Abstract
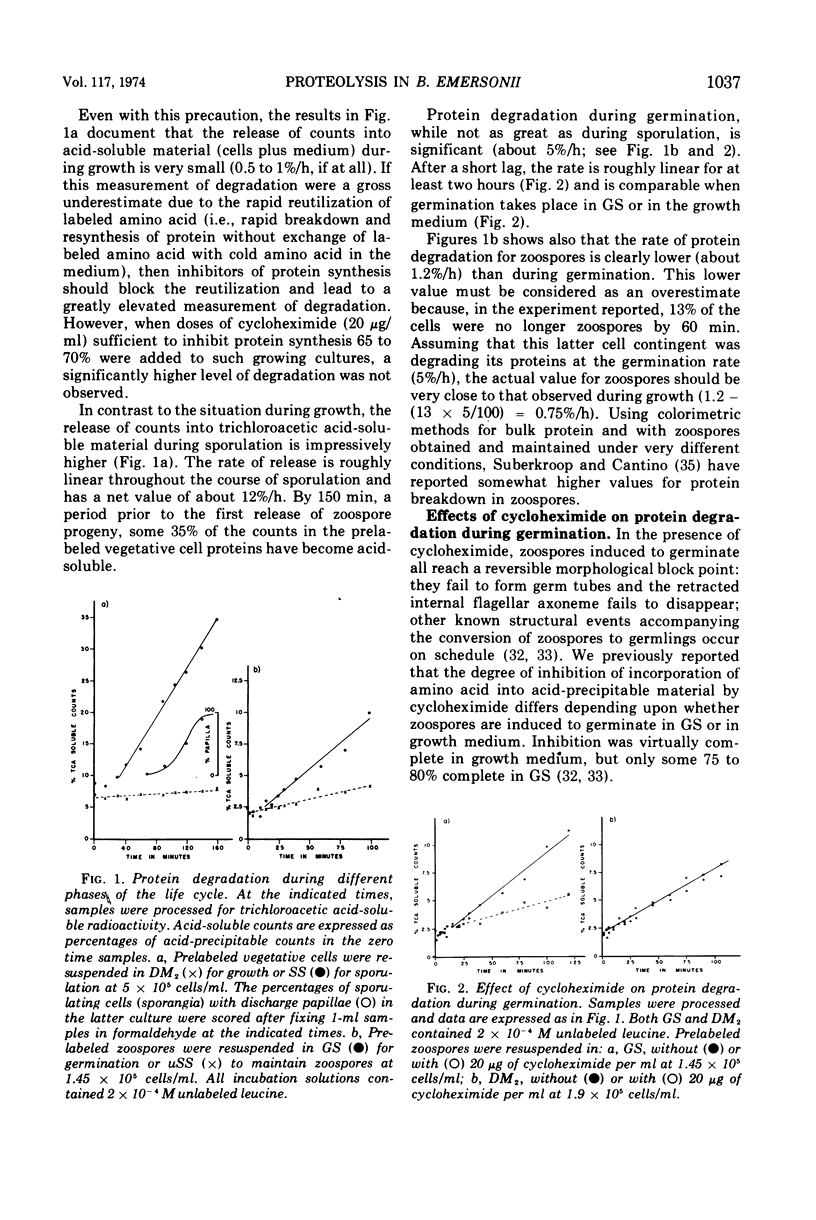
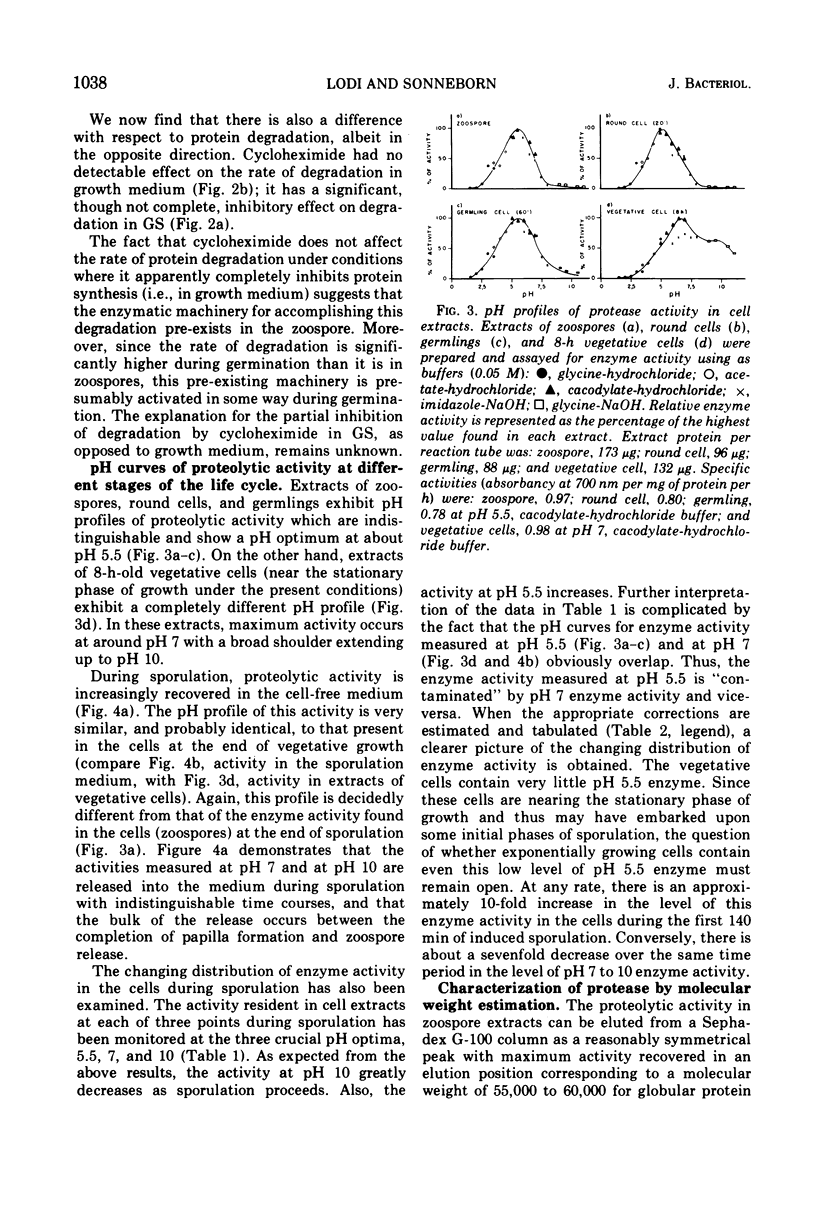
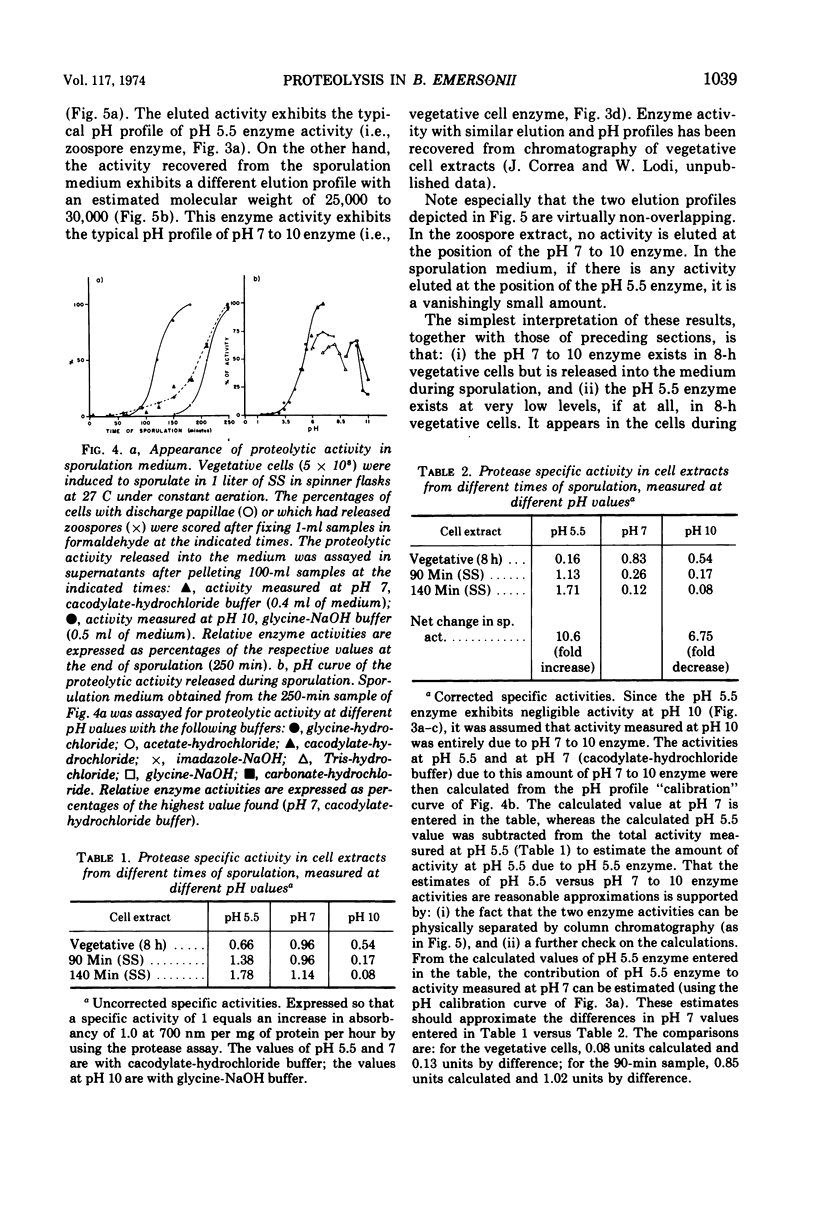
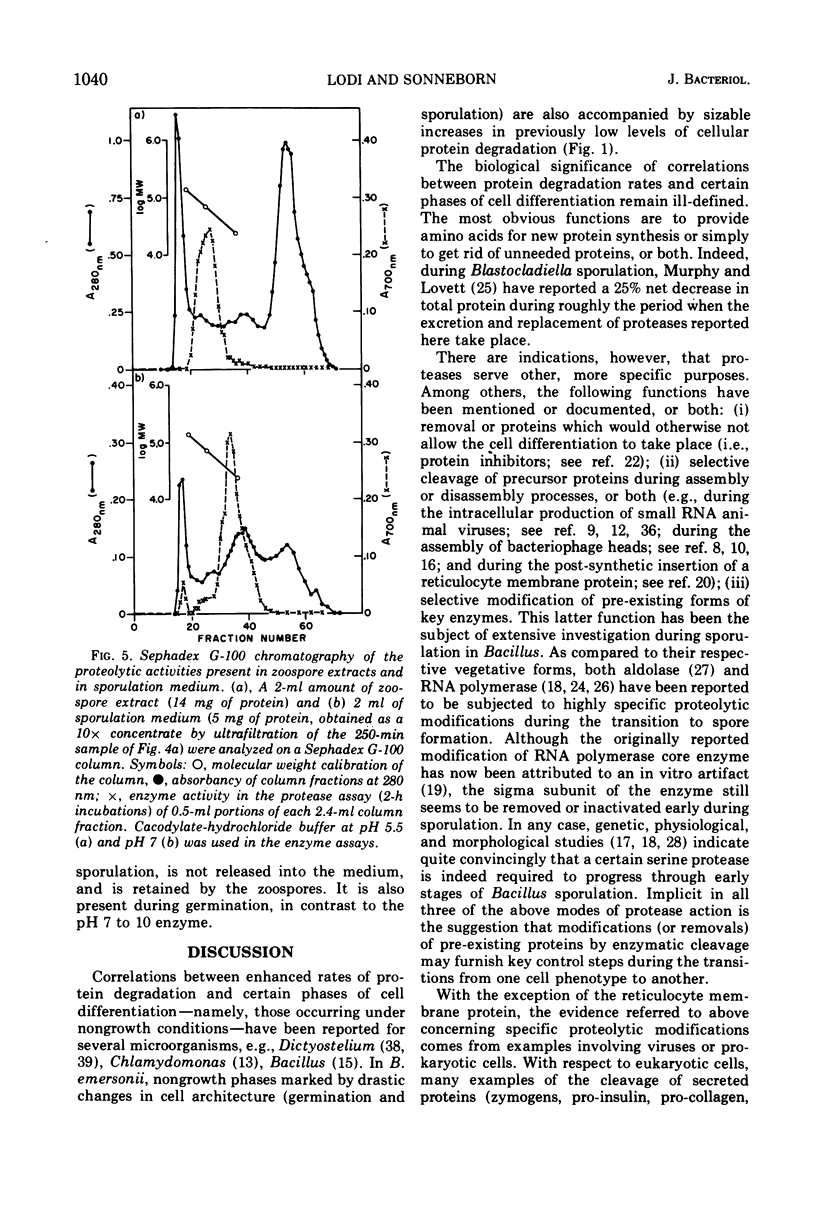
Analysis of protein degradation during the life cycle of Blastocladiella emersonii showed that (i) protein degradation is especially high during two phases of differentiation (sporulation, 12%/h and germination, 5%/h) in contrast with a much smaller degradation rate in the other phases (growth and zoospores, less than 1%/hr); (ii) protein degradation during germination in growth medium, as well as most of the germination process, is quantitatively unaffected by cycloheximide; (iii) a caseinolytic protease (pH optimum 5.5, apparent molecular weight 55,000 to 60,000) is present in extracts of zoospores and germinating cells; (iv) this protease activity is very low (perhaps absent) in extracts of late growth phase cells, but reappears during induced sporulation; (v) a different class of caseinolytic protease activity (pH optima 7 and 10; apparent molecular weight 25,000 to 30,000) is found in cellular extracts of late growth phase and early phases of sporulation; (vi) the latter class of enzyme activity is released into the medium during later phases of sporulation and is replaced in the cells by the former class. Speculations as to the roles of protein degradation in cell differentiation are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib E., Farkas V. The control of morphogenesis: an enzymatic mechanism for the initiation of septum formation in yeast. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2052–2056. doi: 10.1073/pnas.68.9.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabib E., Keller F. A. Chitin and yeast budding. Allosteric inhibition of chitin synthetase by a heat-stable protein from yeast. J Biol Chem. 1971 Jan 10;246(1):167–173. [PubMed] [Google Scholar]
- Cabib E., Ulane R., Bowers B. Yeast chitin synthetase. Separation of the zymogen from its activating factor and recovery of the latter in the vacuole fraction. J Biol Chem. 1973 Feb 25;248(4):1451–1458. [PubMed] [Google Scholar]
- Cabib E., Ulane R. Chitin synthetase activating factor from yeast, a protease. Biochem Biophys Res Commun. 1973 Jan 4;50(1):186–191. doi: 10.1016/0006-291x(73)91081-4. [DOI] [PubMed] [Google Scholar]
- Camargo E. P., Dietrich C. P., Sonneborn D., Strominger J. L. Biosynthesis of chitin in spores and growing cells of Blastocladiella emersonii. J Biol Chem. 1967 Jul 10;242(13):3121–3128. [PubMed] [Google Scholar]
- Cantino E. C., Myers R. B. Concurrent effect of visible light on -particles, chitin synthetase and encystment capacity in zoospores of Blastocladiella emersonii. Arch Mikrobiol. 1972;83(3):203–215. doi: 10.1007/BF00645122. [DOI] [PubMed] [Google Scholar]
- Dickson R. C., Barnes S. L., Eiserling F. A. Structural proteins of bacteriophage T4. J Mol Biol. 1970 Nov 14;53(3):461–474. doi: 10.1016/0022-2836(70)90077-x. [DOI] [PubMed] [Google Scholar]
- Holland J. J., Kiehn E. D. Specific cleavage of viral proteins as steps in the synthesis and maturation of enteroviruses. Proc Natl Acad Sci U S A. 1968 Jul;60(3):1015–1022. doi: 10.1073/pnas.60.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoda J., Cone R. Analysis of T4 phage proteins. I. Conversion of precursor proteins into lower molecular weight peptides during normal capsid formation. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1275–1281. doi: 10.1073/pnas.66.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ITZHAKI R. F., GILL D. M. A MICRO-BIURET METHOD FOR ESTIMATING PROTEINS. Anal Biochem. 1964 Dec;9:401–410. doi: 10.1016/0003-2697(64)90200-3. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Polypeptide cleavages in the formation of poliovirus proteins. Proc Natl Acad Sci U S A. 1968 Sep;61(1):77–84. doi: 10.1073/pnas.61.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. F., Kates J. R., Keller S. J. Protein turnover and macromolecular synthesis during growth and gametic differentiation in Chlamydomonas reinhardtii. Biochim Biophys Acta. 1968 May 21;157(3):589–598. doi: 10.1016/0005-2787(68)90156-1. [DOI] [PubMed] [Google Scholar]
- Keller F. A., Cabib E. Chitin and yeast budding. Properties of chitin synthetase from Saccharomyces carlsbergensis. J Biol Chem. 1971 Jan 10;246(1):160–166. [PubMed] [Google Scholar]
- Kornberg A., Spudich J. A., Nelson D. L., Deutscher M. P. Origin of proteins in sporulation. Annu Rev Biochem. 1968;37:51–78. doi: 10.1146/annurev.bi.37.070168.000411. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leighton T. J., Dor R. H., Warren R. A., Kelln R. A. The relationship of serine protease activity to RNA polymerase modification and sporulation in Bacillus subtilis. J Mol Biol. 1973 May 5;76(1):103–122. doi: 10.1016/0022-2836(73)90083-1. [DOI] [PubMed] [Google Scholar]
- Linn T. G., Greenleaf A. L., Shorenstein R. G., Losick R. Loss of the sigma activity of RNA polymerase of Bacillus subtilis during sporulation. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1865–1869. doi: 10.1073/pnas.70.6.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F. Biosynthesis of reticulocyte membrane proteins by membrane-free polyribosomes. Proc Natl Acad Sci U S A. 1973 May;70(5):1526–1530. doi: 10.1073/pnas.70.5.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett J. S. Reactivation of ribonucleic acid and protein synthesis during germination of Blastocladiella zoospores and the role of the ribosomal nuclear cap. J Bacteriol. 1968 Oct;96(4):962–969. doi: 10.1128/jb.96.4.962-969.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCDONALD C. E., CHEN L. L. THE LOWRY MODIFICATION OF THE FOLIN REAGENT FOR DETERMINATION OF PROTEINASE ACTIVITY. Anal Biochem. 1965 Jan;10:175–177. doi: 10.1016/0003-2697(65)90255-1. [DOI] [PubMed] [Google Scholar]
- Mandelstam J., Waites W. M. Sporulation in Bacillus subtilis. The role of exoprotease. Biochem J. 1968 Oct;109(5):793–801. doi: 10.1042/bj1090793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet J., Kerjan P., Aubert J. P., Szulmajster J. Proteolytic conversion in vitro of B. subtilis vegetative RNA polymerase into the homologous spore enzyme. FEBS Lett. 1972 Jun 1;23(1):47–50. doi: 10.1016/0014-5793(72)80281-3. [DOI] [PubMed] [Google Scholar]
- Murphy M. N., Lovett J. S. RNA and protein synthesis during zoospore differentiation in synchronized cultures of Blastocladiella. Dev Biol. 1966 Aug;14(1):68–95. doi: 10.1016/0012-1606(66)90006-6. [DOI] [PubMed] [Google Scholar]
- Reysset G., Millet J. Characterization of an intracellular protease in B. subtillus during sporulation. Biochem Biophys Res Commun. 1972 Oct 17;49(2):328–334. doi: 10.1016/0006-291x(72)90414-7. [DOI] [PubMed] [Google Scholar]
- Sadoff H. L., Celikkol E., Engelbrecht H. L. Conversion of bacterial aldolase from vegetative to spore form by a sporulation-specific protease. Proc Natl Acad Sci U S A. 1970 Jul;66(3):844–849. doi: 10.1073/pnas.66.3.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santo L., Leighton T. J., Doi R. H. Ultrastructural analysis of sporulation in a conditional serine protease mutant of Bacillus subtilis. J Bacteriol. 1972 Jul;111(1):248–253. doi: 10.1128/jb.111.1.248-253.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmoyer I. R., Lovett J. S. Regulation of protein synthesis in zoospores of Blastocladiella. J Bacteriol. 1969 Nov;100(2):854–864. doi: 10.1128/jb.100.2.854-864.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll D. R., Bromberg R., Sonneborn D. R. Zoospore germination in the water mold. Blastocladiella emersonii. I. Measurement of germination and sequence of subcellular morphological changes. Dev Biol. 1969 Sep;20(3):183–217. doi: 10.1016/0012-1606(69)90012-8. [DOI] [PubMed] [Google Scholar]
- Soll D. R., Sonneborn D. R. Zoospore germination in Blastocladiella emersonii. 3. Structural changes in relation to protein and RNA synthesis. J Cell Sci. 1971 Nov;9(3):679–699. doi: 10.1242/jcs.9.3.679. [DOI] [PubMed] [Google Scholar]
- Soll D. R., Sonneborn D. R. Zoospore germination in Blastocladiella emersonii. IV. Ion control over cell differentiation. J Cell Sci. 1972 Mar;10(2):315–333. doi: 10.1242/jcs.10.2.315. [DOI] [PubMed] [Google Scholar]
- Soll D. R., Sonneborn D. R. Zoospore germination in Blastocladiella emersonii: cell differentiation without protein synthesis? Proc Natl Acad Sci U S A. 1971 Feb;68(2):459–463. doi: 10.1073/pnas.68.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll D. R., Sonneborn D. R. Zoospore germination in the water mold. Blastocladiella emersonii. II. Influence of cellular and environmental variables on germination. Dev Biol. 1969 Sep;20(3):218–235. doi: 10.1016/0012-1606(69)90013-x. [DOI] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr Evidence for large precursor proteins in poliovirus synthesis. Proc Natl Acad Sci U S A. 1968 Mar;59(3):966–971. doi: 10.1073/pnas.59.3.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truesdell L. C., Cantino E. C. The induction and early events of germination in the zoospore of Blastocladiella emersonii. Curr Top Dev Biol. 1971;6(6):1–44. doi: 10.1016/s0070-2153(08)60636-5. [DOI] [PubMed] [Google Scholar]
- WHITE G. J., SUSSMAN M. Metabolism of major cell components during slime mold morphogenesis. Biochim Biophys Acta. 1961 Oct 28;53:285–293. doi: 10.1016/0006-3002(61)90441-3. [DOI] [PubMed] [Google Scholar]
- WRIGHT B. E., ANDERSON M. L. Protein and amino acid turnover during differentiation in the slime mold. I. Utilization of endogenous amino acids and proteins. Biochim Biophys Acta. 1960 Sep 9;43:62–66. doi: 10.1016/0006-3002(60)90407-8. [DOI] [PubMed] [Google Scholar]



