Abstract
Given that hydroxyapatite (HA) biomaterials are highly efficient at adsorbing proadhesive proteins, we questioned whether functionalizing HA with RGD peptides would have any benefit. In this study, we implanted uncoated or RGD-coated HA disks into rat tibiae for 30 minutes to allow endogenous protein adsorption, and then evaluated mesenchymal stem cell (MSC) interactions with the retrieved disks. These experiments revealed that RGD, when presented in combination with adsorbed tibial proteins (including fibronectin, vitronectin and fibrinogen), has a markedly detrimental effect on MSC adhesion and survival. Moreover, analyses of HA disks implanted for 5 days showed that RGD significantly inhibits total bone formation as well as the amount of new bone directly contacting the implant perimeter. Thus, RGD, which is widely believed to promote cell/biomaterial interactions, has a negative effect on HA implant performance. Collectively these results suggest that, for biomaterials that are highly interactive with the tissue microenvironment, the ultimate effects of RGD will depend upon how signaling from this peptide integrates with endogenous processes such as protein adsorption.
Keywords: Bioadsorption, Bone Tissue Engineering, Cell Adhesion, Hydroxyapatite, Osseointegration, RGD peptide
Introduction
Following surgical placement, hard tissue implants are exposed to blood and other body fluids. An implant’s ability to adsorb proteins from these fluids, and present them in conformations which engage osteogenic cell receptors is an important factor in implant osseointegration [1–3]. It has been suggested that hydroxyapatite (HA) biomaterials are particularly efficient at adsorbing pro-adhesive proteins [4–6], which may contribute to HA’s high degree of osseoconductivity. To model in vivo events, we previously coated HA disks with serum to mimic blood, and evaluated protein adsorption and adhesion of human mesenchymal stem cells (MSCs) [7], a cell type that can differentiate along the osteoblast lineage. These studies indicated that HA adsorbs abundant vitronectin (VN) and fibronectin (FN) from serum [4, 7], and that these proteins are adsorbed in conformations that promote the binding of purified integrins and MSCs [4]. Moreover, MSC adhesion to serum-coated HA is mediated by an αv-containing integrin heterodimer [8], a subtype that binds both VN and FN.
Given the importance of osteogenic cell attachment, a common strategy for improving cell/biomaterial interactions is to functionalize material surfaces with biomimetic peptides such as RGD. RGD is the known integrin recognition site within many cell attachment proteins, including FN, VN and Fibrinogen (Fbg) [9–11]. Numerous studies have shown that RGD peptides promote increased binding of osteogenic cells, including MSCs, to many types of biomaterials [3, 12, 13]. For example, we and others have reported that RGD-modified HA stimulates better cell adhesion as compared with naive HA [7, 14–19]. However, in vivo, any biomimetic peptide tethered to the HA surface would be presented to MSCs within the context of an adsorbed protein layer. To model this process in vitro, we previously monitored MSC attachment to HA surfaces coated sequentially with RGD and serum [7]. Surprisingly, we found that disks coated with high concentrations of RGD, followed by serum, supported less cell adhesion and spreading than disks coated with serum alone [7], suggesting that the presence of RGD had some inhibitory effect on MSC interactions with HA. Importantly, this effect was observed with three variants of RGD; a linear peptide (GRGDdSP) [7], a cyclic peptide (GPenGRGDSPCA) [7], and a peptide expressing an HA-binding domain (EEEEEEEGPenGRGDSPCA) [19].
The pro-adhesive proteins FN and VN are known to be abundant within both serum and blood, however, there are significant differences in the concentration of other molecules within these fluids. Thus, the use of serum as an in vitro model for the blood overcoating that occurs during implantation requires validation. To address this issue, we monitored the adhesion of MSCs to uncoated or RGD-coated HA disks that had been briefly implanted into tibial osteotomies, to allow for protein adsorption from within the bone milieu. In addition, disks were implanted into tibiae for longer time intervals to evaluate bone growth at the implant interface. Our results indicate that, when presented within the context of an adsorbed protein layer, RGD has a detrimental effect on both MSC adhesion and new bone synthesis at the implant site.
Materials and Methods
Peptide preparation
RGD peptides (GPenGRGDSPCA, 948.1g/mol, American Peptide) were reconstituted in ddH2O at 1mg/mL, aliquotted and stored at −20°C
Disk preparation
Clinical grade HA powder (Fisher Scientific) was pressed into disks as previously described for in vitro studies [7], or using a 3mm steel hardened die, under 1000 psi for in vivo studies. Pressed disks were coated with RGD peptide as previously described [7]. The disks were subsequently washed with phosphate-buffered saline (PBS) to remove unbound peptide, and warmed to 37°C prior to incubation with cells, or insertion into tibial osteotomies.
Cell culture
As previously described [4], MSCs were isolated from human bone marrow samples with approval from the University of Alabama Institutional Review Board. Cells from passages 3–13 were used for all experiments.
Animal surgeries and histology
Bone formation on HA implants was evaluated using a rat tibial implant model due, in part, to the relative ease and inexpensive of this system, as well as the comparability of the model to humans. Rat tibial implantation has been extensively employed in investigations of implant integration, including those focused on RGD-modified biomaterials. For our studies, 6–8 month-old male Sprague-Dawley rats were anesthetized with isoflourane, and a 3.25mm × 2.1mm osteotomy was created in the proximal tibia using a Vetroson dental drill fitted with a size 8 burr. HA disks were inserted into the osteotomies (without additional fixation) and left in place for either 30 minutes or 5 days. Only one implant was placed per animal. Implants were placed into the intramedullary region of the bone, although variability in parameters such as the size of individual tibiae and surgical technique did sometimes influence the exact location of disk placement. All experiments were executed in accordance with guidelines established by the University of Alabama Institutional Animal Care and Use Committee.
HA disks implanted for 30 minutes were retrieved from the osteotomies and then washed extensively in PBS with agitation. The disks were subsequently subjected to cell adhesion assays as described below. At least 5 disks were implanted and analyzed for each of the three treatment groups (uncoated HA, 1 µg/ml RGD coated HA, and 1000 µg/ml coated RGD).
For the 5-day implants, tibiae were retrieved (with disks in place), and embedded in either paraffin for hematoxylin and eosin (H&E) staining, or in poly(methyl methacrylate) for Goldner’s trichrome staining. For H&E staining, three implants were evaluated per treatment group (9 animals total). For Goldner’s trichrome, which stains mineralized tissue green, 5 implants were analyzed for each of the three treatment groups (15 animals total), with at least two tissue sections per implant evaluated.
The amount of total new bone surrounding 5-day implants, as well as the amount of bone in direct contact with the implant perimeter, were quantified from Goldner’s stained sections using Bioquant imaging software. Briefly, images of the tibiae, with the implant centered in the field, were taken at a 4X magnification. The area of the tissue in the field, with the area of the implant removed, was quantified to determine total tissue area. The area of new bone formation, as evidenced by the green staining (excluding the pre-existing cortical bone), was then measured, and quantified in relation to the total tissue area. For perimeter contact measurements, the perimeter of the implant was quantified. The areas of contact between the implant and the new bone were then measured and quantified in relation to the total perimeter of the implant.
Adhesion and morphology of MSCs seeded onto implanted HA disks
Disks retrieved from tibial osteotomies after a 30 minute implantation were washed to remove debris and loosely-bound proteins. Human MSCs were seeded onto the disks in serum-free media and allowed to adhere for 1 hr. Following this incubation, unbound cells were removed with three PBS washes with agitation unless otherwise indicated. The adherent cells were subsequently fixed in 3.7% formaldehyde, permeabilized with 0.2% Triton-X-100, and stained with phalloidin-Alexa 488 and DAPI (Molecular Probes). The samples were mounted with 4.7mM n-propyl-gallate, and visualized using a Nikon fluorescent microscope. Cell adhesion was quantified by counting the number of cells per microscopic field.
Western blotting of desorbed tibial proteins
Retrieved disks were washed, and proteins remaining on the surface were solubilized in boiling SDS-buffer (50mM Tris buffer, 2% SDS, 5% β-mercaptoethanol) for 30 minutes with agitation. Desorbed proteins were resolved by SDS-PAGE, transferred to PVDF membranes, and then blotted with antibodies against fibronectin (Chemicon), vitronectin (Santa Cruz), or fibrinogen (Abcam). An HRP-conjugated secondary antibody was subsequently added and proteins were detected by enhanced chemiluminescence (Amersham Life Sciences).
Blockade of cell adhesion by soluble RGD peptides
RGD release from the HA surface was monitored through multiple reaction monitoring – liquid chromatography mass spectrometry (MRM-LCMS). Briefly, disks were coated with RGD peptide, washed, and incubated for 1 hour in serum-free media to reproduce conditions of a cell adhesion assay. The media was then retrieved, and the amount of peptide in solution was determined by comparing readings to a standard curve. To determine the amount of RGD peptide required for blockade of cell attachment, MSCs (pre-labeled with a fluorescent dye, CMFDA, Molecular Probes) were seeded onto FBS-coated HA disks in serum-free media containing varying concentrations of soluble RGD peptide. After 1 hr, cells were lysed in 1% TX-100 in 50mM Tris to release the fluorescent dye into solution, and fluorescence was quantified on a fluorometer.
ELISA
HA disks were coated with RGD, FBS or sequentially-coated RGD/FBS as previously described [7]. Following the coatings, the disks were washed and blocked with denatured BSA. Disks were incubated with purified human α5β1 or αvβ3 (Chemicon) for 1 hr. Disks were then washed and exposed to antibodies for α5β1 or αvβ3 (Chemicon); followed by an HRP-conjugated secondary antibody. A colorimetric substrate was added, and the absorbance read at 450 nm.
Caspase 3 activation
MSCs were seeded onto HA disks previously coated with RGD, FBS or RGD/FBS. After 24 hours at 37°C, disks were washed and treated with boiling SDS-buffer to solubilize the adherent cells. Cellular proteins were resolved on a 17% polyacrylamide gel, transferred to PVDF membranes, and active (cleaved) caspase 3 was detected using an antibody from Cell Signaling.
Statistical analysis
For cell adhesion assays performed on retrieved disks, at least 5 implants per treatment group were evaluated. For measurements of bone formation, 5 implants per treatment group were subjected to Bioquant software analyses of Goldner’s trichrome-stained sections, with at least 2 sections per implant analyzed. For all other graphical data, at least 3 independent experiments were performed, with each experiment performed in triplicate. Data were plotted as mean + s.e.m., and a One-Way ANOVA parametric analysis was used to calculate statistics. A confidence level of 95% (p<0.05) was considered significant.
Results
MSC adhesion to HA disks coated with proteins from the tibial microenvironment
Our prior studies indicated that MSCs adhere and spread better on HA disks coated with serum as compared with uncoated or RGD-coated surfaces [7], presumably due to the presence of adsorbed serum FN and/or VN. In addition, we found that when disks were sequentially coated with RGD/serum, RGD inhibited cell adhesion to adsorbed serum proteins [7]. To determine whether similar cell responses were elicited by endogenous proteins, HA disks were implanted into rat tibial osteotomies for 30 minutes to allow protein adsorption; the disks were then retrieved, washed, and MSCs were seeded onto the disks and allowed to adhere. Prior to implantation, disks were pre-coated with either low or high concentrations of RGD (1 µg/ml or 1000 µg/ml, respectively), or alternately left uncoated. A comparison of MSC adhesion on the retrieved disks indicated that disks initially left uncoated, then overcoated with endogenous tibial proteins, promoted significantly greater MSC adhesion than either of the disks that had been pre-coated with RGD prior to implantation (Figs.1a and b). As a control, we also evaluated MSC adhesion on uncoated or RGD-coated disks that had not been placed into tibiae. As shown (Fig.1c), RGD alone was not able to induce cell spreading, a response that reflects full integrin activation and also contributes to strong cell adhesion [20, 21]. Taken together the results in Figure 1 suggest that MSCs adhere and spread better on adsorbed endogenous proteins than on RGD alone, and importantly, when RGD is combined with endogenous proteins, RGD appears to have a strong inhibitory effect on MSC attachment.
Figure 1. RGD peptides inhibit cell adhesion to HA disks coated with proteins from the tibial microenvironment.
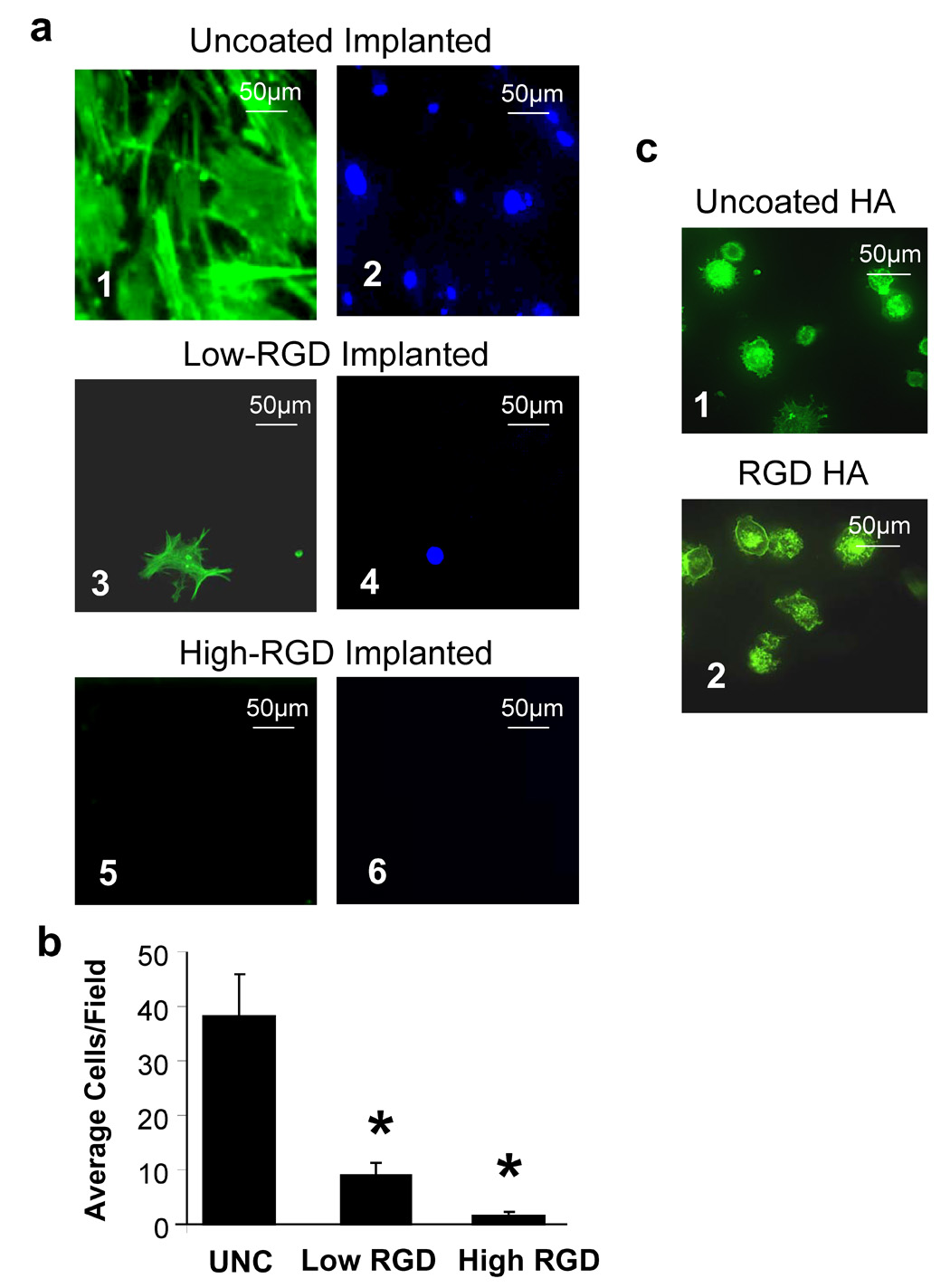
a, Representative images of MSCs adherent to HA disks retrieved from tibial osteotomies. Prior to implantation, disks were left uncoated (panels 1,2), or coated with either 1µg/mL RGD (“low RGD”, panels 3,4), or 1000 µg/mL RGD (“high RGD”, panels 5,6). Cells were double-labeled with phalloidin-Alexa 488 (green stain, panels 1, 3 and 5) and DAPI (blue stain, panels 2, 4, 6) b, Cells adherent to the retrieved disks were quantified by counting the average number of cells per field. * denotes significant difference from uncoated samples. c, Phalloidin-stained cells adherent to uncoated (panel 1), or RGD-coated (panel 2), HA disks in the absence of implantation.
Effect of RGD on the adsorption of proadhesive proteins
We speculated that poor cell attachment to retrieved HA disks pre-coated with RGD might have resulted from RGD blockade of protein binding sites on HA, thus reducing the amount of adsorbed endogenous proteins. To evaluate protein adsorption, HA disks retrieved from tibiae were incubated in SDS buffer to desorb proteins, and the amounts of FN, VN and Fbg were assessed by Western blotting. As shown (Fig.2), low concentrations of RGD pre-coatings did not have any inhibitory effect on the adsorption of FN, VN or Fbg, although the high RGD coatings did slightly diminish VN and Fbg deposition. Thus, MSC adhesion to disks retrieved from tibiae was inhibited by the presence of RGD peptides despite an abundance of proadhesive proteins on the HA surface. As well, the marked inhibition of cell adhesion by low concentrations of RGD (see Figs.1a and b), which do not block protein adsorption (Fig.2), suggests that diminished protein adsorption is not the major mechanism by which RGD attenuates MSC binding to implanted HA disks.
Figure 2. Pre-coating HA with RGD has a minimal effect on the adsorption of proadhesive proteins from the tibial microenvironment.
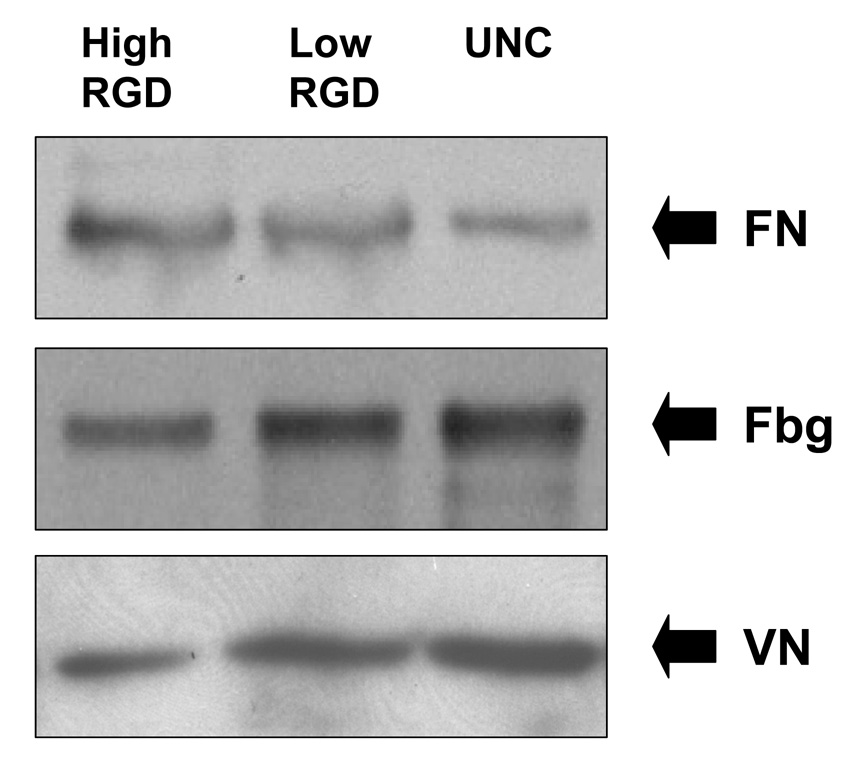
Western blots of fibronectin (FN), fibrinogen (Fbg) and vitronectin (VN) following desorption from HA disks that were implanted into tibiae for 30 minutes.
Strength of cell attachment on RGD-coated HA
We hypothesized that RGD peptides on HA might compete with adsorbed proteins for integrins on the MSC surface. RGD peptides are known to promote weaker integrin activation than full-length adhesive proteins [20, 21], therefore it follows that if a majority of integrin receptors was bound with RGD rather than FN or VN, this might result in attenuated integrin signaling and weaker cell attachment. Consistent with standard methods for monitoring cell adhesion, our protocol includes a wash step at the end of the attachment interval to remove unbound cells. It was possible that, in the case of RGD-modified HA, loosely-bound cells were also removed during this step. To test this, MSCs were allowed to adhere to RGD-modified retrieved disks, and then disks were washed very gently. This experiment was performed side-by-side with our standard protocol, which includes several washes with agitation. As shown in Fig.3, more MSCs were present on the gently-washed retrieved disks (“low stringency wash”) as compared with disks subjected to a standard wash protocol. Importantly, even after a gentle wash, there were fewer cells, and these were significantly less spread, than cells adherent to disks coated with endogenous proteins only (compare Fig.3 with Fig.1a, panel 1). These data suggest that disks coated with endogenous proteins only (i.e., no RGD) stimulate greater integrin activation and stronger cell adhesion than disks coated with RGD prior to implantation.
Figure 3. The presence of RGD weakens cell attachment to retrieved HA disks.
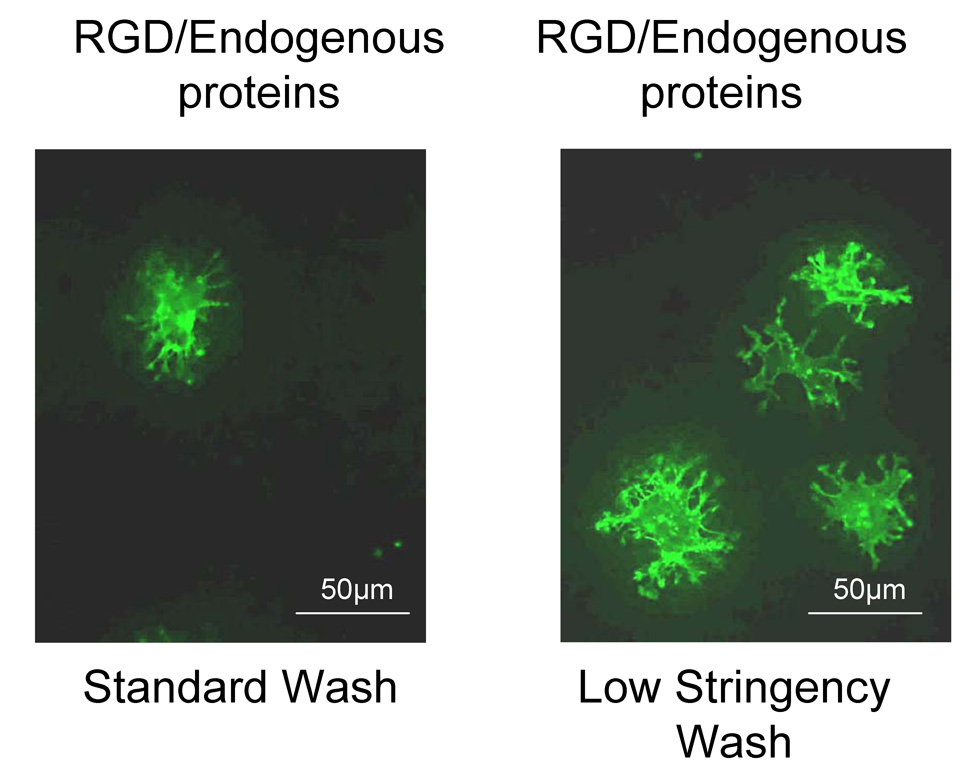
Representative images of cells adherent to retrieved disks following exposure to either a standard or low stringency wash protocol.
Effect of RGD on new bone synthesis and bone/implant contact
The adhesion of osteogenic cells to orthopaedic and dental biomaterials is a significant factor in implant osseointegration. To test whether the weak cell adhesion associated with RGD pre-coatings (Fig.3) had any effect on implant integration, uncoated and RGD-coated HA disks were placed in tibial osteotomies for 5 days. The tibiae, with implants in place, were then retrieved, and new bone deposition on the HA surface was measured by either H & E staining or Goldner’s Trichrome. Images of H & E-stained sections (Fig.4a) showed trabecular-like bone (pink staining) in apposition to the perimeter of HA disks that were left uncoated prior to implantation. In contrast, there was a marked dearth of bone-like tissue surrounding RGD-coated implants. To more definitively assess bone formation, sections stained with Goldner’s trichrome, which is highly specific for mineralized tissue (green staining), were subjected to Bioquant imaging analysis. Specifically, Bioquant software was used to quantify the total amount of newly-synthesized bone in the vicinity of the implant, as well as the percentage of the implant surface that was in direct contact with bone. As shown in Figs.4b and c, both the low and high RGD peptide coatings significantly inhibited the total amount of new bone formed, as well the amount of bone directly contacting the HA surface.
Figure 4. RGD peptides inhibit osseointegration of HA implants.

a, Representative images of tibiae with embedded HA disks following a 5-day implantation. Sections were stained with hematoxylin and eosin. b, Representative images of 5-day implants stained with Goldner’s trichrome, which stains mineralized tissue green c, The amount of total new bone surrounding the implant (white bars), and the amount of bone directly contacting the perimeter of the implant (black bars) were quantified using Bioquant software. * denotes significant difference from uncoated samples.
Influence of RGD on integrin binding sites within adsorbed proteins
There are multiple mechanisms by which the presence of RGD in combination with adsorbed endogenous proteins might contribute to diminished cell attachment. We next tested the hypothesis that RGD peptides on the HA surface cause a disruption in conformation of adsorbed FN and VN, thus diminishing the accessibility of the integrin binding site within these proteins. Because of the large number of samples required for mechanistic studies, we used serum as an in vitro model for the overcoating of blood that happens in vivo on the implant surface. To this end, disks were pre-coated with RGD, serum (FBS), or a sequential RGD/FBS coating, and then the binding of purified integrin receptors to the disks was quantified by ELISA. We evaluated the binding of two integrins, αvβ3 which binds to VN (in addition to other matrix molecules including FN), and α5β1, which binds to FN. Results from these experiments revealed that both αvβ3 and α5β1 integrins bound significantly better to FBS-coated surfaces than to RGD-coated surfaces (Fig.5a and b), consistent with the fact that full-length FN and VN are known to promote stronger integrin binding than the isolated RGD sequence [20, 21]. However, there was no significant decrease in integrin binding to RGD/FBS sequential-coatings as compared to FBS alone (Fig.5a and b), suggesting that the presence of RGD on the HA surface does not disrupt the availability of the integrin binding site on adsorbed proadhesive proteins.
Figure 5. RGD peptides do not disrupt accessibility of integrin binding sites on adsorbed proadhesive proteins.
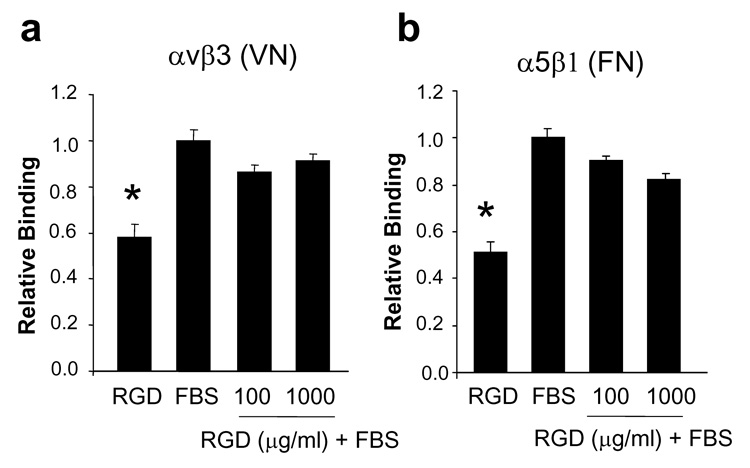
a, Purified αvβ3 integrin binding to HA disks coated with RGD, FBS, or sequential RGD/FBS. b, Purified α5β1 integrin binding to disks coated with RGD, FBS, or sequential RGD/FBS. * in panels a and b denotes difference from FBS-coated samples.
RGD release from the HA surface
We next questioned whether RGD peptides might be released from the HA surface in sufficient quantities to bind MSCs in solution and block cell attachment. To examine this possibility, HA disks were pre-coated with RGD peptide, and then incubated in serum-free media to reproduce the conditions of a cell adhesion assay. At the end of this incubation, the solution was collected and the concentration of released RGD peptide was determined by MRM-LCMS, through comparison with a standard curve. It was found that approximately 100–200 ng/mL of peptide were released into solution (data not shown). To determine if this amount of soluble RGD was sufficient to block cell adhesion to protein-coated HA, MSCs were seeded onto FBS-coated HA disks in media containing varying concentrations of soluble RGD to allow blockade of integrin receptors. MSC adhesion was then quantified as previously described [7, 19]. Results from these experiments showed that RGD concentrations up to, and including, 1 µg/ml had no significant effect on cell adhesion (Fig.6). Thus, the amount of RGD released from the HA surface under the conditions of our adhesion assays is many-fold less than the amount required to significantly diminish MSC attachment to protein-coated HA.
Figure 6. RGD peptides released from the surface of HA do not significantly inhibit cell adhesion.
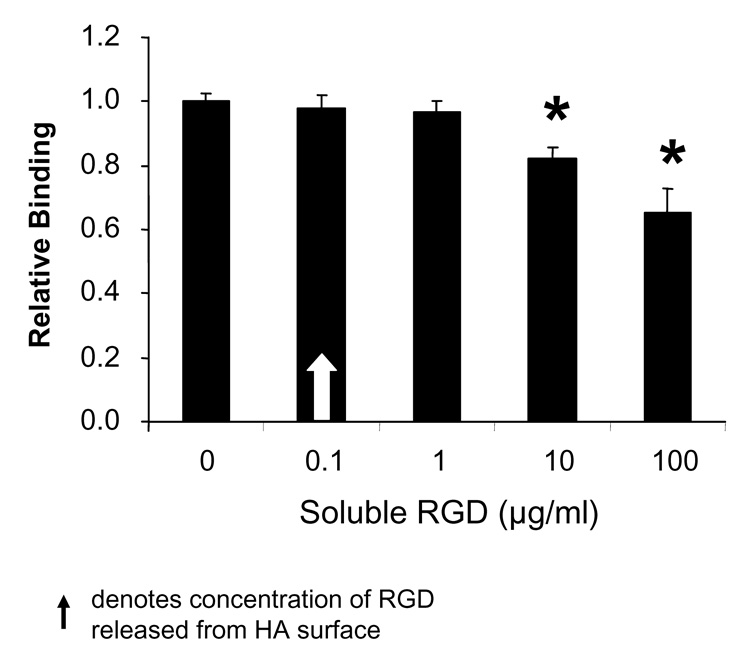
Cell adhesion to FBS-coated HA disks in the presence of varying concentrations of soluble RGD. * denotes significant difference from cell adhesion in the absence of soluble RGD peptide.
Cell apoptosis on RGD-coated HA
While the collective results described above suggested that RGD inhibits implant integration through inducing weak cell attachment, we also questioned whether RGD might affect cell survival. Interestingly, it was reported that adherent cells that either have unliganded integrins, or integrins bound to inappropriate ligands, undergo apoptosis [22]. Accordingly, we speculated that cells adherent to RGD for extended intervals might perceive RGD as an “inappropriate” signaling ligand. To test this hypothesis, MSCs were seeded onto HA disks coated with either FBS or sequentially coated with RGD/FBS, and apoptosis was evaluated by monitoring caspase 3 activation. Pre-coating HA disks with RGD induced significantly greater caspase 3 activation (Fig.7), indicating that the presence of RGD on the HA surface, when presented in the context of adsorbed proteins, induces apoptosis.
Figure 7. RGD peptides initiate apoptotic signaling cascades.
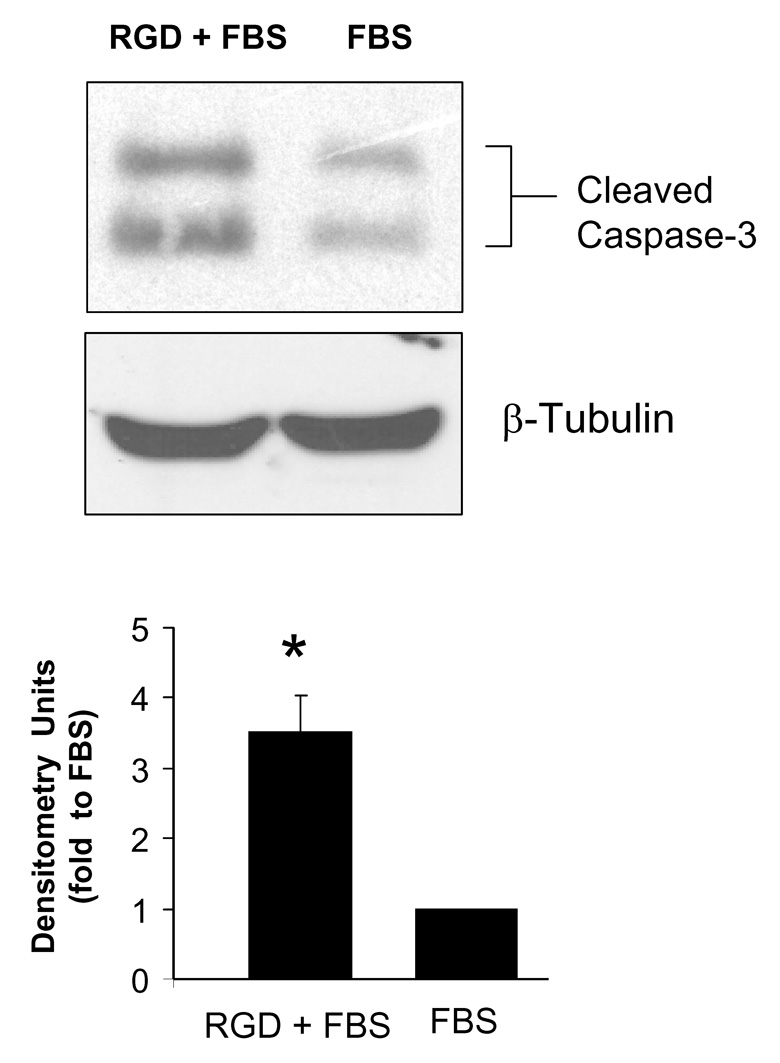
a, Representative western blot of active (cleaved) caspase 3 in cells grown for 24 hours on HA disks coated with either FBS or sequentially coated with RGD/FBS. b, Densitometric analysis of western blots. * denotes significant difference from FBS
Discussion
HA is highly osseoconductive, and we hypothesize that this is partially due to the fact that calcium-phosphate biomaterials adsorb proteins from the microenvironment that assist in bone regeneration. To model protein adsorption from body fluids, many investigators have characterized protein adsorption from serum. We and others have shown that HA adsorbs more FN and VN from serum than materials such as titanium, stainless steel, or poly(l-lactic acid) [4–6], and preincubation of HA with either protein significantly enhances osteogenic cell attachment [7, 23, 24] Moreover, adsorption of serum proteins protects cells from apoptosis [6], presumably through induction of cell survival signals elicited by engaged and activated integrins. Cell adhesion to serum-coated HA surfaces is RGD-dependent [25], and inhibited by function-blocking antibodies against the αv integrin subunit [8], suggesting that cells adhere via adsorbed FN and/or VN. Our current results show that HA adsorbs abundant FN, VN, and Fbg within the first 30 minutes of implantation in the tibial environment, and that adsorption of endogenous proteins is required for optimal MSC adhesion and spreading.
FN, VN and Fbg, representing the most abundant adhesion-promoting proteins in blood [26–28] bind to integrins through an RGD-dependent mechanism [9, 10]. However, in addition to the requisite RGD sequence, there are multiple other domains within these proteins that bind to integrins and either synergistically or additively stimulate integrin signaling [11, 29, 30]. Hence, the RGD sequence by itself elicits weaker integrin activation than full length adhesion proteins [20, 21]. For example, integrin binding to full length FN and VN activates the downstream signaling molecules FAK and ERK [31], leading to the induction of osteogenic gene expression [32], alkaline phosphatase activity, calcium deposition [33, 34], and runx2 activation [33]. In contrast, cell adhesion to RGD was shown to activate FAK, but not ERK [35]. In light of these observations, our initial prediction was that RGD peptides would have little effect on cell adhesion to HA implants, given that HA would adsorb adhesion proteins in vivo, and that molecular cues from these adsorbed proteins would likely over-ride signaling from RGD. To test this hypothesis, we implanted uncoated or RGD-coated HA disks in tibiae to allow endogenous protein adsorption, retrieved the disks and then monitored MSC attachment. Surprisingly, we found that RGD peptides negatively impacted cell adhesion. The mechanisms underlying this finding are not currently understood, however our results appear to argue against several possibilities. First, the presence of RGD coatings on the HA disks had little effect on the adsorption of FN, VN or Fbg from the tibial microenvironment. Thus, cell adhesion was attenuated despite the presence of abundant adhesion proteins on the HA surface. Secondly, in vitro ELISA-type assays using purified α5β1 and αvβ3 receptors indicated that RGD pre-coatings did not significantly disrupt the accessibility of integrin binding sites on the full-length proteins, suggesting that loss of cell adhesion was not due to conformational-disruption of adsorbed proteins. Finally, the blockade in cell attachment did not appear to be due to release of soluble RGD peptides from the HA surface, a process which could theoretically block cell attachment to HA by saturating the integrins of cells still in suspension.
Our working hypothesis is that there is competition between RGD and adsorbed proteins for cell surface integrins, and that, as the concentration of RGD increases, more integrins become bound with RGD rather than full-length proteins. In turn, this causes attenuated integrin signaling, leading to a lack of full cell spreading and weaker overall cell attachment (see Figure 8 for model). This concept is supported by current results showing that wash steps more readily removed cells from disks coated sequentially with RGD/endogenous proteins as compared with endogenous proteins alone. Additionally, the cells that did remain bound to RGD-modified surfaces following wash steps were more poorly spread than those attached to HA disks coated with endogenous proteins only.
Figure 8. Model describing negative effects of RGD on implant osseointegration.
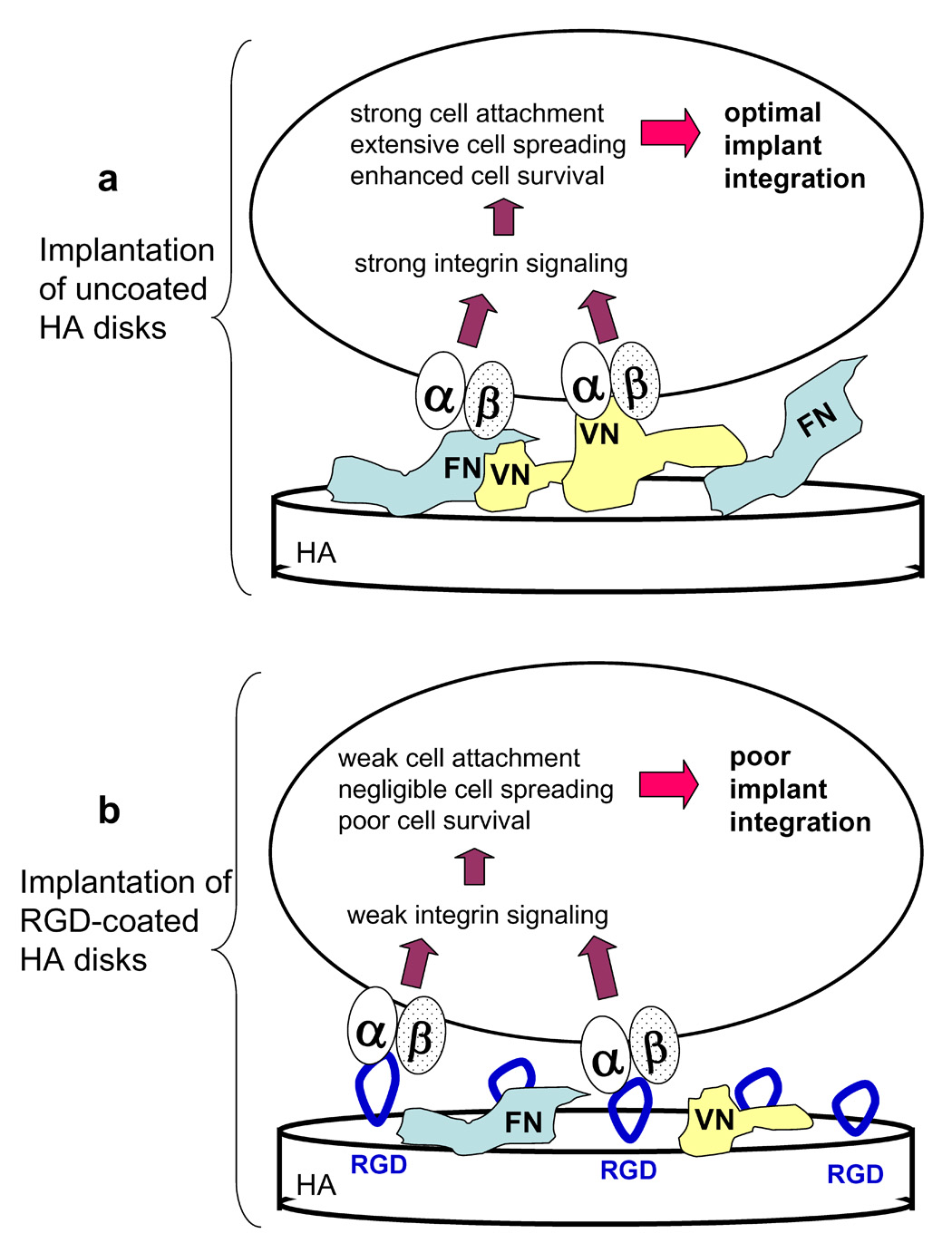
a, When adsorbed native matrix proteins, such as FN and VN, bind to integrin receptors on the MSC surface, this induces robust integrin-dependent signaling, leading to strong cell adhesion, cell spreading and initiation of survival signals. These events are crucial for osteoblastic differentiation of MSCs and deposition of a bone matrix on the implant surface. b, When RGD peptides are coupled to the biomaterial surface, there is competition between RGD and native adsorbed proteins for binding to integrin receptors. If a high proportion of integrins are bound with RGD rather than native proteins, then weak integrin signaling will ensue, resulting in poor cell adhesion and spreading, increased cell apoptosis, and ultimately, poor osseointegration.
Initial cell attachment is an essential first step in osseointegration, however there are many other factors that ultimately influence implant fixation. To test the effects of RGD on implant integration, we placed uncoated and RGD-coated HA disks in tibiae, and then monitored bone formation 5 days later. These experiments revealed that RGD peptides had a strong inhibitory effect on both the total amount of new bone formed, and the amount of bone directly contacting the implant perimeter. These data are consistent with the hypothesis that RGD inhibits osteogenic cell attachment, however we speculated that other events may also play a role in this process. We were particularly intrigued by work from Stupack et al. which described a phenomenon known as “integrin-mediated death”, a process whereby adherent cells with unliganded integrins or integrins bound with “inappropriate” ligands undergo apoptosis [22]. Based on this work, we questioned whether RGD peptides might be saturating integrin receptors, preventing binding to full-length FN, VN or Fbg, and that in turn, cells might perceive RGD as an inappropriate signaling ligand. Indeed, we found that the presence of RGD peptides caused greater activation of the apoptotic marker, caspase 3. Thus, our collective results suggest that RGD peptides, by competing with adsorbed proteins for integrin receptors, have a negative effect on implant integration by reducing both the initial attachment and survival of osteogenic cells on HA surfaces.
Interestingly, despite extensive in vitro results describing a beneficial effect for RGD, the number of animal studies aimed at assessing the performance of RGD-modified biomaterials is limited. In general, these studies support the view that RGD increases implant integration [36–42]. However, in some instances, RGD peptides either had no effect on new bone synthesis [43], or were actually detrimental [44]. For example, RGD peptides were reported to inhibit peri-implant bone formation on polymer-coated titanium surfaces [44]. Clearly there are multiple factors that could influence the bioactivity of RGD in vivo including peptide density, the amino acid sequences flanking the RGD domain, and the stability of peptide bonding to the material surface. However, in addition to these factors, we hypothesize that interactive processes between the material surface and host tissue may have contributed to some of the variable results previously reported for in vivo studies using RGD.
Conclusions
The broad implication of the current investigation is that the potential benefits of RGD with regard to implant osseointegration will likely be context-dependent. For biomaterials that are highly interactive with the tissue microenvironment, the effects of RGD will depend upon how signaling from these peptides integrates with endogenous processes such as protein adsorption. Accordingly, there is a compelling need to study and characterize these endogenous processes in order to gain meaningful predictive information about biomaterials performance. This concept is strikingly illustrated by the fact that, in the absence of adsorbed proteins, RGD consistently improves cell adhesion to HA, whereas in contrast, RGD is markedly detrimental when presented in combination with adsorbed proteins.
Acknowledgments
This research was supported by NIH/NIAMS grant R01AR51539 (SLB) and a Predoctoral NRSA from NIBIB (KMH). The authors gratefully acknowledge the Bone Histomorphometry Core Facility for their assistance with tissue processing and staining and the Mass Spectrometry Core Facility for assistance with MRM-LCMS (Purchase of the API-4000 mass spectrometer was provided by a grant to Dr. Stephen Barnes from the UAB Health Services Foundation. The operation of the Shared Facility was provided by a NCI Core Support grant to the UAB Comprehensive Cancer Center (P30 CA13148)).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Garcia AJ. Get a grip: integrins in cell-biomaterial interactions. Biomaterials. 2005;26(36):7525–7529. doi: 10.1016/j.biomaterials.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 2.Bagambisa FB, Kappert HF, Schilli W. Cellular and molecular biological events at the implant interface. J Craniomaxillofac Surg. 1994;22(1):12–17. doi: 10.1016/s1010-5182(05)80290-2. [DOI] [PubMed] [Google Scholar]
- 3.LeBaron RG, Athanasiou KA. Extracellular matrix cell adhesion peptides: functional applications in orthopedic materials. Tissue Eng. 2000;6(2):85–103. doi: 10.1089/107632700320720. [DOI] [PubMed] [Google Scholar]
- 4.Kilpadi KL, Chang PL, Bellis SL. Hydroxylapatite binds more serum proteins, purified integrins, and osteoblast precursor cells than titanium or steel. J Biomed Mater Res. 2001;57(2):258–267. doi: 10.1002/1097-4636(200111)57:2<258::aid-jbm1166>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 5.Matsuura T, Hosokawa R, Okamoto K, Kimoto T, Akagawa Y. Diverse mechanisms of osteoblast spreading on hydroxyapatite and titanium. Biomaterials. 2000;21(11):1121–1127. doi: 10.1016/s0142-9612(99)00264-1. [DOI] [PubMed] [Google Scholar]
- 6.Woo KM, Seo J, Zhang R, Ma PX. Suppression of apoptosis by enhanced protein adsorption on polymer/hydroxyapatite composite scaffolds. Biomaterials. 2007;28(16):2622–2630. doi: 10.1016/j.biomaterials.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sawyer AA, Hennessy KM, Bellis SL. Regulation of mesenchymal stem cell attachment and spreading on hydroxyapatite by RGD peptides and adsorbed serum proteins. Biomaterials. 2005;26(13):1467–1475. doi: 10.1016/j.biomaterials.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Kilpadi KL, Sawyer AA, Prince CW, Chang PL, Bellis SL. Primary human marrow stromal cells and Saos-2 osteosarcoma cells use different mechanisms to adhere to hydroxylapatite. J Biomed Mater Res A. 2004;68(2):273–285. doi: 10.1002/jbm.a.20043. [DOI] [PubMed] [Google Scholar]
- 9.Wilson CJ, Clegg RE, Leavesley DI, Pearcy MJ. Mediation of biomaterial-cell interactions by adsorbed proteins: a review. Tissue Eng. 2005;11(1–2):1–18. doi: 10.1089/ten.2005.11.1. [DOI] [PubMed] [Google Scholar]
- 10.Ruoslahti E. The RGD story: a personal account. Matrix Biol. 2003;22(6):459–465. doi: 10.1016/s0945-053x(03)00083-0. [DOI] [PubMed] [Google Scholar]
- 11.Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 12.Garcia AJ, Keselowsky BG. Biomimetic surfaces for control of cell adhesion to facilitate bone formation. Crit Rev Eukaryot Gene Expr. 2002;12(2):151–162. doi: 10.1615/critreveukaryotgeneexpr.v12.i2.50. [DOI] [PubMed] [Google Scholar]
- 13.Hersel U, Dahmen C, Kessler H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials. 2003;24(24):4385–4415. doi: 10.1016/s0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- 14.Balasundaram G, Sato M, Webster TJ. Using hydroxyapatite nanoparticles and decreased crystallinity to promote osteoblast adhesion similar to functionalizing with RGD. Biomaterials. 2006;27(14):2798–2805. doi: 10.1016/j.biomaterials.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Fujisawa R, Mizuno M, Nodasaka Y, Kuboki Y. Attachment of osteoblastic cells to hydroxyapatite crystals by a synthetic peptide (Glu7-Pro-Arg-Gly-Asp-Thr) containing two functional sequences of bone sialoprotein. Matrix Biol. 1997;16(1):28. doi: 10.1016/s0945-053x(97)90113-x. [DOI] [PubMed] [Google Scholar]
- 16.Roessler S, Born R, Scharnweber D, Worch H, Sewing A, Dard M. Biomimetic coatings functionalized with adhesion peptides for dental implants. J Mater Sci Mater Med. 2001;12(10–12):871–877. doi: 10.1023/a:1012807621414. [DOI] [PubMed] [Google Scholar]
- 17.Itoh D, Yoneda S, Kuroda S, Kondo H, Umezawa A, Ohya K, et al. Enhancement of osteogenesis on hydroxyapatite surface coated with synthetic peptide (EEEEEEEPRGDT) in vitro. J Biomed Mater Res. 2002;62(2):292–298. doi: 10.1002/jbm.10338. [DOI] [PubMed] [Google Scholar]
- 18.Gilbert M, Shaw WJ, Long JR, Nelson K, Drobny GP, Giachelli CM, et al. Chimeric peptides of statherin and osteopontin that bind hydroxyapatite and mediate cell adhesion. J Biol Chem. 2000;275(21):16213–16218. doi: 10.1074/jbc.M001773200. [DOI] [PubMed] [Google Scholar]
- 19.Sawyer AA, Weeks DM, Kelpke SS, McCracken MS, Bellis SL. The effect of the addition of a polyglutamate motif to RGD on peptide tethering to hydroxyapatite and the promotion of mesenchymal stem cell adhesion. Biomaterials. 2005;26(34):7046–7056. doi: 10.1016/j.biomaterials.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Hautanen A, Gailit J, Mann DM, Ruoslahti E. Effects of modifications of the RGD sequence and its context on recognition by the fibronectin receptor. J Biol Chem. 1989;264(3):1437–1442. [PubMed] [Google Scholar]
- 21.Pierschbacher M, Hayman EG, Ruoslahti E. Synthetic peptide with cell attachment activity of fibronectin. Proc Natl Acad Sci U S A. 1983;80(5):1224–1227. doi: 10.1073/pnas.80.5.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stupack DG, Puente XS, Boutsaboualoy S, Storgard CM, Cheresh DA. Apoptosis of adherent cells by recruitment of caspase-8 to unligated integrins. J Cell Biol. 2001;155(3):459–470. doi: 10.1083/jcb.200106070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alves CM, Yang Y, Carnes DL, Ong JL, Sylvia VL, Dean DD, et al. Modulating bone cells response onto starch-based biomaterials by surface plasma treatment and protein adsorption. Biomaterials. 2007;28(2):307–315. doi: 10.1016/j.biomaterials.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 24.Deligianni D, Korovessis P, Porte-Derrieu MC, Amedee J. Fibronectin preadsorbed on hydroxyapatite together with rough surface structure increases osteoblasts' "in vitro": the theoretical usefulness of fibronectin preadsorption on hydroxyapatite to increase permanent stability and longevity in spine implants. J Spinal Disord Tech. 2005;18(3):257–262. [PubMed] [Google Scholar]
- 25.Okamoto K, Matsuura T, Hosokawa R, Akagawa Y. RGD peptides regulate the specific adhesion scheme of osteoblasts to hydroxyapatite but not to titanium. J Dent Res. 1998;77(3):481–487. doi: 10.1177/00220345980770030701. [DOI] [PubMed] [Google Scholar]
- 26.Preissner KT. Structure and biological role of vitronectin. Annu Rev Cell Biol. 1991;7:275–310. doi: 10.1146/annurev.cb.07.110191.001423. [DOI] [PubMed] [Google Scholar]
- 27.Dahlback B. Blood coagulation. Lancet. 2000;355(9215):1627–1632. doi: 10.1016/S0140-6736(00)02225-X. [DOI] [PubMed] [Google Scholar]
- 28.Mosher DF. Physiology of fibronectin. Annu Rev Med. 1984;35:561–575. doi: 10.1146/annurev.me.35.020184.003021. [DOI] [PubMed] [Google Scholar]
- 29.Johansson S, Svineng G, Wennerberg K, Armulik A, Lohikangas L. Fibronectin-integrin interactions. Front Biosci. 1997;2:d126–d146. doi: 10.2741/a178. [DOI] [PubMed] [Google Scholar]
- 30.Akiyama SK. Integrins in cell adhesion and signaling. Hum Cell. 1996;9(3):181–186. [PubMed] [Google Scholar]
- 31.Kamarajan P, Kapila YL. An altered fibronectin matrix induces anoikis of human squamous cell carcinoma cells by suppressing integrin alpha v levels and phosphorylation of FAK and ERK. Apoptosis. 2007;12(12):2221–2231. doi: 10.1007/s10495-007-0138-9. [DOI] [PubMed] [Google Scholar]
- 32.Carvalho RS, Schaffer JL, Gerstenfeld LC. Osteoblasts induce osteopontin expression in response to attachment on fibronectin: demonstration of a common role for integrin receptors in the signal transduction processes of cell attachment and mechanical stimulation. J Cell Biochem. 1998;70(3):376–390. doi: 10.1002/(sici)1097-4644(19980901)70:3<376::aid-jcb11>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 33.Salasznyk RM, Klees RF, Hughlock MK, Plopper GE. ERK signaling pathways regulate the osteogenic differentiation of human mesenchymal stem cells on collagen I and vitronectin. Cell Commun Adhes. 2004;11(5–6):137–153. doi: 10.1080/15419060500242836. [DOI] [PubMed] [Google Scholar]
- 34.Salasznyk RM, Klees RF, Williams WA, Boskey A, Plopper GE. Focal adhesion kinase signaling pathways regulate the osteogenic differentiation of human mesenchymal stem cells. Exp Cell Res. 2007;313(1):22–37. doi: 10.1016/j.yexcr.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilbert M, Giachelli CM, Stayton PS. Biomimetic peptides that engage specific integrin-dependent signaling pathways and bind to calcium phosphate surfaces. J Biomed Mater Res A. 2003;67(1):69–77. doi: 10.1002/jbm.a.10053. [DOI] [PubMed] [Google Scholar]
- 36.Elmengaard B, Bechtold JE, Soballe K. In vivo study of the effect of RGD treatment on bone ongrowth on press-fit titanium alloy implants. Biomaterials. 2005;26(17):3521–3526. doi: 10.1016/j.biomaterials.2004.09.039. [DOI] [PubMed] [Google Scholar]
- 37.Elmengaard B, Bechtold JE, Soballe K. In vivo effects of RGD-coated titanium implants inserted in two bone-gap models. J Biomed Mater Res A. 2005;75(2):249–255. doi: 10.1002/jbm.a.30301. [DOI] [PubMed] [Google Scholar]
- 38.Ferris DM, Moodie GD, Dimond PM, Gioranni CW, Ehrlich MG, Valentini RF. RGD-coated titanium implants stimulate increased bone formation in vivo. Biomaterials. 1999;20(23–24):2323–2331. doi: 10.1016/s0142-9612(99)00161-1. [DOI] [PubMed] [Google Scholar]
- 39.Kroese-Deutman HC, van den Dolder J, Spauwen PH, Jansen JA. Influence of RGD-loaded titanium implants on bone formation in vivo. Tissue Eng. 2005;11(11–12):1867–1875. doi: 10.1089/ten.2005.11.1867. [DOI] [PubMed] [Google Scholar]
- 40.Kantlehner M, Schaffner P, Finsinger D, Meyer J, Jonczyk A, Diefenbach B, et al. Surface coating with cyclic RGD peptides stimulates osteoblast adhesion and proliferation as well as bone formation. Chembiochem. 2000;1(2):107–114. doi: 10.1002/1439-7633(20000818)1:2<107::AID-CBIC107>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 41.Schaffner P, Meyer J, Dard M, Wenz R, Nies B, Verrier S, et al. Induced tissue integration of bone implants by coating with bone selective RGD-peptides in vitro and in vivo studies. J Mater Sci Mater Med. 1999;10(12):837–839. doi: 10.1023/a:1008904513304. [DOI] [PubMed] [Google Scholar]
- 42.Germanier Y, Tosatti S, Broggini N, Textor M, Buser D. Enhanced bone apposition around biofunctionalized sandblasted and acid-etched titanium implant surfaces. A histomorphometric study in miniature pigs. Clin Oral Implants Res. 2006;17(3):251–257. doi: 10.1111/j.1600-0501.2005.01222.x. [DOI] [PubMed] [Google Scholar]
- 43.Barber TA, Ho JE, De Ranieri A, Virdi AS, Sumner DR, Healy KE. Peri-implant bone formation and implant integration strength of peptide-modified p(AAM-co-EG/AAC) interpenetrating polymer network-coated titanium implants. J Biomed Mater Res A. 2007;80(2):306–320. doi: 10.1002/jbm.a.30927. [DOI] [PubMed] [Google Scholar]
- 44.Ho JE, Barber TA, Virdi AS, Sumner DR, Healy KE. The effect of enzymatically degradable IPN coatings on peri-implant bone formation and implant fixation. J Biomed Mater Res A. 2007;81(3):720–727. doi: 10.1002/jbm.a.31008. [DOI] [PubMed] [Google Scholar]


