Google Scholar]
- 30.Scheibner KA, Lutz MA, Boodoo S, Fenton MJ, Powell JD, Horton MR. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J Immunol. 2006;177:1272–1281. doi: 10.4049/jimmunol.177.2.1272. [DOI] [PubMed] [Google Scholar]
- 31.Seong SY, Matzinger P. Hydrophobicity: an ancient damage-associated molecular pattern that initiates innate immune responses. Nat Rev Immunol. 2004;4:469–478. doi: 10.1038/nri1372. [DOI] [PubMed] [Google Scholar]
- 32.Stein T, Morris JS, Davies CR, Weber-Hall SJ, Duffy MA, Heath VJ, Bell AK, Ferrier RK, Sandilands GP, Gusterson BA. Involution of the mouse mammary gland is associated with an immune cascade and an acute-phase response, involving LBP, CD14 and STAT3. Breast Cancer Res. 2004;6:R75–91. doi: 10.1186/bcr753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Srivastava PK. Therapeutic cancer vaccines. Curr Opin Immunol. 2006;18:201–205. doi: 10.1016/j.coi.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 34.Takeuchi N, Hiraoka S, Zhou XY, Nagafuku M, Ono S, Tsujimura T, Nakazawa M, Yura Y, Hamaoka T, Fujiwara H. Anti-HER-2/neu immune responses are induced before the development of clinical tumors but declined following tumorigenesis in HER-2/neu transgenic mice. Cancer Res. 2004;64:7588–7595. doi: 10.1158/0008-5472.CAN-04-1081. [DOI] [PubMed] [Google Scholar]
- 35.Warger T, Hilf N, Rechtsteiner G, Haselmayer P, Carrick DM, Jonuleit H, von Landenberg P, Rammensee HG, Nicchitta CV, Radsak MP, Schild H. Interaction of TLR2 and TLR4 ligands with the N-terminal domain of Gp96 amplifies innate and adaptive immune responses. J Biol Chem. 2006;281:22545–22553. doi: 10.1074/jbc.M502900200. [DOI] [PubMed] [Google Scholar]