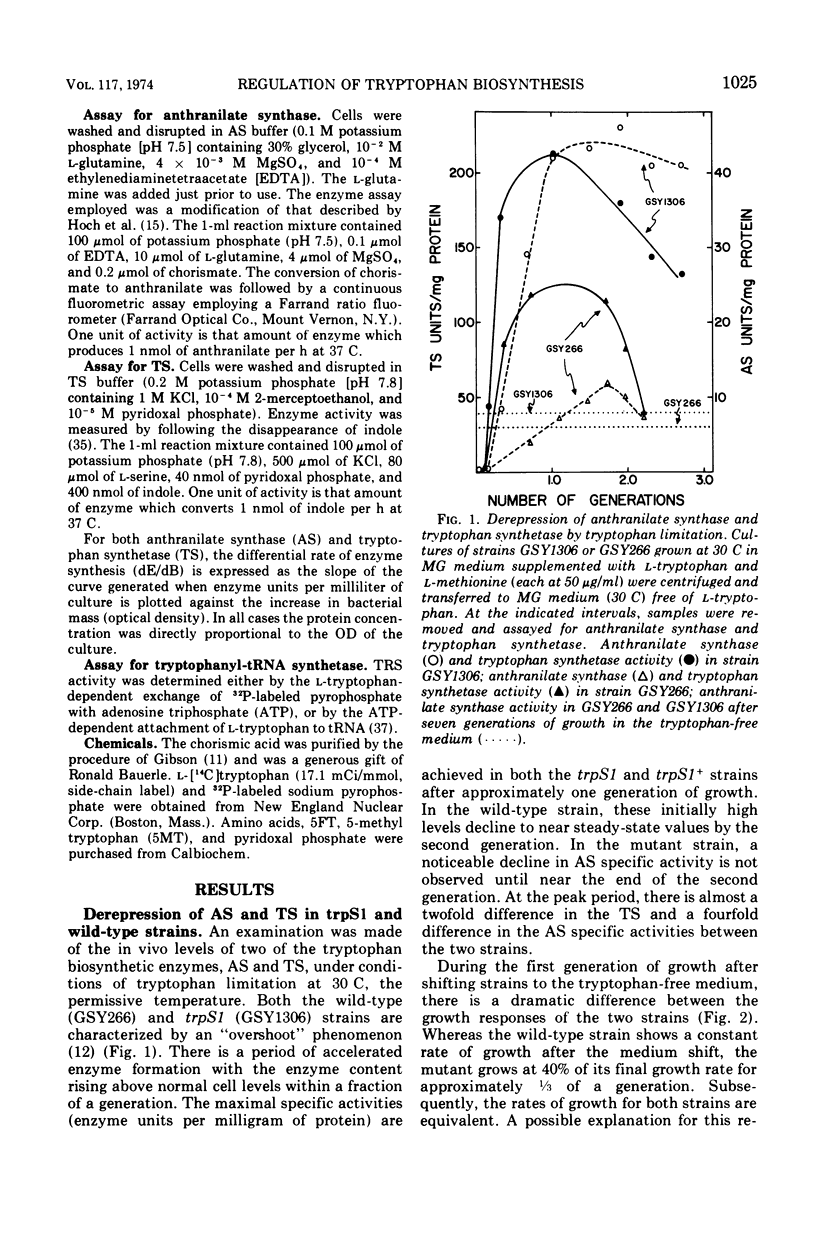
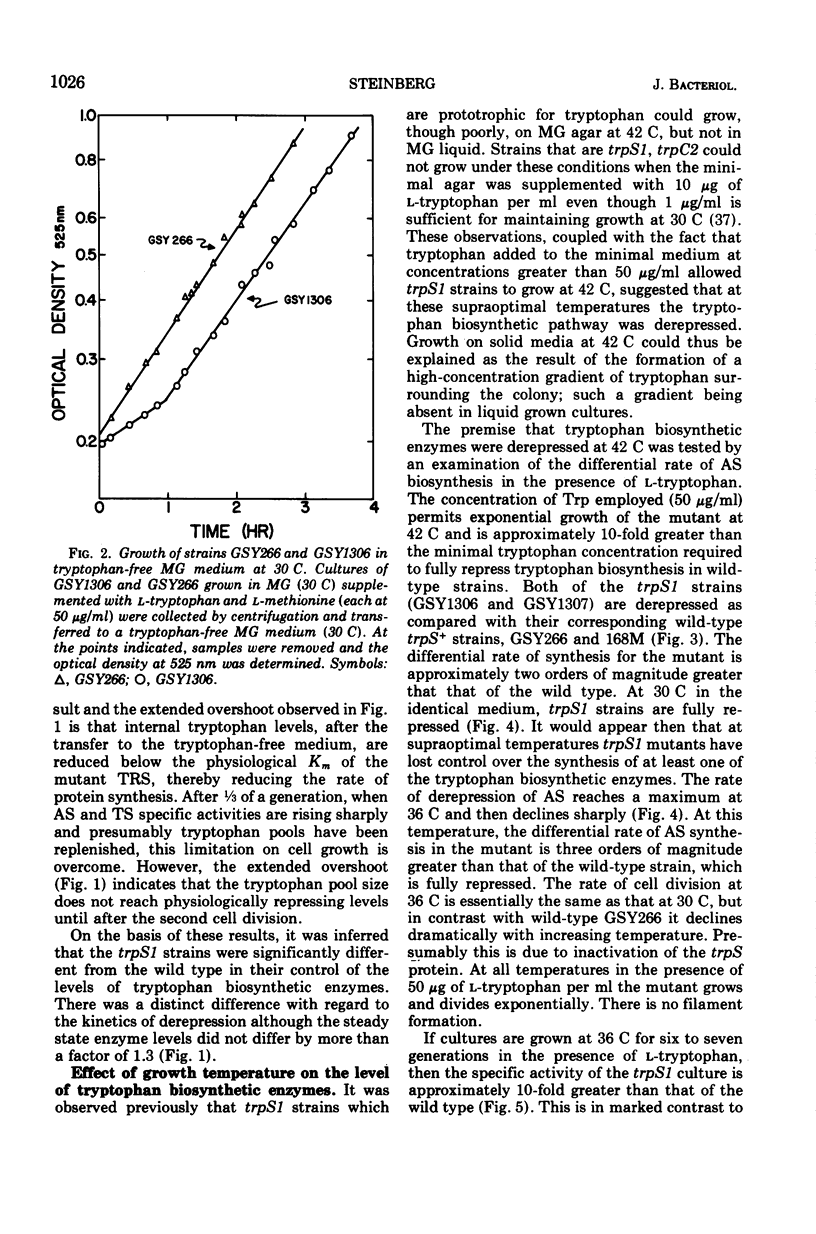
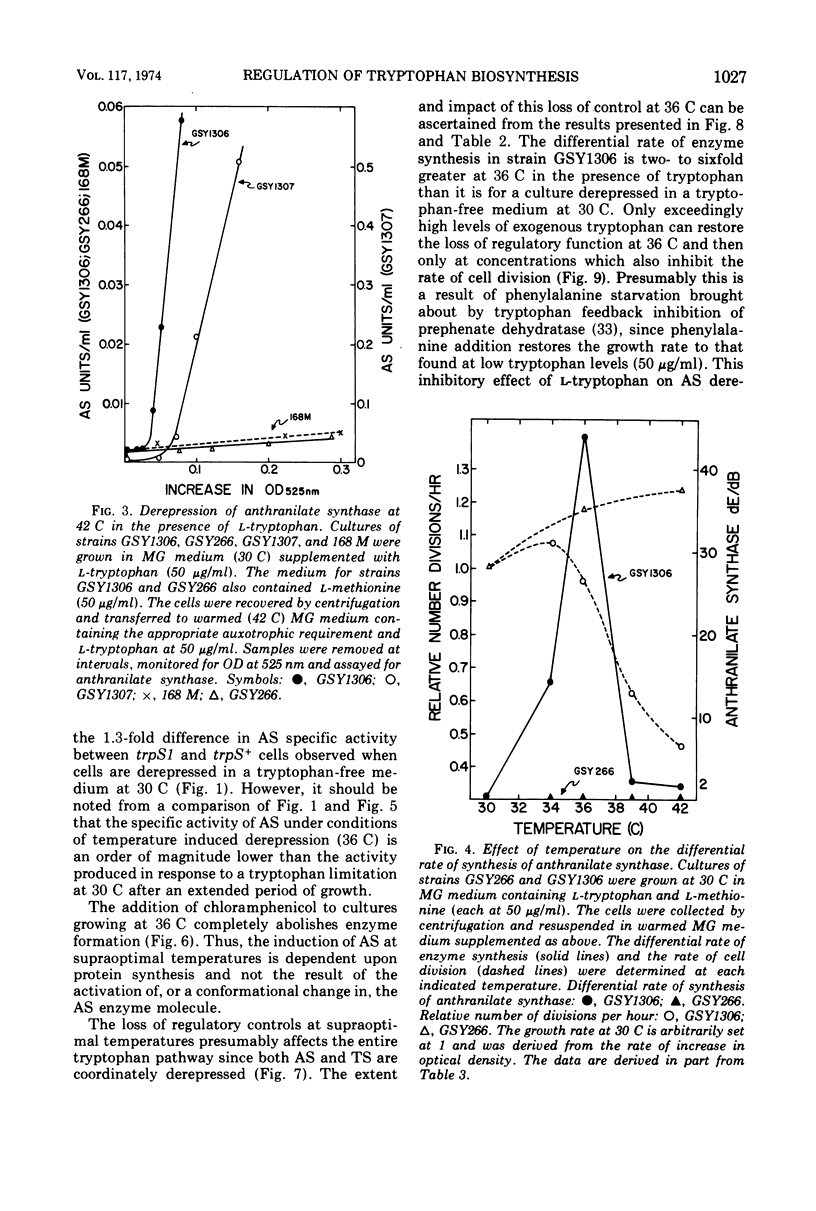
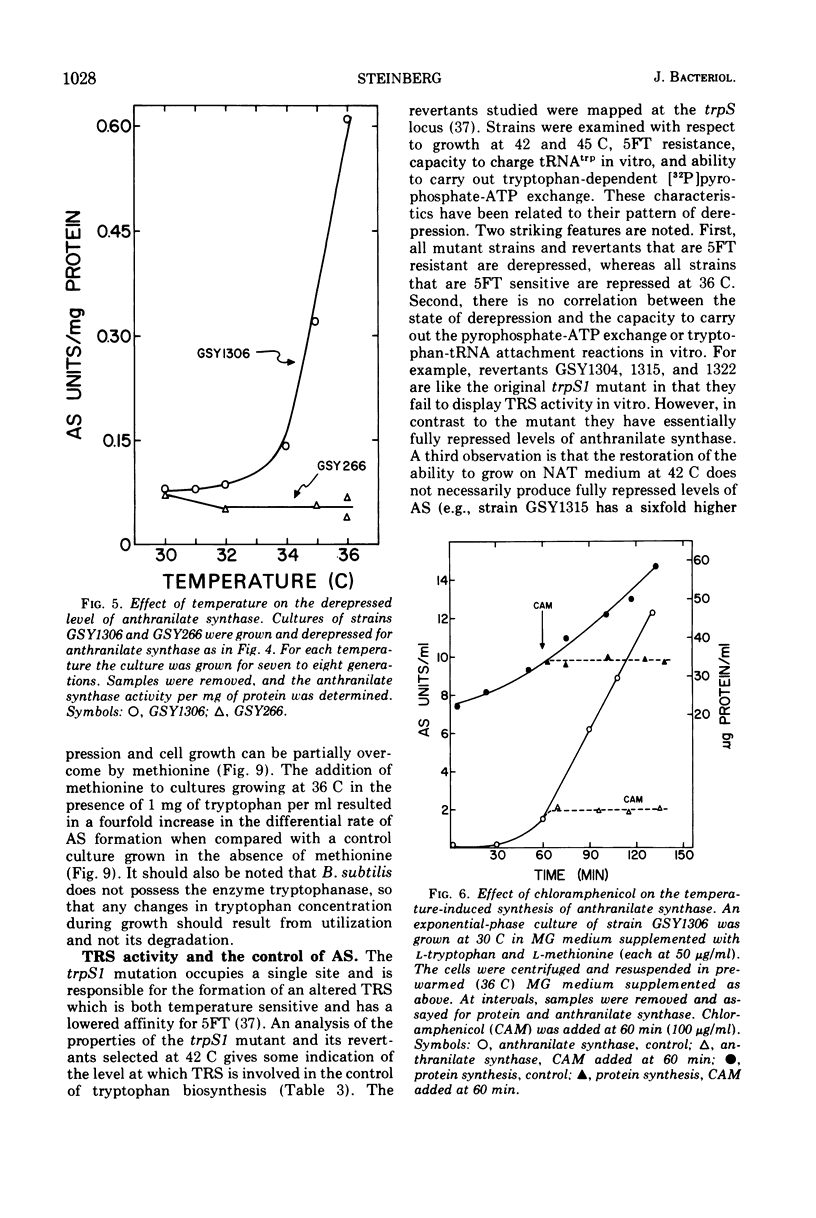
Abstract
A tryptophanyl-transfer ribonucleic acid (tRNA) synthetase (l-tryptophan: tRNA ligase adenosine monophosphate, EC 6.1.1.2) mutant (trpS1) of Bacillus subtilis is derepressed for enzymes of the tryptophan biosynthetic pathway at temperatures which reduce the growth rate but still allow exponential growth. Derepression of anthranilate synthase in a tryptophan-supplemented medium (50 μg/ml) is maximal at 36 C, and the differential rate of synthesis is 600- to 2,000-fold greater than that of the wild-type strain or trpS1 revertants. A study of the derepression pattern in the mutant and its revertants indicates that the 5-fluorotryptophan recognition site of the tryptophanyl-tRNA synthetase is an integral part of the repression mechanism. Evidence for a second locus, unlinked to the trpS1 locus, which functions in the repression of tryptophan biosynthetic enzymes is presented.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander R. R., Calvo J. M., Freundlich M. Mutants of Salmonella typhimurium with an altered leucyl-transfer ribonucleic acid synthetase. J Bacteriol. 1971 Apr;106(1):213–220. doi: 10.1128/jb.106.1.213-220.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostopoulos C., Crawford I. P. Le groupe des gènes régissant la biosynthèse du tryptophane chez Bacillus subtilis. C R Acad Sci Hebd Seances Acad Sci D. 1967 Jul 3;265(1):93–96. [PubMed] [Google Scholar]
- Anderson J. J., Neidhardt F. C. Growth-linked instability of a mutant valyl-transfer ribonucleic acid synthetase in Escherichia coli. J Bacteriol. 1972 Jan;109(1):315–325. doi: 10.1128/jb.109.1.315-325.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlati S., Ciferri O. Incorporation of 5-methyl- and 5-hydroxy-tryptophan into the protein of Bacillus subtilis. J Bacteriol. 1970 Jan;101(1):166–172. doi: 10.1128/jb.101.1.166-172.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt J. M., Umbarger H. E. On the role of isoleucyl-tRNA synthetase in multivalent repression. Biochem Genet. 1972 Apr;6(2):99–118. doi: 10.1007/BF00486395. [DOI] [PubMed] [Google Scholar]
- Camakaris J., Pittard J. Repression of 3-deoxy-D-arabinoheptulosonic acid-7-phosphate synthetase (trp) and enzymes of the tryptophan pathway in Escherichia coli K-12. J Bacteriol. 1971 Aug;107(2):406–414. doi: 10.1128/jb.107.2.406-414.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang G. W., Roth J. R., Ames B. N. Histidine regulation in Salmonella typhimurium. 8. Mutations of the hisT gene. J Bacteriol. 1971 Oct;108(1):410–414. doi: 10.1128/jb.108.1.410-414.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle W. F., Yanofsky C. Mutants of Escherichia coli with an altered tryptophanyl-transfer ribonucleic acid synthetase. J Bacteriol. 1968 Apr;95(4):1283–1294. doi: 10.1128/jb.95.4.1283-1294.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EIDLIC L., NEIDHARDT F. C. PROTEIN AND NUCLEIC ACID SYNTHESIS IN TWO MUTANTS OF ESCHERICHIA COLI WITH TEMPERATURE-SENSITIVE AMINOACYL RIBONUCLEIC ACID SYNTHETASES. J Bacteriol. 1965 Mar;89:706–711. doi: 10.1128/jb.89.3.706-711.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EIDLIC L., NEIDHARDT F. C. ROLE OF VALYL-SRNA SYNTHETASE IN ENZYME REPRESSION. Proc Natl Acad Sci U S A. 1965 Mar;53:539–543. doi: 10.1073/pnas.53.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORINI L., MAAS W. K. The potential for the formation of a biosynthetic enzyme in Escherichia coli. Biochim Biophys Acta. 1957 Jul;25(1):208–209. doi: 10.1016/0006-3002(57)90450-x. [DOI] [PubMed] [Google Scholar]
- Hatfield G. W., Burns R. O. Specific binding of leucyl transfer RNA to an immature form of L-threonine deaminase: its implications in repression. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1027–1035. doi: 10.1073/pnas.66.4.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinonen J., Artz S. W., Zalkin H. Regulation of the tyrosine biosynthetic enzymes in Salmonella typhimurium: analysis of the involvement of tyrosyl-transfer ribonucleic acid and tyrosyl-transfer ribonucleic acid synthetase. J Bacteriol. 1972 Dec;112(3):1254–1263. doi: 10.1128/jb.112.3.1254-1263.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch S. O., Anagnostopoulos C., Crawford I. P. Enzymes of the tryptophan operon of Bacillus subtilis. Biochem Biophys Res Commun. 1969 Jun 27;35(6):838–844. doi: 10.1016/0006-291x(69)90700-1. [DOI] [PubMed] [Google Scholar]
- Hoch S. O., Roth C. W., Crawford I. P., Nester E. W. Control of tryptophan biosynthesis by the methyltryptophan resistance gene in Bacillus subtilis. J Bacteriol. 1971 Jan;105(1):38–45. doi: 10.1128/jb.105.1.38-45.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Hiraga S., Yura T. Temperature-sensitive repression of the tryptophan operon in Escherichia coli. J Bacteriol. 1969 Jul;99(1):279–286. doi: 10.1128/jb.99.1.279-286.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Hiraga S., Yura T. Tryptophanyl transfer RNA synthetase and expression of the tryptophan operon in the trpS mutants of Escherichia coli. Genetics. 1969 Mar;61(3):521–538. doi: 10.1093/genetics/61.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K. Regulatory mechanism of the tryptophan operon in Escherichia coli: possible interaction between trpR and trpS gene products. Mol Gen Genet. 1972;115(4):349–363. doi: 10.1007/BF00333173. [DOI] [PubMed] [Google Scholar]
- Kano Y., Matsushiro A., Shimura Y. Isolation of the novel regulatory mutants of the tryptophan biosynthetic system in Escherichia coli. Mol Gen Genet. 1968;102(1):15–26. doi: 10.1007/BF00341866. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewis J. A., Ames B. N. Histidine regulation in Salmonella typhimurium. XI. The percentage of transfer RNA His charged in vivo and its relation to the repression of the histidine operon. J Mol Biol. 1972 Apr 28;66(1):131–142. doi: 10.1016/s0022-2836(72)80011-1. [DOI] [PubMed] [Google Scholar]
- Lorence J. H., Nester E. W. Multiple molecular forms of chorismate mutase in Bacillus subtillis. Biochemistry. 1967 May;6(5):1541–1553. doi: 10.1021/bi00857a041. [DOI] [PubMed] [Google Scholar]
- Low B., Gates F., Goldstein T., Söll D. Isolation and partial characterization of temperature-sensitive Escherichia coli mutants with altered leucyl- and seryl-transfer ribonucleic acid synthetases. J Bacteriol. 1971 Nov;108(2):742–750. doi: 10.1128/jb.108.2.742-750.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin C. S., Magee P. T., Hartwell L. H. Role of isoleucyl-transfer ribonucleic acid synthetase in ribonucleic acid synthesis and enzyme repression in yeast. J Bacteriol. 1969 Nov;100(2):579–584. doi: 10.1128/jb.100.2.579-584.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosteller R. D., Yanofsky C. Evidence that tryptophanyl transfer ribonucleic acid is not the corepressor of the tryptophan operon of Escherichia coli. J Bacteriol. 1971 Jan;105(1):268–275. doi: 10.1128/jb.105.1.268-275.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazario M., Kinsey J. A., Ahmad M. Neurospora mutant deficient in tryptophanyl-transfer ribonucleic acid synthetase activity. J Bacteriol. 1971 Jan;105(1):121–126. doi: 10.1128/jb.105.1.121-126.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt F. C. Roles of amino acid activating enzymes in cellular physiology. Bacteriol Rev. 1966 Dec;30(4):701–719. doi: 10.1128/br.30.4.701-719.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker J., Neidhardt F. C. Metabolic regulation of aminoacyl-tRNA synthetase formation in bacteria. Biochem Biophys Res Commun. 1972 Oct 17;49(2):495–501. doi: 10.1016/0006-291x(72)90438-x. [DOI] [PubMed] [Google Scholar]
- Rebello J. L., Jensen R. A. Metabolic interlock. The multi-metabolite control of prephenate dehydratase activity in Bacillus subtilis. J Biol Chem. 1970 Aug 10;245(15):3738–3744. [PubMed] [Google Scholar]
- Roth J. R., Ames B. N. Histidine regulatory mutants in Salmonella typhimurium II. Histidine regulatory mutants having altered histidyl-tRNA synthetase. J Mol Biol. 1966 Dec 28;22(2):325–333. doi: 10.1016/0022-2836(66)90135-5. [DOI] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg W., Anagnostopoulos C. Biochemical and genetic characterization of a temperature-sensitive, tryptophanyl-transfer ribonucleic acid synthetase mutant of Bacillus subtilis. J Bacteriol. 1971 Jan;105(1):6–19. doi: 10.1128/jb.105.1.6-19.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel T., Meyers M., Kovach J. S., Goldberger R. F. Specificity of interaction between the first enzyme for histidine biosynthesis and aminoacylated histidine transfer ribonucleic acid. J Bacteriol. 1972 Oct;112(1):126–130. doi: 10.1128/jb.112.1.126-130.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vold B. S. Analysis of isoaccepting transfer ribonucleic acid species of Bacillus subtilis: chromatographic differences between transfer ribonucleic acids from spores and cells in exponential growth. J Bacteriol. 1973 Feb;113(2):825–833. doi: 10.1128/jb.113.2.825-833.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitt D. D., Carlton B. C. Non-coordinate regulation in 5-methyl tryptophan-resistant mutants of Bacillus subtilis. Biochem Biophys Res Commun. 1968 Nov 25;33(4):636–642. doi: 10.1016/0006-291x(68)90343-4. [DOI] [PubMed] [Google Scholar]
- Williams A. L., Williams L. S. Control of arginine biosynthesis in Escherichia coli: characterization of arginyl-transfer ribonucleic acid synthetase mutants. J Bacteriol. 1973 Mar;113(3):1433–1441. doi: 10.1128/jb.113.3.1433-1441.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L. S., Neidhardt F. C. Synthesis and inactivation of aminoacyl-transfer RNA synthetases during growth of Escherichia coli. J Mol Biol. 1969 Aug 14;43(3):529–550. doi: 10.1016/0022-2836(69)90357-x. [DOI] [PubMed] [Google Scholar]


