Abstract
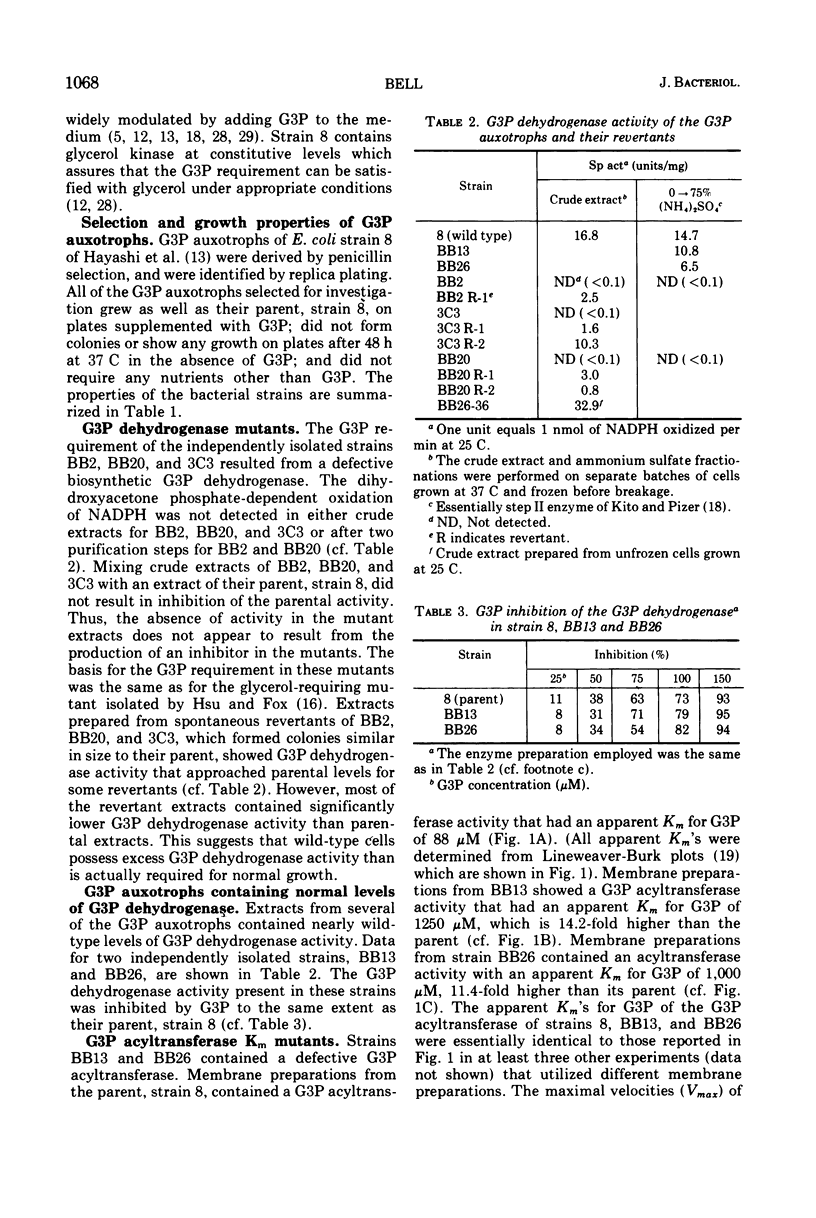
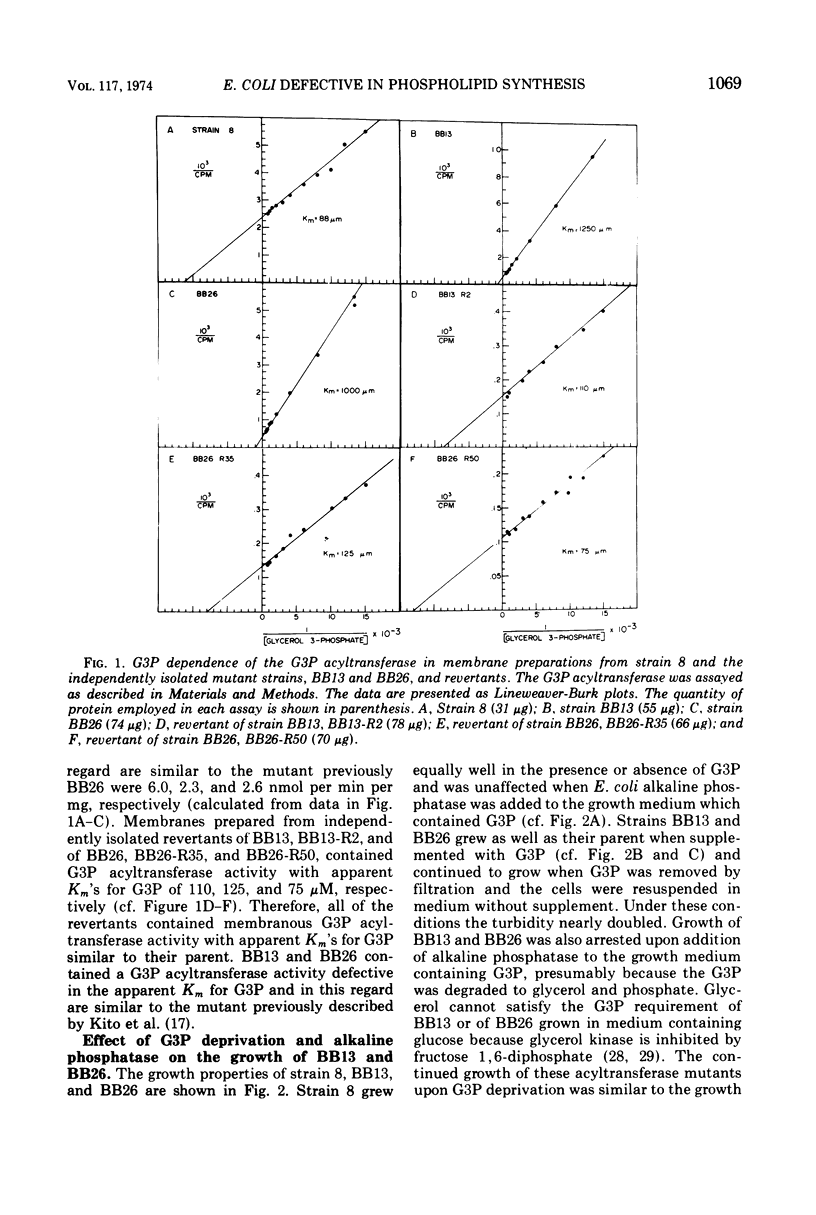
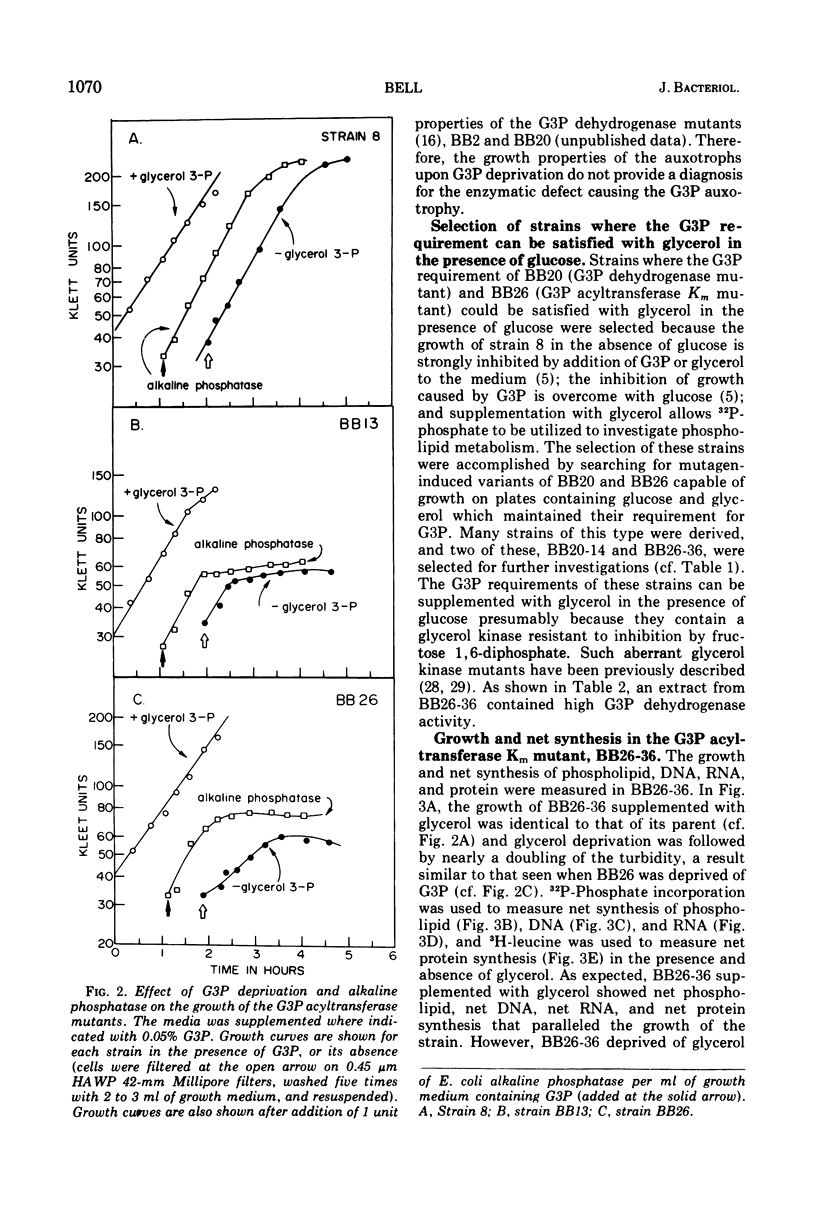
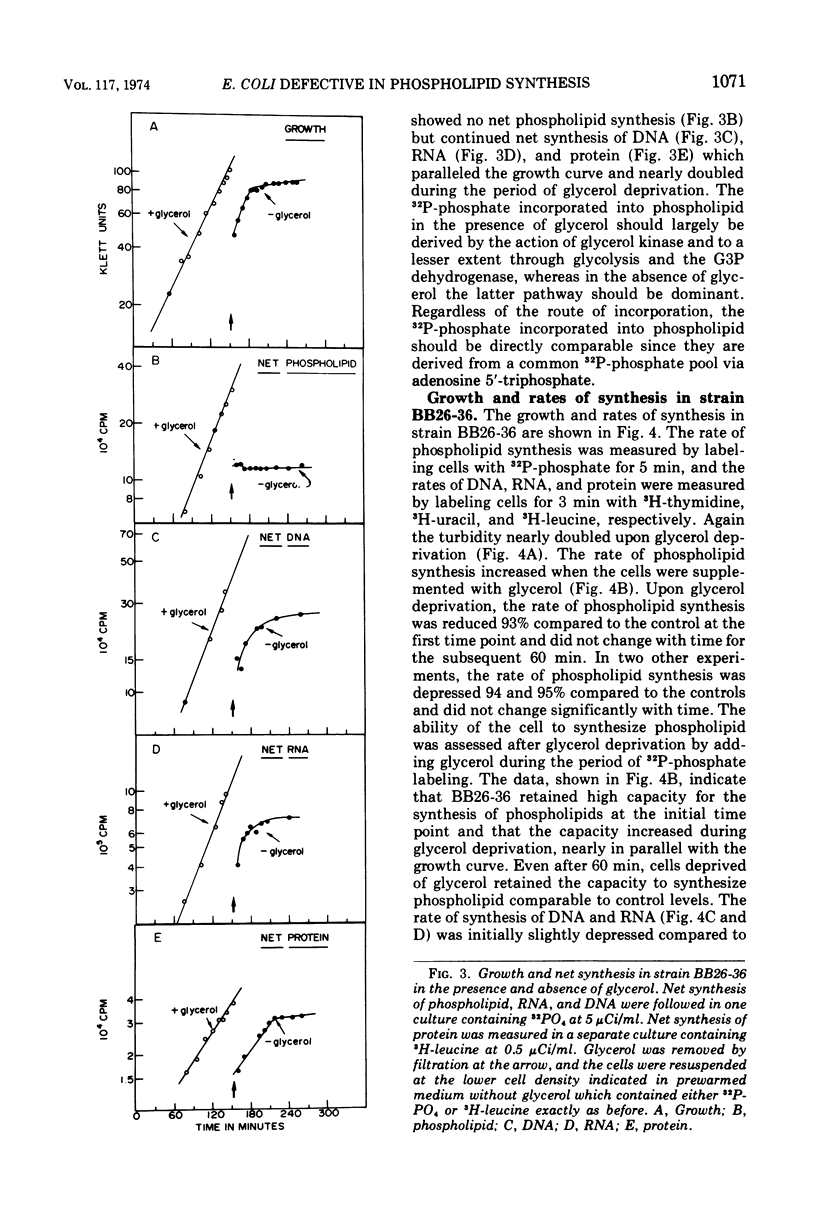
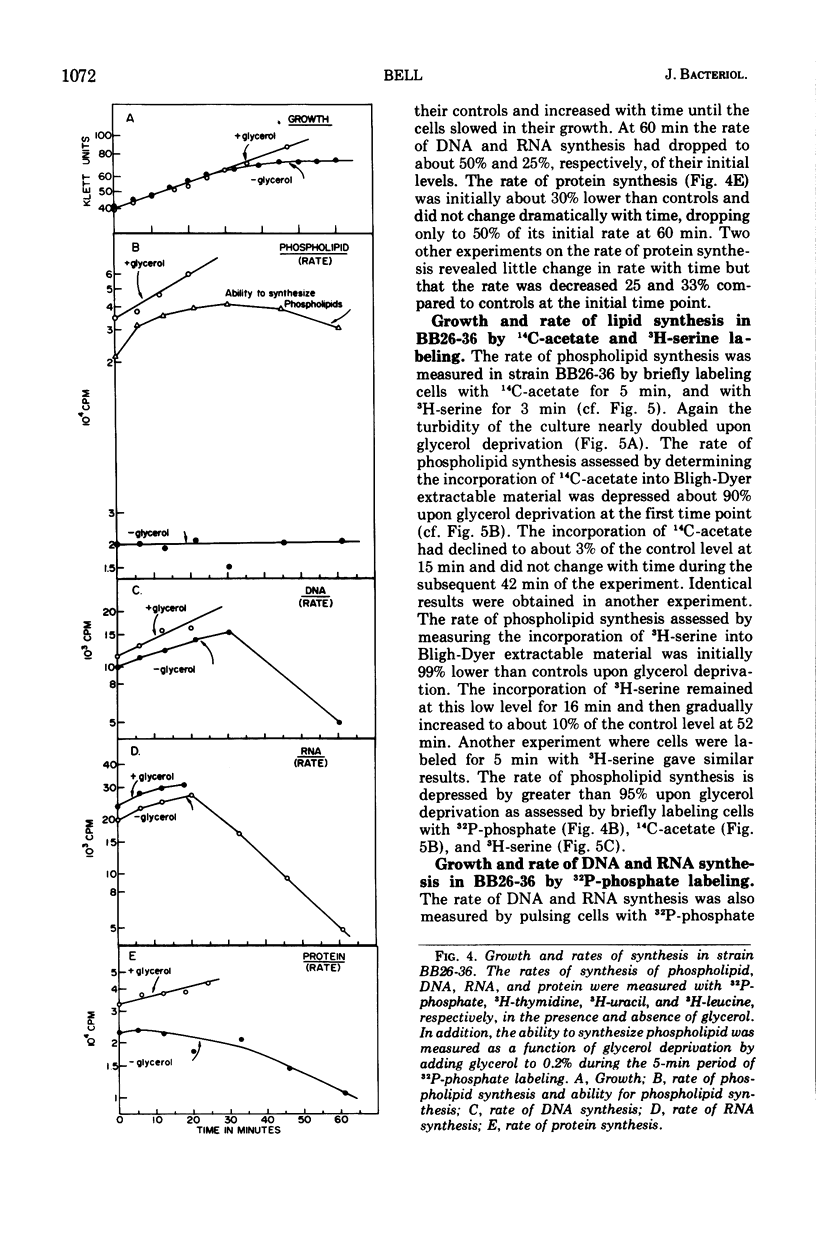
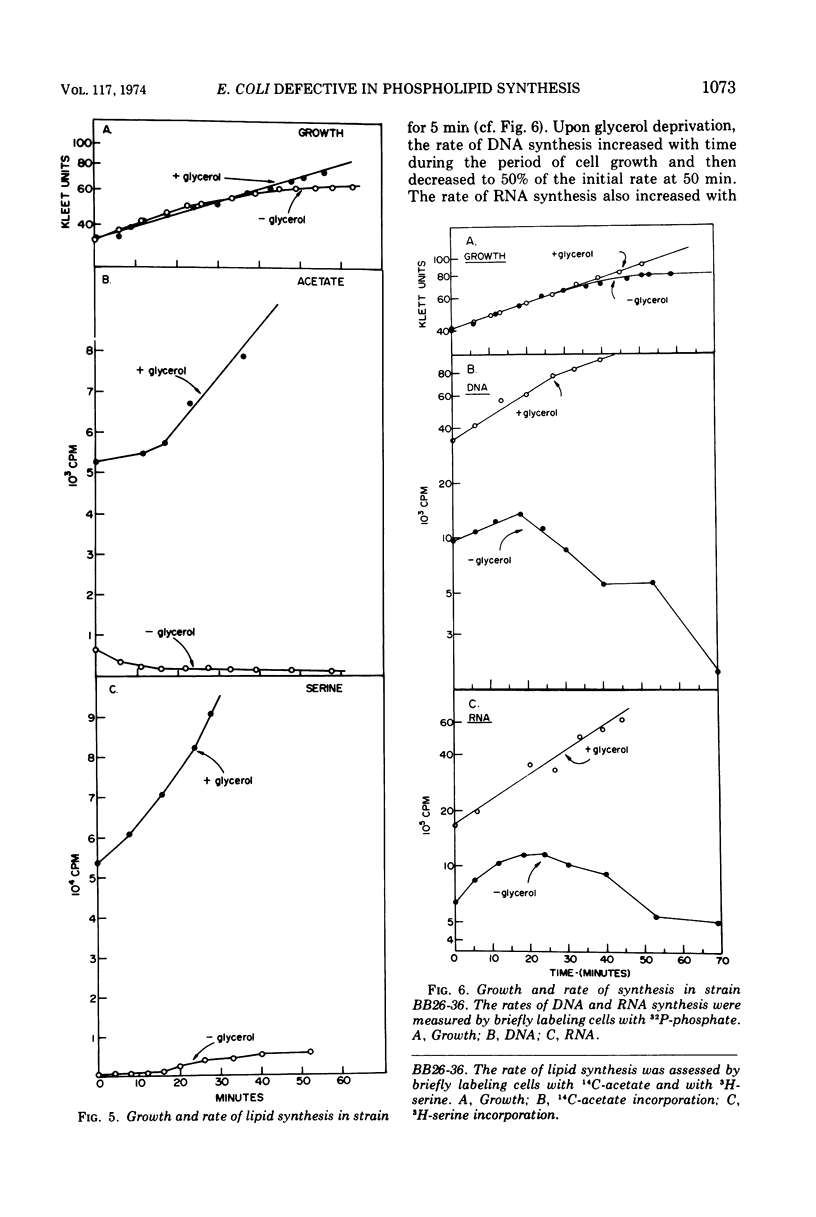
sn-Glycerol 3-phosphate (G3P) auxotrophs of Escherichia coli have been selected from a strain which cannot aerobically catabolize G3P. The auxotrophy resulted from loss of the biosynthetic G3P dehydrogenase (EC 1.1.1.8) or from a defective membranous G3P acyltransferase. The apparent Km of the acyltransferase for G3P was 11- to 14-fold higher (from about 90 μm to 1,000 to 1,250 μm) in membrane preparations from the mutants than those of the parent. All extracts prepared from revertants of the G3P dehydrogenase mutants showed G3P dehydrogenase activity, but most contained less than 10% of the wild-type level. Membrane preparations from revertants of the acyltransferase mutants had apparent Km's for G3P similar to that of the parent. Strains have been derived in which the G3P requirement can be satisfied with glycerol in the presence of glucose, presumably because the glycerol kinase was desensitized to inhibition by fructose 1,6-diphosphate. Investigations on the growth and macromolecular synthesis in a G3P acyltransferase Km mutant revealed that upon glycerol deprivation, net phospholipid synthesis stopped immediately; growth continued for about one doubling; net ribonucleic acid (RNA), deoxyribonucleic acid (DNA), and protein nearly doubled paralleling the growth curve; the rate of phospholipid synthesis assessed by labeling cells with 32P-phosphate, 14C-acetate, or 3H-serine was reduced greater than 90%; the rates of RNA and DNA synthesis increased as the cells grew and then decreased as the cells stopped growing; the rate of protein synthesis showed no increase and declined more slowly than the rates of RNA and DNA synthesis when the cells stopped growing. The cells retained and gained in the capacity to synthesize phospholipids upon glycerol deprivation. These data indicate that net phospholipid synthesis is not required for continued macromolecular synthesis for about one doubling, and that the rates of these processes are not coupled during this time period.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bell R. M., Mavis R. D., Osborn M. J., Vagelos P. R. Enzymes of phospholipid metabolism: localization in the cytoplasmic and outer membrane of the cell envelope of Escherichia coli and Salmonella typhimurium. Biochim Biophys Acta. 1971 Dec 3;249(2):628–635. doi: 10.1016/0005-2736(71)90144-1. [DOI] [PubMed] [Google Scholar]
- Cozzarelli N. R., Koch J. P., Hayashi S., Lin E. C. Growth stasis by accumulated L-alpha-glycerophosphate in Escherichia coli. J Bacteriol. 1965 Nov;90(5):1325–1329. doi: 10.1128/jb.90.5.1325-1329.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Birge C. H., Vagelos P. R. Evidence for two genes specifically involved in unsaturated fatty acid biosynthesis in Escherichia coli. J Bacteriol. 1969 Nov;100(2):601–604. doi: 10.1128/jb.100.2.601-604.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Godson G. N. Mutants of Escherichia coli with temperature-sensitive lesions in membrane phospholipid synthesis: genetic analysis of glycerol-3-phosphate acyltransferase mutants. Mol Gen Genet. 1972;116(3):199–210. doi: 10.1007/BF00269765. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Ray T. K., Vagelos P. R. Selection and characterization of an E. coli mutant defective in membrane lipid biosynthesis. Proc Natl Acad Sci U S A. 1970 Mar;65(3):737–744. doi: 10.1073/pnas.65.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Vagelos P. R. Metabolism and function of the membrane phospholipids of Escherichia coli. Biochim Biophys Acta. 1972 Feb 14;265(1):25–60. doi: 10.1016/0304-4157(72)90018-4. [DOI] [PubMed] [Google Scholar]
- Fox C. F. A lipid requirement for induction of lactose transport in Escherichia coli. Proc Natl Acad Sci U S A. 1969 Jul;63(3):850–855. doi: 10.1073/pnas.63.3.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser M., Bayer W. H., Bell R. M., Vagelos P. R. Regulation of macromolecular biosynthesis in a mutant of Escherichia coli defective in membrane phospholipid biosynthesis. Proc Natl Acad Sci U S A. 1973 Feb;70(2):385–389. doi: 10.1073/pnas.70.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYASHI S., KOCH J. P., LIN E. C. ACTIVE TRANSPORT OF L-ALPHA-GLYCEROPHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1964 Sep;239:3098–3105. [PubMed] [Google Scholar]
- HAYASHI S., LIN E. C. CAPTURE OF GLYCEROL BY CELLS OF ESCHERICHIA COLI. Biochim Biophys Acta. 1965 Mar 29;94:479–487. doi: 10.1016/0926-6585(65)90056-7. [DOI] [PubMed] [Google Scholar]
- HIRSCHMANN H. The nature of substrate asymmetry in stereoselective reactions. J Biol Chem. 1960 Oct;235:2762–2767. [PubMed] [Google Scholar]
- Henning U., Dennert G., Rehn K., Deppe G. Effects of oleate starvation in a fatty acid auxotroph of Escherichia coli K-12. J Bacteriol. 1969 May;98(2):784–796. doi: 10.1128/jb.98.2.784-796.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C. C., Fox C. F. Induction of the lactose transport system in a lipid-synthesis-defective mutant of Escherichia coli. J Bacteriol. 1970 Aug;103(2):410–416. doi: 10.1128/jb.103.2.410-416.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kito M., Lubin M., Pizer L. I. A mutant of Escherichia coli defective in phosphatidic acid synthesis. Biochem Biophys Res Commun. 1969 Feb 21;34(4):454–458. doi: 10.1016/0006-291x(69)90403-3. [DOI] [PubMed] [Google Scholar]
- Kito M., Pizer L. I. Purification and regulatory properties of the biosynthetic L-glycerol 3-phosphate dehydrogenase from Escherichia coli. J Biol Chem. 1969 Jun 25;244(12):3316–3323. [PubMed] [Google Scholar]
- MUNKRES K. D., RICHARDS F. M. THE PURIFICATION AND PROPERTIES OF NEUROSPORA MALATE DEHYDROGENASE. Arch Biochem Biophys. 1965 Mar;109:466–479. doi: 10.1016/0003-9861(65)90391-7. [DOI] [PubMed] [Google Scholar]
- Mavis R. D., Bell R. M., Vagelos P. R. Effect of phospholipase C hydrolysis of membrane phospholipids on membranous enzymes. J Biol Chem. 1972 May 10;247(9):2835–2841. [PubMed] [Google Scholar]
- Mindich L. Induction of Staphylococcus aureus Lactose Permease in the Absence of Glycerolipid Synthesis. Proc Natl Acad Sci U S A. 1971 Feb;68(2):420–424. doi: 10.1073/pnas.68.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindich L. Membrane synthesis in Bacillus subtilis. I. Isolation and properties of strains bearing mutations in glycerol metabolism. J Mol Biol. 1970 Apr 28;49(2):415–432. doi: 10.1016/0022-2836(70)90254-8. [DOI] [PubMed] [Google Scholar]
- Mindich L. Membrane synthesis in Bacillus subtilis. II. Integration of membrane proteins in the absence of lipid synthesis. J Mol Biol. 1970 Apr 28;49(2):433–439. doi: 10.1016/0022-2836(70)90255-x. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- White D. A., Albright F. R., Lennarz W. J., Schnaitman C. A. Distribution of phospholipid-synthesizing enzymes in the wall and membrane subfractions of the envelope of Escherichia coli. Biochim Biophys Acta. 1971 Dec 3;249(2):636–642. doi: 10.1016/0005-2736(71)90145-3. [DOI] [PubMed] [Google Scholar]
- Willecke K., Mindich L. Induction of citrate transport in Bacillus subtilis during the absence of phospholipid synthesis. J Bacteriol. 1971 May;106(2):514–518. doi: 10.1128/jb.106.2.514-518.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaig N., Kistler W. S., Lin E. C. Glycerol kinase, the pacemaker for the dissimilation of glycerol in Escherichia coli. J Bacteriol. 1970 Jun;102(3):753–759. doi: 10.1128/jb.102.3.753-759.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwaig N., Lin E. C. Feedback inhibition of glycerol kinase, a catabolic enzyme in Escherichia coli. Science. 1966 Aug 12;153(3737):755–757. doi: 10.1126/science.153.3737.755. [DOI] [PubMed] [Google Scholar]


