Abstract
Bronchioloalveolar carcinoma (BAC), a subtype of lung adenocarcinoma (ADC) without stromal, vascular, or pleural invasion, is considered an in situ tumor with a 100% survival rate. However, the histological criteria for invasion remain controversial. BAC-like areas may accompany otherwise invasive adenocarcinoma, referred to as mixed type adenocarcinoma with BAC features (AWBF). AWBF are considered to evolve from BAC, representing a paradigm for malignant progression in ADC. However, the supporting molecular evidence remains forthcoming. Here, we have studied the genomic changes of BAC and AWBF by array comparative genomic hybridization (CGH). We used submegabase-resolution tiling set array CGH to compare the genomic profiles of 14 BAC or BAC with focal area suspicious for invasion with those of 15 AWBF. Threshold-filtering and frequency-scoring analysis found that genomic profiles of noninvasive and focally invasive BAC are indistinguishable and show fewer aberrations than tumor cells in BAC-like areas of AWBF. These aberrations occurred mainly at the subtelomeric chromosomal regions. Increased genomic alterations were noted between BAC-like and invasive areas of AWBF. We identified 113 genes that best differentiated BAC from AWBF and were considered candidate marker genes for tumor invasion and progression. Correlative gene expression analyses demonstrated a high percentage of them to be poor prognosis markers in early stage ADC. Quantitative PCR also validated the amplification and overexpression of PDCD6 and TERT on chromosome 5p and the prognostic significance of PDCD6 in early stage ADC patients. We identified candidate genes that may be responsible for and are potential markers for malignant progression in AWBF.
Keywords: array comparative genomic hybridization, bronchioloalveolar carcinoma, microarray, non-small-cell lung carcinoma, prognostic markers
Lung adenocarcinoma (ADC) accounts for ≈35% of all lung cancers and has an overall 5-year survival of 17% (1). The recent World Health Organization (WHO) classification recognized a particular subtype, bronchioloalveolar carcinoma (BAC), for its noninvasive features and excellent prognosis (2). BAC has a distinct histological pattern of tumor cells growing along preexisting alveolar framework without evidence of stromal, pleural, or vascular invasion. Yet, some invasive ADC, classified as mixed type, may have components or large areas of BAC-like pattern. Multistage development of adenocarcinoma putatively involves progression from atypical adenomatous hyperplasia (AAH) through BAC to invasive mixed type ADC with BAC features (AWBF) (3–5). Mice that express oncogenic KRAS develop histological changes that range from mild hyperplasia/dysplasia analogous to atypical adenomatous hyperplasia to alveolar adenomas and ultimately display overt ADC (6, 7). BAC-associated tumors have gained significant attention for their potentially greater sensitivity to treatment by epidermal growth factor receptor (EGFR) inhibitors (8). The initial studies that recognized BAC as a distinct entity reported 5-year survival rates of 100% (9–11), but more recent studies have reported lower 5-year survival rates of 83–86% for resected stage I BAC patients (12–14). These rates possibly reflect difficulties in applying the histological criteria of invasion in BAC or AWBF. Some studies have also reported that BAC with focal areas of microinvasion may also have excellent prognosis similar to noninvasive BAC (11, 15). The identification of genes/proteins that may distinguish BAC from AWBF and are predictors of ADC with poor prognosis could be useful for the establishment of molecular pathological classification of lung ADC. In this study, we have used array comparative genomic hybridization (CGH) to test our hypothesis that BAC is molecularly distinguishable from AWBF by their differential genomic profiles and that marker genes for invasion and/or poor prognosis may be identified.
Results
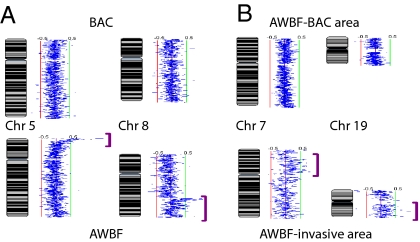
Most chromosomal changes in both BAC and AWBF were subtle, indicating low levels of genomic alteration as well as partial attenuation by contaminating nonneoplastic host cells. The profiles of BAC and BAC with focal areas suspicious for invasion were indistinguishable and showed low copy gains at 1p, 2q, 5p, 7p, 11p, 11q, 12q, 16p, 16q, 17q, 20q, and 21q (Fig. 1B). Copy gains typically occurred at the subtelomeric regions. AWBF had similar chromosomal changes but with greater variability and frequency and longer segmental alterations. Deletions were also more common in AWBF and were observed mainly on 3p and 5q and to a lesser extent on 4q and 6q. In two patients with synchronous BAC and invasive AWBF, the BAC-like area of the latter showed greater aberrations than the BAC (Fig. 2A). In two other AWBF, greater alterations were also noted in BAC-like areas compared with invasive areas (Fig. 2B). Normal lung samples showed no alteration of these regions.
Fig. 1.
BAC compared with AWBF by histology, frequency scoring, and threshold filtering. (A1) BAC showing typical growth pattern of tumoral cells along the preexisting alveolar scaffold without evidence of invasion. (H&E staining; ×100.) (A2) AWBF has both BAC-like and invasive areas. (H&E staining; ×16.) (Inset) High-power view of the invasive component. (H&E staining; ×100.) (B) Frequency scoring of BAC (green) compared with AWBF (red) illustrates the percentage of cases at which a change in genomic content has occurred in each of the study groups. Some changes were shared by BAC and AWBF (yellow). The presentation is per array loci; gains are represented by the colors on the right, and deletions are represented by the colors on the left. Vertical black thick and thin lines represent 100% and 50% of the samples, respectively. Blue arrows highlight the chromosomal areas of most frequent changes in BAC. (C) Unsupervised hierarchical clustering (Genesis software) of 119 clones selected by threshold filtering shows complete segregation of the two study groups: BAC (green rectangle on the right) and AWBF (red rectangle on the left). The color code of data corresponds to log2 ratio of array CGH signals.
Fig. 2.
Increase in genomic instability. Shown is a karyotypic presentation of the log2 ratio of array CGH signals (SeeGH software). Normal genomic content is represented by the midline (blue); clonal gains deviate to the right (green lines) and deletions to the left (red lines). The lower images show progression of genomic instability represented by more chromosomal aberrations than the upper images (purple brackets). (A) Synchronous BAC and AWBF in the same patient. (B) AWBF sampled in BAC and invasive areas.
Using threshold-filtering, we identified 119 clones that distinguished BAC from AWBF. Hierarchical clustering of all cases using these clones separated BAC from AWBF samples (Fig. 1C). In addition, a Fisher's Exact Test comparing the frequency of genomic changes between the BAC and AWBF groups yielded a list of 517 clones that best differentiated the two lesions. Integrating these two analyses was accomplished by applying a 10-clone “window” to identify shared regions [supporting information (SI) Text]. The result was a list of 256 candidate clones of high interest, from which a shorter list of 58 clones with gains in AWBF compared with BAC was selected. These clones included 113 unique amplified genes (Table S1) that could represent invasion and tumor progression markers for AWBF.
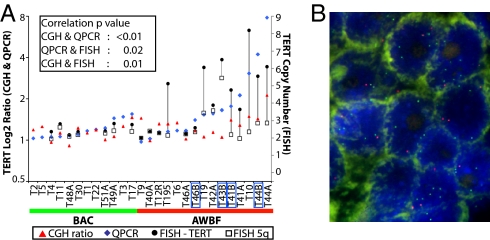
Quantitative polymerase chain reaction (QPCR) validated the gene content changes in 33 of the 113 candidate marker genes; 25 genes (75.8%) (Table S2) showed significantly higher gene copy number in AWBF compared with BAC. Among the evaluated genes were TERT and PDCD6, which we selected for further validation by QPCR and/or FISH based on their location on chromosome 5p, which showed prominent genomic changes (Fig. 1B). Measurement of both genes by QPCR demonstrated significant differences in gene copy number between BAC and AWBF (P = 0.03 for TERT and P = 0.02 for PDCD6), consistent with the array CGH results (Fig. 3A). Using FISH, we also studied the gene copy of TERT and chromosome 5 in 21 tumors. The correlation coefficients were 0.76 between array CGH and QPCR, 0.50 between QPCR and FISH, and 0.53 between array CGH and FISH (Fig. 4). FISH appears the most sensitive in detecting the amplification levels and revealed the existence of chromosome 5 polysomy, especially in AWBF. Furthermore, FISH showed increased signal in the invasive area of AWBF compared with the BAC-like area in two samples, T41 and T46 (Fig. 4A). The coefficient of correlation for PDCD6 amplification between array CGH and QPCR was 0.94.
Fig. 3.
PDCD6 validation and markers of poor prognosis. (A) QPCR performed on genomic DNA showed statistically significant differences in PDCD6 gene copy number between BAC and AWBF (P = 0.01), confirming the array CGH analysis that identified its amplification. (B) QPCR performed on 10 paired cDNAs of ADC-normal samples. PDCD6 was significantly overexpressed in tumor compared with normal lung tissue (P < 0.01), with a mean 3-fold higher expression. (C) Multivariate analysis adjusting for stage, histology, and differentiation that relied on QPCR of cDNA from 85 NSCLC samples found that PDCD6 was an independent poor prognostic factor for overall survival in stage I–II ADC patients. (D–F) Kaplan-Meier survival curves of SERPINE1 (D), GNB2 (E), and ST13 (F), based on gene expression data from the Duke database. Expression data were dichotomized at the median.
Fig. 4.
TERT validation by QPCR and FISH. (A) TERT content measured by array CGH and QPCR on genomic DNA (relative to normal control) and FISH (mean gene copy number per nucleus) show high correlation between the different methods. The black lines connecting copy number of TERT (filled circles) and 5q (open squares) are drawn to highlight the difference in copy number between the two probes. The blue boxes mark the AWBF sampled in invasive areas. Samples T41A, T44A, and T46A represent the BAC-like area, and T41B, T44B, and T46B represent the invasive area of AWBF. (B) FISH performed on AWBF in BAC area (sample T195) using the dual-color FISH probe mix that contains the hTERT locus (5p15, green signal) and the control D5S89 probe (5q31, red signal). Gain of TERT is reflected by the increased number of green compared with red foci.
Using real-time QPCR (RT-QPCR), we showed that in 10 separate pairs of invasive ADC and their corresponding nonneoplastic lung tissues, PDCD6 was overexpressed in tumor compared with normal lung tissue (P < 0.01), with a mean 3-fold increase in expression (Fig. 3B). In a series of 85 resected (stage I–IIIA) non-small-cell lung carcinoma (NSCLC) samples, PDCD6 overexpression was an independent poor prognostic factor for overall survival in stage I–II ADC patients [hazard ratio (HR) = 4.94, 95% C.I. 1.22–8.52, P = 0.02] (Fig. 3C) as well as for the entire cohort of stage I–II NSCLC patients (HR = 3.82, 95% C.I. 1.26–11.6, P = 0.03).
We next performed a correlative gene expression study using external and our own lung ADC gene expression microarray datasets, starting with the 113 amplified genes. Analysis of the Toronto, Harvard, and Michigan datasets discovered that 35%, 33%, and 29% of the genes were overexpressed; a fraction are expected to be based on gene amplification. These datasets included only 87, 59, and 42 of the 113 genes, respectively, and overexpression was noted in 42%, 36%, and 34% of them (Table 1 and Table S1). These results indicate a slight enrichment of the candidate amplified gene list for overexpression.
Table 1.
Correlative gene expression validation results for the candidate amplified genes
| Toronto | Harvard (17) | Michigan (18) | |
|---|---|---|---|
| No. of ADC samples | 39 | 127 | 86 |
| No. of normal lung samples | 10 | 17 | 10 |
| Array type | U133A | U95A | HuGeneFL |
| No. of genes/probe sets | 13,840/22,215 | 9,513/12,625 | 5,945/7,129 |
| No. of genes/probe sets up-regulated | 4,885/6,251 | 3,175/3,635 | 1,692/1,909 |
| No. of genes/probe sets down-regulated | 3,076/3,998 | 1,887/2,119 | 929/946 |
| % of genes up-regulated | 35.29 | 33.37 | 28.46 |
| % FDR used | 4.06 | 3.98 | 4.98 |
| No. of genes from 113 gene list that are present on array | 87 | 59 | 42 |
| No. of up-regulated genes that match 113 gene list | 38 | 22 | 15 |
| No. of genes that match by mistake based on FDR | 1.54 | 0.88 | 0.75 |
| Expected no. of up-regulated genes based on observed % of up-regulated genes | 30 | 19 | 12 |
| Validation rate, % | 41.90 | 35.80 | 33.93 |
FDR, false discovery rate.
Univariate analysis of the Duke microarray dataset showed that 10,023 of 54,675 (18%) probe sets were prognostic for overall survival (P < 0.05), with 4,879 (9%) overexpressed genes associated with poor prognosis. Among our 113 candidate-amplified genes, 112 were represented by 227 probe sets on the U133 plus 2 array. The expression of 46/227 (20%) probe sets was significantly associated with prognosis and thus was not significantly different from the percentage of all microarray probe sets that were prognostic (P = 0.507). However, 34 of the 227 probe sets (15%), representing 27/113 (24%) putatively amplified and overexpressed genes (Table 2) were associated with poor prognosis. This percentage is significantly higher than the 9% of all probe sets (P = 0.002) with such association. The most prognostic overexpressed genes included SERPINE1 (HR = 6.02, 95% C.I. 1.98–16.23, P = 0.001), GNB2 (HR = 5.8, 95% C.I. 1.83–14.52, P = 0.002), and ST13 (HR = 5.37, 95% C.I. 1.67–13.05, P = 0.003), (Fig. 3 D–F).
Table 2.
Markers of poor prognosis in early stage lung ADC, as identified in silico in the Duke microarray expression dataset (19)
| Gene symbol | Gene name | Hazard ratio | 95% C.I. | P |
|---|---|---|---|---|
| AP1S1 | Adaptor-related protein complex 1, σ 1 subunit | 4.59 | 1.77–11.91 | 0.002 |
| AP4M1 | Adaptor-related protein complex 4, μ 1 subunit | 3.68 | 1.5–9.01 | 0.004 |
| BRD9 | Bromodomain containing 9 | 3.89 | 1.1–13.8 | 0.035 |
| CCDC21 | Coiled-coil domain containing 21 | 6.46 | 1.15–36.14 | 0.034 |
| CCL8 | Chemokine (C-C motif) ligand 8 | 1.74 | 1–3.04 | 0.050 |
| COPS6 | COP9 constitutive photomorphogenic homolog subunit 6 (Arabidopsis) | 2.95 | 1.4–6.22 | 0.004 |
| CSDE1 | Cold shock domain containing E1, RNA-binding | 3.61 | 1.1–11.84 | 0.034 |
| EP300 | E1A binding protein p300 | 6.15 | 1.23–30.63 | 0.027 |
| GNB2 | Guanine nucleotide binding protein (G protein), β polypeptide 2 | 6.69 | 2.1–21.29 | 0.001 |
| HIPK1 | Homeodomain interacting protein kinase 1 | 2.98 | 1.1–8.11 | 0.032 |
| HRSP12 | Heat-responsive protein 12 | 2.98 | 1.19–7.45 | 0.020 |
| LAPTM4B | Lysosomal associated protein transmembrane 4β | 1.47 | 1.01–2.13 | 0.044 |
| MCM7 | MCM7 minichromosome maintenance deficient 7 (Saccharomyces cerevisiae) | 2.74 | 1.45–5.19 | 0.002 |
| MGC4677 | Hypothetical protein MGC4677 | 3.97 | 1.89–8.31 | <0.001 |
| OLFM2 | Olfactomedin 2 | 4.45 | 1.5–13.15 | 0.007 |
| POP7 | Processing of precursor 7, ribonuclease P subunit (S. cerevisiae) | 3.25 | 1.2–8.79 | 0.020 |
| PPA1 | Pyrophosphatase (inorganic) 1 | 3.06 | 1.31–7.16 | 0.010 |
| RABL4 | RAB, member of RAS oncogene family-like 4 | 3.88 | 1.12–13.43 | 0.032 |
| RPL30 | Ribosomal protein L30 | 19.70 | 2.44–158.85 | 0.005 |
| SERPINE1 | Serpin peptidase inhibitor, clade E (nexin, plasminogen activator inhibitor type 1), member 1 | 3.43 | 1.84–6.4 | <0.001 |
| SH3BGRL3 | SH3 domain binding glutamic acid-rich protein like 3 | 5.01 | 1.75–14.35 | 0.003 |
| SLC25A17 | Solute carrier family 25 (mitochondrial carrier), member 17 | 3.68 | 1.32–10.26 | 0.013 |
| ST13 | Suppression of tumorigenicity 13 | 2.02 | 1.21–3.37 | 0.007 |
| TAF6 | TAF6 RNA polymerase II, TATA box binding protein (TBP)-associated factor, 80 kDa | 3.99 | 1.52–10.52 | 0.005 |
| TLE3 | Transducin-like enhancer of split 3 (E(sp1) homolog, Drosophila) | 5.09 | 1.45–17.83 | 0.011 |
| TOB2 | Transducer of ERBB2, 2 | 3.37 | 1.01–11.32 | 0.049 |
| ZNF561 | Zinc finger protein 561 | 8.20 | 1.41–47.74 | 0.019 |
Using frequency scoring, we identified the most common deletions (SI Text). The majority of deleted clones in AWBF were on 3p and 5q, and they showed more continuity in their chromosomal location than on the other chromosomes. The deleted clones on chromosome 3p and 5q included 149 genes (Table S3), among which are FHIT and DLEC1. Similar to the candidate gained genes, correlative gene expression analysis using external and our own lung ADC datasets found that 22%, 20%, and 16% of the genes in the Toronto, Harvard, and Michigan datasets were down-regulated. Among the 149 candidate genes with loss, only 113, 84, and 48, respectively, were represented in these three datasets. Down-regulation was found in 45%, 26%, and 20% of them (Table S4). These results also showed an enrichment of the candidate deleted gene list for down-regulation.
Discussion
We have demonstrated that the genomic profile of BAC is distinguishable from that of invasive AWBF, with the latter displaying greater genomic aberrations. We have also demonstrated that there is progression at the genomic level from BAC-like to invasive areas of AWBF. The 113 differentially gained genes in AWBF compared with BAC could represent candidate marker genes for tumor invasion and malignant progression. Correlative gene expression studies on microarray datasets suggest that a high percentage of these genes are prognostic markers for early stage ADC patients. Using QPCR, we validated the common amplification of 25 genes including TERT and PDCD6 and found PDCD6 overexpression to be an independent prognostic marker for poor overall survival in early stage ADC. Further validation could potentially lead to use of these genes as markers for differentiating aggressive AWBF from noninvasive and prognostically excellent BAC.
There have been many attempts to classify lung carcinomas at the molecular level through various techniques, including metaphase CGH studies (16), gene expression (17–19), and array CGH analyses (20). Our whole-genome submegabase array study shows that BAC is characterized by alterations at subtelomeric chromosomal regions. Such aberrations could be explained mechanistically by the breakage/fusion/bridge (BFB) cycle (21). Normally telomeres protect chromosome ends and prevent their fusion; however, telomere loss may lead to chromosome instability and cyclic fusion, the formation of a chromosomal bridge and its breakage in proximity to the site of initial fusion, of sister chromatids during replication. This self-perpetuating process resolves through net gain of telomere by translocation of the ends of another chromosome, by small subtelomeric duplications of the end of the same chromosome, or by direct telomere addition. Consequently, gene amplification and deletions occur preferentially at the subtelomeric chromosomal regions. BFB also predicts progressive accumulation of genetic alterations, as observed in both BAC and AWBF. It has been shown that, as BFB progresses and more chromosomal abnormalities accumulate, the breakpoints are more interstitial (21).
The differential genomic changes noted between BAC and invasive AWBF provide important evidence for a better understanding of the pathogenesis of ADC. We have used two independent algorithms to enhance the certainty of the profile that distinguishes BAC from invasive AWBF. Our inability to clearly differentiate BAC from BAC with focal area of invasion at the genomic level suggests that both may have a similar behavior with low metastatic potential and that early invasion is likely determined at gene expression levels by epigenetic mechanisms. The finding also suggests that BAC or BAC with focal invasion, which are negative for the overexpression of identified marker genes, could potentially be grouped into a single diagnostic entity with excellent prognosis (11, 15).
The 113 candidate marker genes that we identified may represent part of the “signature of chromosomal instability” for invasion and malignant progression in AWBF (22). The correlative gene expression validation rate (≈35%) in the Harvard and Michigan datasets was limited by the low number of probe sets in the microarray platform that matched the genomic gene list (less than half). Nevertheless, it confirms the importance of some of our candidate markers in lung carcinoma (Table S1) and the overexpression of others such as SAR1A (23), SYCP1 (24), and MCM7 (22), which have been linked to other malignancies as well as lung cancer. We have previously reported the poor prognostic significance of TERT gene amplification in NSCLC (25). Our present findings extend the importance of TERT amplification to AWBF and increased TERT gene copy because of chromosome 5 polysomy.
PDCD6, programmed cell death 6, or apoptosis-linked gene 2 (ALG-2) is located on chromosome 5pter–5p15.2 and is in close proximity to TERT. It encodes a 191-aa protein that was originally considered pro-apoptotic (26). PDCD6 belongs to the penta-EF hand Ca2+-binding protein family (27) and is ubiquitously expressed in the body. PDCD6 is required for T cell receptor-, glucocorticoid- (26), and FAS- (28) induced cell death. It interacts with the SH3-binding domain containing pro-apoptotic protein AIP1 (ALG-2-interacting protein-1) (29), peflin (30), and annexin XI (31) in a Ca2+-dependent way as well as with DAPK1 (death-associated protein kinase 1) (32). During FAS-induced apoptosis, PDCD6, which is a 22-kDa protein, is cleaved in its N-terminus to yield a 19-kDa protein and translocates from the cytoplasmic membrane to the cytosol (28). More recent work questioned the need of PDCD6 for apoptosis, as it may be compensated by other functionally redundant proteins (33). Immunohistochemical staining has revealed high expression of PDCD6 in primary tumors compared with normal tissues of the breast, liver, and lung (34, 35). Both nuclear and cytoplasmic overexpression have been reported for lung cancer, especially metastatic ADC, indicating that it plays a role in survival pathways (35). We confirm that PDCD6 is significantly overexpressed in lung ADC (35). Moreover, we have also demonstrated that PDCD6 is a poor prognostic factor in both early stage NSCLC and ADC and thus may serve as one of the markers to differentiate more indolent from aggressive AWBF.
Potti et al. (19) reported a genomic strategy to refine prognosis for early stage NSCLC and identify patients at high risk of relapse after initial surgery. They constructed a lung metagene model based on gene expression data and showed that its prognostic accuracy surpasses that of a model based on traditional clinical data. Their model was applied to all histologic types of early stage disease but did not consider BAC as a special entity. Although none of the 122 genes in the published metagenes matched our 113 genes, analysis of our genes in their dataset showed that the overexpression of 27 genes (24%) was associated with poor prognosis in early stage ADC patients. Significantly higher gene copy number in AWBF compared with BAC was confirmed by QPCR on genomic DNA in 74% of these genes (20 of 27 genes, Table S2).
The 27 putative markers that we identified include serpin peptidase inhibitor, clade E, member 1 (SERPINE1); guanine nucleotide-binding protein β-2 (GNB2); and suppression of tumorigenicity 13 (ST13). SERPINE1, also known as plasminogen activator inhibitor-1, is the primary physiological inhibitor of both tissue-type plasminogen activator (tPA) and urokinase-like PA (uPA) and thus promotes the stabilization and formation of thrombi. In addition to regulating the fibrinolytic system, SERPINE1 has de-adhesive properties and is capable of inducing cell detachment that is dependent on the presence of complexes of uPA:uPA-receptor matrix-engaged integrins (36). Interestingly, SERPINE1 high expression has been linked previously with poor prognosis in a number of malignancies (37), including lung ADC (17). High expression of SERPINE1 may activate cellular scattering, promote migration, and possibly enhance metastatic spread, all of which could account for the poor prognosis observed. Our study relates the high expression to amplification present at the genomic level. SERPINE1 is located on the same locus, 7q21.3–q22, as GNB2, which is a prognostic marker for lung ADC. GNB2 is the second of five possible genes encoding the β subunit of G proteins. As of yet, no other study associates GNB2 with lung cancer, but it is well established that G protein-coupled receptors can promote cancer progression and metastasis in a variety of tumors including NSCLC (38). ST13, whose aliases are P48, HOP, and Hsc70-interacting protein, acts as a cochaperone of heat-shock protein 70 (Hsp70) to stabilize its activity (39). Hsp70 is known to promote survival in cancer cells (40), thus making it reasonable to hypothesize that ST13 amplification would lead to tumor progression.
For the most part, the function and role in lung cancer of the remaining genes from the 113 candidate gene list are poorly characterized, and their involvement in the progression of lung ADC is worthy of further study. These genes are also promising markers for poor prognosis in early lung ADC and could serve as potential targets for future therapy. The finding that gene losses at certain chromosomal regions are more prominent in AWBF than BAC indicates that gene deletions may also play an important role in the progression of ADC. Our putatively deleted genes require further validation.
Materials and Methods
Study Materials.
The study protocol was approved by the University Health Network Research Ethics Board and included 26 resected lung cancers (1996–2005) classified histologically as nonmucinous BAC or invasive-AWBF. For each case, the histology slides were reviewed independently by the study pathologists (S.A.-R. and M.-S.T.) and tumors were classified according to the 2004 WHO criteria (2). Twelve cases were classified as AWBF when they had not only prominent nonmucinous BAC-like pattern (>50% of the tumor) but also frank invasive ADC of other histological types, such as acinar, papillary, or solid (Fig. 1A). Fourteen cases were considered noninvasive BAC or BAC with possible focal microinvasive area. In 11 of the AWBF cases, tissue from the BAC-like area was sampled, and in three, additional tissue from a frankly invasive area was sampled separately. One case involved sampling from the invasive area only. Clinical characteristics of the samples are provided in Table S5. Eight corresponding normal lung tissues were selected arbitrarily as normal controls. For mRNA expression studies, we used matched tumor and normal tissues from the University Health Network snap-frozen lung tumor bank (41).
Tissue Sampling, DNA Isolation, and Array CGH.
DNA was isolated from formalin-fixed, paraffin-embedded tissue. Guided by hematoxylin and eosin (H&E)-stained sections, we marked representative paraffin blocks with tumor areas containing >50% tumor cell nuclei were marked and cored them by using the needle for tissue array (Beecher Instruments). The process of tissue sampling, DNA isolation, and array CGH is detailed in the SI Text.
Array CGH Data Analysis.
Array CGH data analysis was based on two independent algorithms, threshold-filtering and frequency-scoring (42), using multiple software tools including SeeGH (43), Genesis (44), aCGH-Smooth (45), and Frequency-Plot (42). The algorithms and the overlap between them are described in SI Text. Our analysis concentrated on clone gains rather than losses because clone gains involved more chromosomes, their prevalence was higher (Fig. 1B), and occasionally they were of higher copy number (not limited to just two copies per clone).
Validation by RT-QPCR.
Gene copy numbers were evaluated for the DNA used in the array CGH studies by RT-QPCR, using primer sets for target and housekeeping genes. The evaluation of 33 genes, including TERT and PDCD6, was performed on all of the array CGH samples except two BACs (Tables S2 and S6). The mRNA expression study was carried out on two groups of samples: 10 pairs of matched ADC and their adjacent normal lung tissue and 85 NSCLC samples. Primer sets design, reaction conditions and analysis description, and patients' demographic information are included in SI Text and Tables S7 and S8.
Validation by FISH.
The 21 cases studied by FISH included 7 BAC with or without suspicion for invasion and 14 AWBF; 3 of the latter were scored in both their BAC and invasive areas. An additional case of AWBF was scored only in the invasive area. Among these cases was one with synchronous BAC and invasive AWBF sampled from the BAC area. FISH failed in 6 samples. The FISH protocol is detailed in the SI Text.
The Toronto DNA Microarray Dataset.
RNA was extracted by the phenol/chloroform method from 39 adenocarcinomas (Table S9) and 10 normal lung tissue samples. RNA quality was assessed by gel electrophoresis and Bioanalyzer (Agilent). cRNA synthesis, hybridization, and scanning were performed according to the manufacturer's protocol. The adenocarcinoma RNA was profiled on the U133A chip (Affymetrix) and the normal lung RNA on the U133A2 chip (Affymetrix). To ensure the compatibility of these two platforms, four of the 39 adenocarcinomas were reprofiled on the U133A2 chip.
Correlative Gene Expression Study.
We validated the 113 amplified genes and the 149 deleted genes from array CGH analysis on the Toronto microarray dataset and on two publicly available lung cancer microarray expression datasets (17, 18), referred to as “Harvard” and “Michigan,” respectively. For a detailed description of the analytic process and a summary of the validation see the SI Text and Tables S1 and S4.
In addition, univariate analysis was performed on microarray expression data of stage I ADC patient samples from a third dataset referred to as “Duke” (19) to identify prognostic markers and compare them with the 113 candidate markers, as detailed in the SI Text and Table S7.
Statistical Analysis.
The Mann–Whitney test was used to compare the genomic copy number of 33 genes including TERT and PDCD6 (Table S2). Pearson correlation coefficients assessed the correlation between array CGH, QPCR, and FISH results. The Wilcoxon signed rank test was used to compare PDCD6 expression in the paired ADC-normal samples. Survival analysis of PDCD6 mRNA of 85 NSCLC patients and 34 stage I ADC patients from the Duke dataset is described in the SI Text.
Supplementary Material
Acknowledgments.
We thank Ni Liu, Olga Ludkovski, Paul Boutros, and Dr. Jeremy Squire for assistance and advice. This work was supported by the Canadian Cancer Society and National Cancer Institute of Canada Grant 015184, Genome Canada/British Columbia, Ontario Institute of Cancer Research, and the Canadian Institutes for Health Research. S.A.-R. is a fellow of the Canadian Institutes for Health Research Training Program for Clinician Scientists in Molecular Oncologic Pathology (STP-53912) and a recipient of the Ontario Cancer Institute Knudson Research Fellowship and the National Cancer Institute of Canada Terry Fox Foundation Clinical Research Fellowship. B.P.C. is supported by a scholarship from the Michael Smith Foundation for Health Research. M.-S.T. is the M. Qasim Choksi Chair in Lung Cancer Translational Research. I.J. is the recipient of the Canada Research Chair in Integrative Computational Biology.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.D.M. is a guest editor invited by the Editorial Board.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no. GSE11945).
This article contains supporting information online at www.pnas.org/cgi/content/full/0709618105/DCSupplemental.
References
- 1.Travis WD, Travis LB, Devesa SS. Lung Cancer. Cancer. 1995;75:191–202. doi: 10.1002/1097-0142(19950101)75:1+<191::aid-cncr2820751307>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 2.Travis WD, Brambilla E, Müller-Hermelink HK, Harris CC, editors. WHO Classification of Tumors: Pathology and Genetics of Tumors of the Lung, Pleura, Thymus and Heart. Lyon: IARC Press; 2004. pp. 35–44. [Google Scholar]
- 3.Kitamura H, Kameda Y, Ito T, Hayashi H. Atypical adenomatous hyperplasia of the lung. Implications for the pathogenesis of peripheral lung adenocarcinoma. Am J Clin Pathol. 1999;111:610–622. doi: 10.1093/ajcp/111.5.610. [DOI] [PubMed] [Google Scholar]
- 4.Kim CF, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 5.Jackson EL, et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001;15:3243–3248. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson L, et al. Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature. 2001;410:1111–1116. doi: 10.1038/35074129. [DOI] [PubMed] [Google Scholar]
- 7.Guerra C, et al. Tumor induction by an endogenous K-ras oncogene is highly dependent on cellular context. Cancer Cell. 2003;4:111–120. doi: 10.1016/s1535-6108(03)00191-0. [DOI] [PubMed] [Google Scholar]
- 8.Miller VA, et al. Bronchioloalveolar pathologic subtype and smoking history predict sensitivity to gefitinib in advanced non-small-cell lung cancer. J Clin Oncol. 2004;22:1103–1109. doi: 10.1200/JCO.2004.08.158. [DOI] [PubMed] [Google Scholar]
- 9.Yokose T, et al. Favorable and unfavorable morphological prognostic factors in peripheral adenocarcinoma of the lung 3 cm or less in diameter. Lung Cancer. 2000;29:179–188. doi: 10.1016/s0169-5002(00)00103-3. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki K, et al. Prognostic significance of the size of central fibrosis in peripheral adenocarcinoma of the lung. Ann Thorac Surg. 2000;69:893–897. doi: 10.1016/s0003-4975(99)01331-4. [DOI] [PubMed] [Google Scholar]
- 11.Noguchi M, et al. Small adenocarcinoma of the lung. Histologic characteristics and prognosis. Cancer. 1995;75:2844–2852. doi: 10.1002/1097-0142(19950615)75:12<2844::aid-cncr2820751209>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 12.Rena O, et al. Stage I pure bronchioloalveolar carcinoma: Recurrences, survival, and comparison with adenocarcinoma of the lung. Eur J Cardiothorac Surg. 2003;23:409–414. doi: 10.1016/s1010-7940(02)00830-8. [DOI] [PubMed] [Google Scholar]
- 13.Ebright MI, et al. Clinical pattern and pathologic stage but not histologic features predict outcome for bronchioloalveolar carcinoma. Ann Thorac Surg. 74:1640–1647. doi: 10.1016/s0003-4975(02)03897-3. [DOI] [PubMed] [Google Scholar]
- 14.Breathnach OS, et al. Bronchioloalveolar carcinoma of the lung: Recurrences and survival in patients with stage I disease. J Thorac Cardiovasc Surg. 2001;121:42–47. doi: 10.1067/mtc.2001.110190. [DOI] [PubMed] [Google Scholar]
- 15.Sakurai H, et al. Grade of stromal invasion in small adenocarcinoma of the lung: Histopathological minimal invasion and prognosis. Am J Surg Pathol. 2004;28:198–206. doi: 10.1097/00000478-200402000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Petersen I, Petersen S. Towards a genetic-based classification of human lung cancer. Anal Cell Pathol. 2001;22:111–121. doi: 10.1155/2001/374304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beer DG, et al. Gene-expression profiles predict survival of patients with lung adenocarcinoma. Nat Med. 2002;8:816–824. doi: 10.1038/nm733. [DOI] [PubMed] [Google Scholar]
- 18.Bhattacharjee A, et al. Classification of human lung carcinomas by mRNA expression profiling reveals distinct adenocarcinoma subclasses. Proc Natl Acad Sci USA. 2001;98:13790–13795. doi: 10.1073/pnas.191502998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Potti A, et al. A genomic strategy to refine prognosis in early-stage non-small-cell lung cancer. N Engl J Med. 2006;355:570–580. doi: 10.1056/NEJMoa060467. [DOI] [PubMed] [Google Scholar]
- 20.Shibata T, et al. Genetic classification of lung adenocarcinoma based on array-based comparative genomic hybridization analysis: Its association with clinicopathologic features. Clin Cancer Res. 2005;11:6177–6185. doi: 10.1158/1078-0432.CCR-05-0293. [DOI] [PubMed] [Google Scholar]
- 21.Murnane JP, Sabatier L. Chromosome rearrangements resulting from telomere dysfunction and their role in cancer. BioEssays. 2004;26:1164–1174. doi: 10.1002/bies.20125. [DOI] [PubMed] [Google Scholar]
- 22.Carter SL, Eklund AC, Kohane IS, Harris LN, Szallasi Z. A signature of chromosomal instability inferred from gene expression profiles predicts clinical outcome in multiple human cancers. Nat Genet. 2006;38:1043–1048. doi: 10.1038/ng1861. [DOI] [PubMed] [Google Scholar]
- 23.Difilippantonio S, et al. Gene expression profiles in human non-small and small-cell lung cancers. Eur J Cancer. 2003;39:1936–1947. doi: 10.1016/s0959-8049(03)00419-2. [DOI] [PubMed] [Google Scholar]
- 24.Tureci O, et al. Identification of a meiosis-specific protein as a member of the class of cancer/testis antigens. Proc Natl Acad Sci USA. 1998;95:5211–5216. doi: 10.1073/pnas.95.9.5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu CQ, et al. Amplification of telomerase (hTERT) gene is a poor prognostic marker in non-small-cell lung cancer. Br J Cancer. 2006;94:1452–1459. doi: 10.1038/sj.bjc.6603110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vito P, Lacana E, D'Adamio L. Interfering with apoptosis: Ca2+-Binding protein ALG-2 and Alzheimer's disease gene ALG-3. Science. 1996;271:521–525. doi: 10.1126/science.271.5248.521. [DOI] [PubMed] [Google Scholar]
- 27.Maki M, Narayana SV, Hitomi K. A growing family of the Ca2+-binding proteins with five EF-hand motifs. Biochem J. 1997;328:718–720. [PMC free article] [PubMed] [Google Scholar]
- 28.Jung YS, et al. Apoptosis-linked gene 2 binds to the death domain of Fas and dissociates from Fas during Fas-mediated apoptosis in Jurkat cells. Biochem Biophys Res Commun. 2001;288:420–426. doi: 10.1006/bbrc.2001.5769. [DOI] [PubMed] [Google Scholar]
- 29.Vito P, Pellegrini L, Guiet C, D'Adamio L. Cloning of AIP1, a novel protein that associates with the apoptosis-linked gene ALG-2 in a Ca2+-dependent reaction. J Biol Chem. 1999;274:1533–1540. doi: 10.1074/jbc.274.3.1533. [DOI] [PubMed] [Google Scholar]
- 30.Kitaura Y, Matsumoto S, Satoh H, Hitomi K, Maki M. Peflin and ALG-2, members of the penta-EF-hand protein family, form a heterodimer that dissociates in a Ca2+-dependent manner. J Biol Chem. 2001;276:14053–14058. doi: 10.1074/jbc.M008649200. [DOI] [PubMed] [Google Scholar]
- 31.Satoh H, Shibata H, Nakano Y, Kitaura Y, Maki M. ALG-2 interacts with the amino-terminal domain of annexin XI in a Ca2+-dependent manner. Biochem Biophys Res Commun. 2002;291:1166–1172. doi: 10.1006/bbrc.2002.6600. [DOI] [PubMed] [Google Scholar]
- 32.Lee JH, Rho SB, Chun T. Programmed cell death 6 (PDCD6) protein interacts with death-associated protein kinase 1 (DAPk1): Additive effect on apoptosis via caspase-3 dependent pathway. Biotechnol Lett. 2005;27:1011–1015. doi: 10.1007/s10529-005-7869-x. [DOI] [PubMed] [Google Scholar]
- 33.Jang IK, Hu R, Lacana E, D'Adamio L, Gu H. Apoptosis-linked gene 2-deficient mice exhibit normal T-cell development and function. Mol Cell Biol. 2002;22:4094–4100. doi: 10.1128/MCB.22.12.4094-4100.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krebs J, Saremaslani P, Caduff R. ALG-2: A Ca2+-binding modulator protein involved in cell proliferation and in cell death. Biochim Biophys Acta. 2002;1600:68–73. doi: 10.1016/s1570-9639(02)00446-6. [DOI] [PubMed] [Google Scholar]
- 35.la Cour JM, et al. Up-regulation of ALG-2 in hepatomas and lung cancer tissue. Am J Pathol. 2003;163:81–89. doi: 10.1016/S0002-9440(10)63632-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Czekay RP, Loskutoff DJ. Unexpected role of plasminogen activator inhibitor 1 in cell adhesion and detachment. Exp Biol Med (Maywood) 2004;229:1090–1096. doi: 10.1177/153537020422901102. [DOI] [PubMed] [Google Scholar]
- 37.Andreasen PA, Kjoller L, Christensen L, Duffy MJ. The urokinase-type plasminogen activator system in cancer metastasis: A review. Int J Cancer. 1997;72:1–22. doi: 10.1002/(sici)1097-0215(19970703)72:1<1::aid-ijc1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 38.Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nat Rev Cancer. 2007;7:79–94. doi: 10.1038/nrc2069. [DOI] [PubMed] [Google Scholar]
- 39.Nollen EA, et al. Modulation of in vivo HSP70 chaperone activity by Hip and Bag-1. J Biol Chem. 2001;276:4677–4682. doi: 10.1074/jbc.M009745200. [DOI] [PubMed] [Google Scholar]
- 40.Ravagnan L, et al. Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat Cell Biol. 2001;3:839–843. doi: 10.1038/ncb0901-839. [DOI] [PubMed] [Google Scholar]
- 41.Barsyte-Lovejoy D, et al. The c-Myc oncogene directly induces the H19 noncoding RNA by allele-specific binding to potentiate tumorigenesis. Cancer Res. 2006;66:5330–5337. doi: 10.1158/0008-5472.CAN-06-0037. [DOI] [PubMed] [Google Scholar]
- 42.Coe BP, et al. Gain of a region on 7p22.3, containing MAD1L1, is the most frequent event in small-cell lung cancer cell lines. Genes Chromosomes Cancer. 2006;45:11–19. doi: 10.1002/gcc.20260. [DOI] [PubMed] [Google Scholar]
- 43.Chi B, DeLeeuw RJ, Coe BP, MacAulay C, Lam WL. SeeGH–A software tool for visualization of whole genome array comparative genomic hybridization data. BMC Bioinformatics. 2004;5:13. doi: 10.1186/1471-2105-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sturn A, Quackenbush J, Trajanoski Z. Genesis: Cluster analysis of microarray data. Bioinformatics. 2002;18:207–208. doi: 10.1093/bioinformatics/18.1.207. [DOI] [PubMed] [Google Scholar]
- 45.Jong K, Marchiori E, Meijer G, Vaart AV, Ylstra B. Breakpoint identification and smoothing of array comparative genomic hybridization data. Bioinformatics. 2004;20:3636–3637. doi: 10.1093/bioinformatics/bth355. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.