Abstract
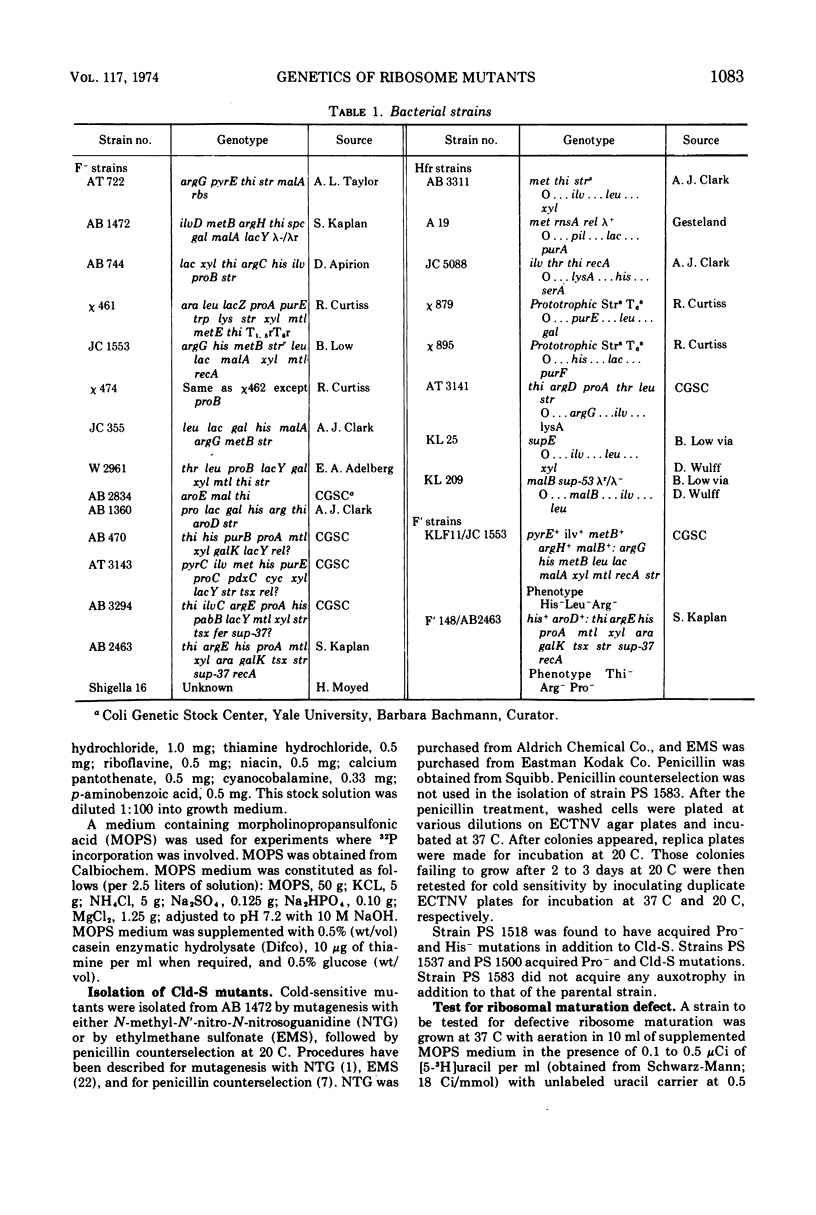
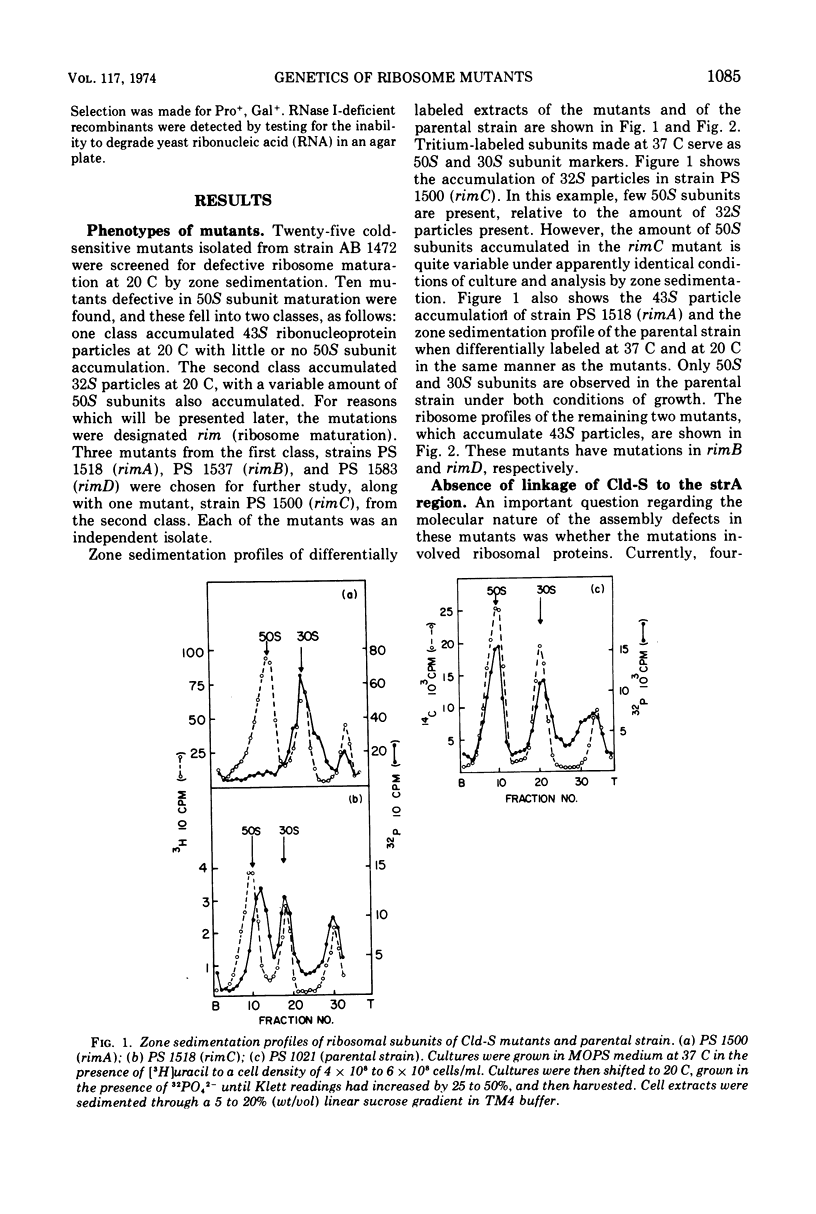
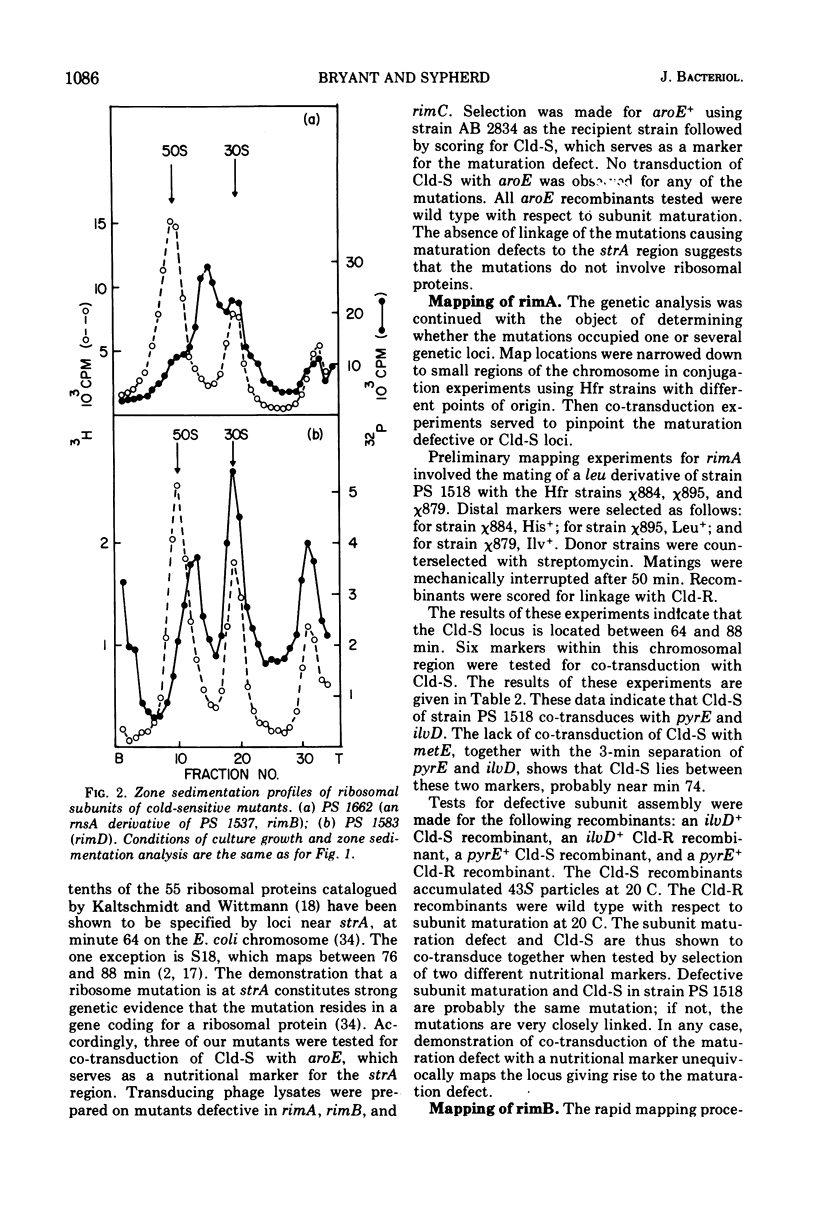
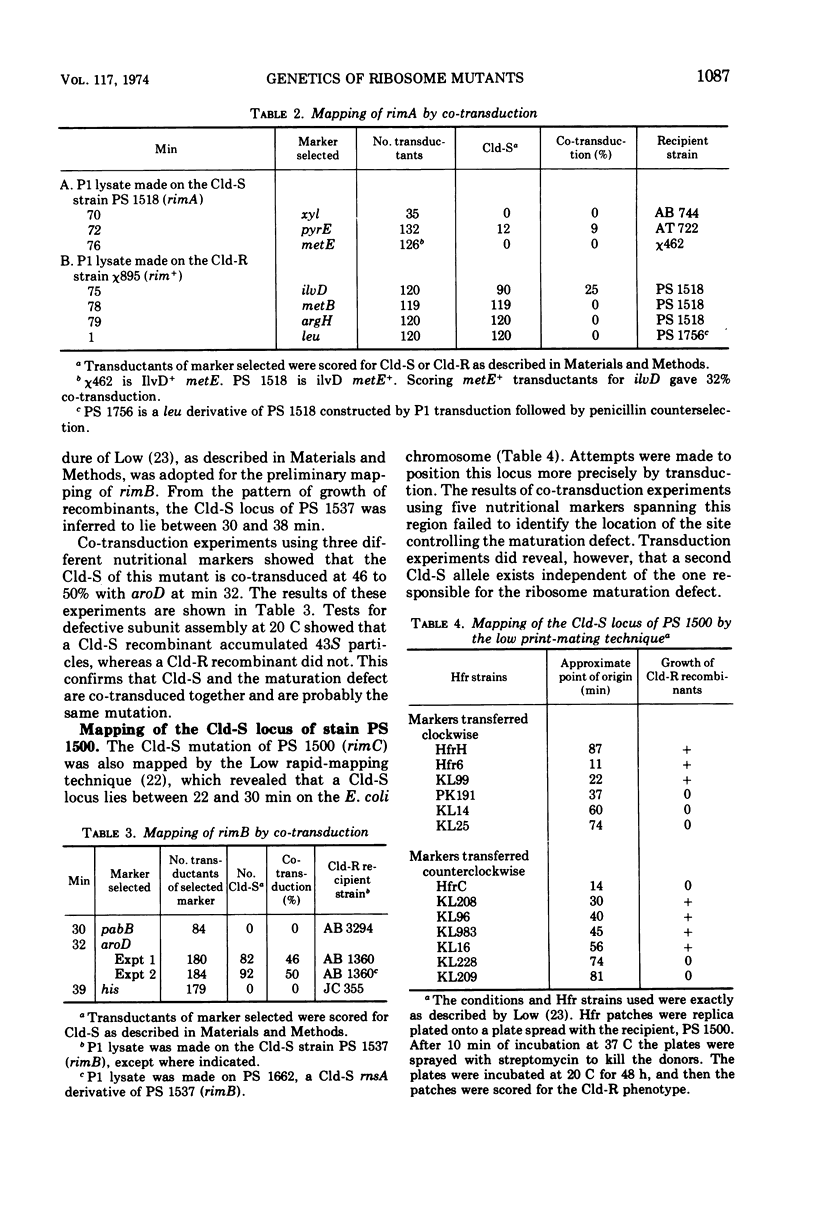
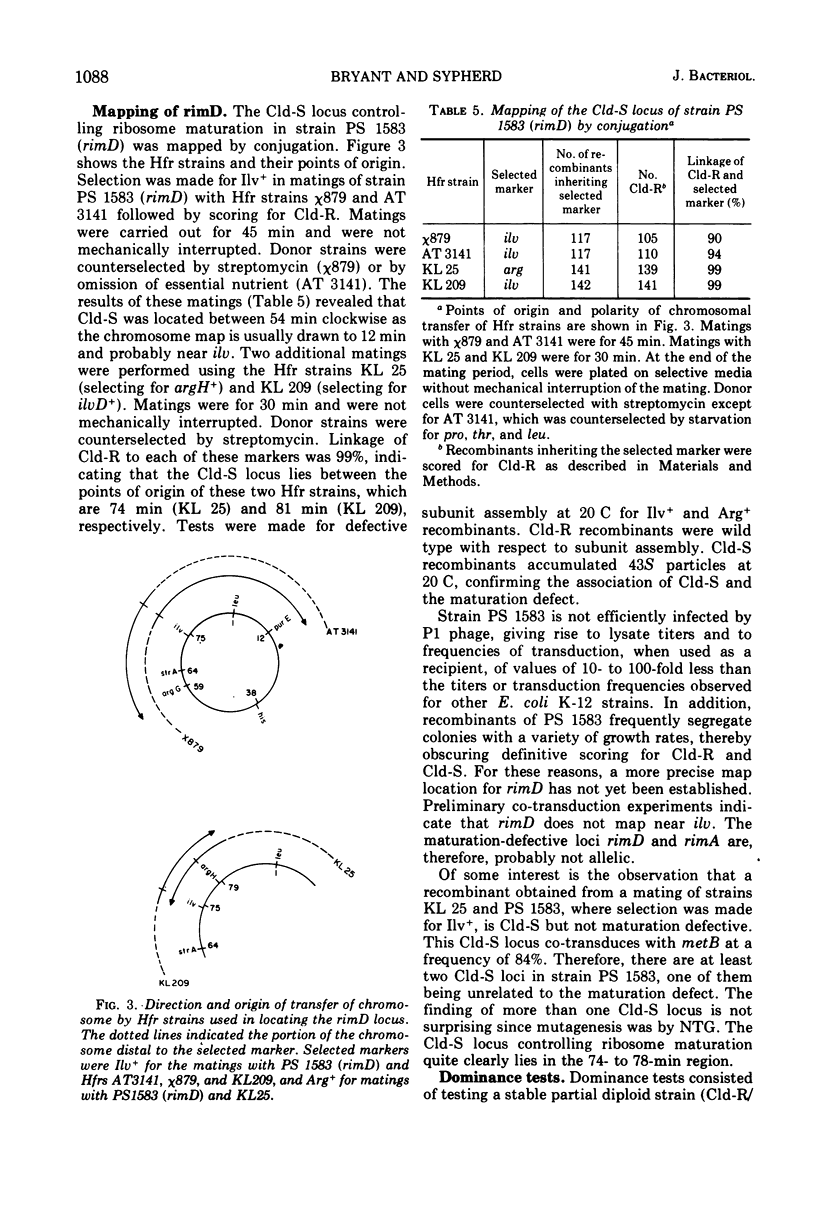
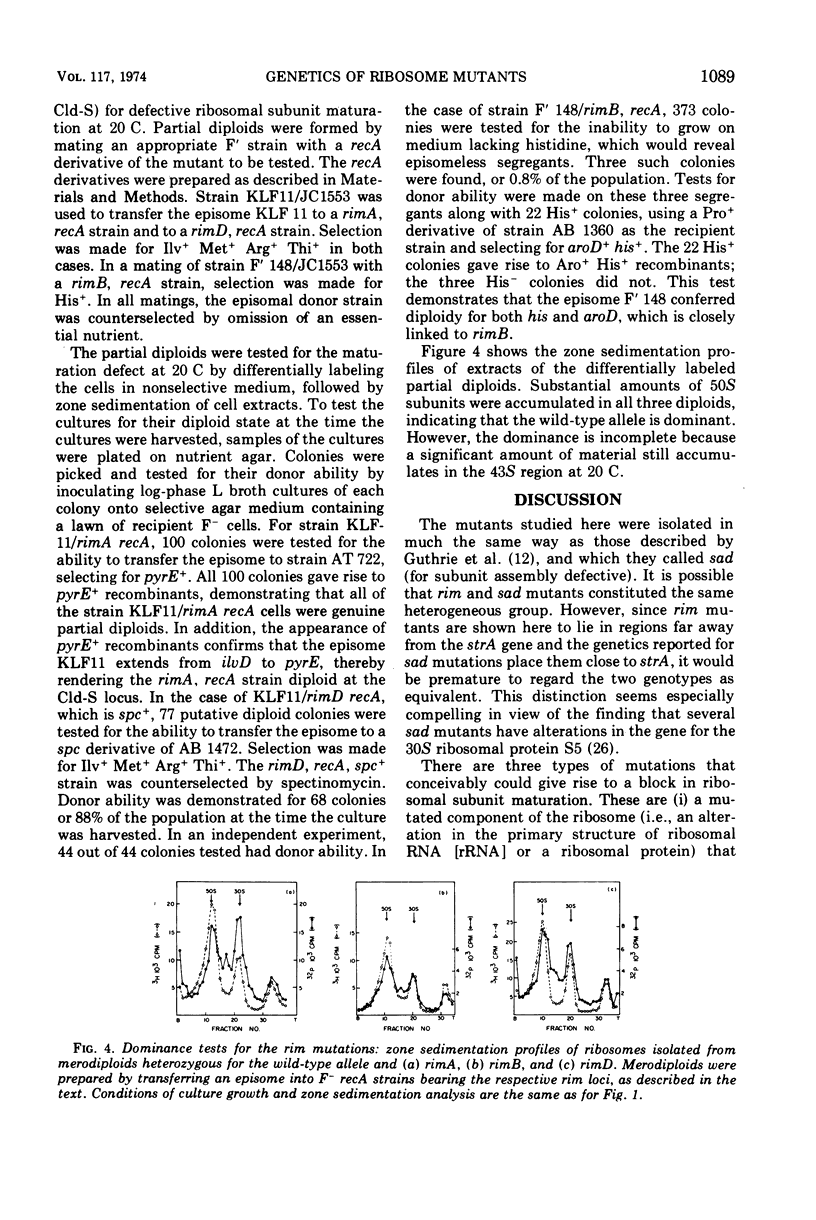
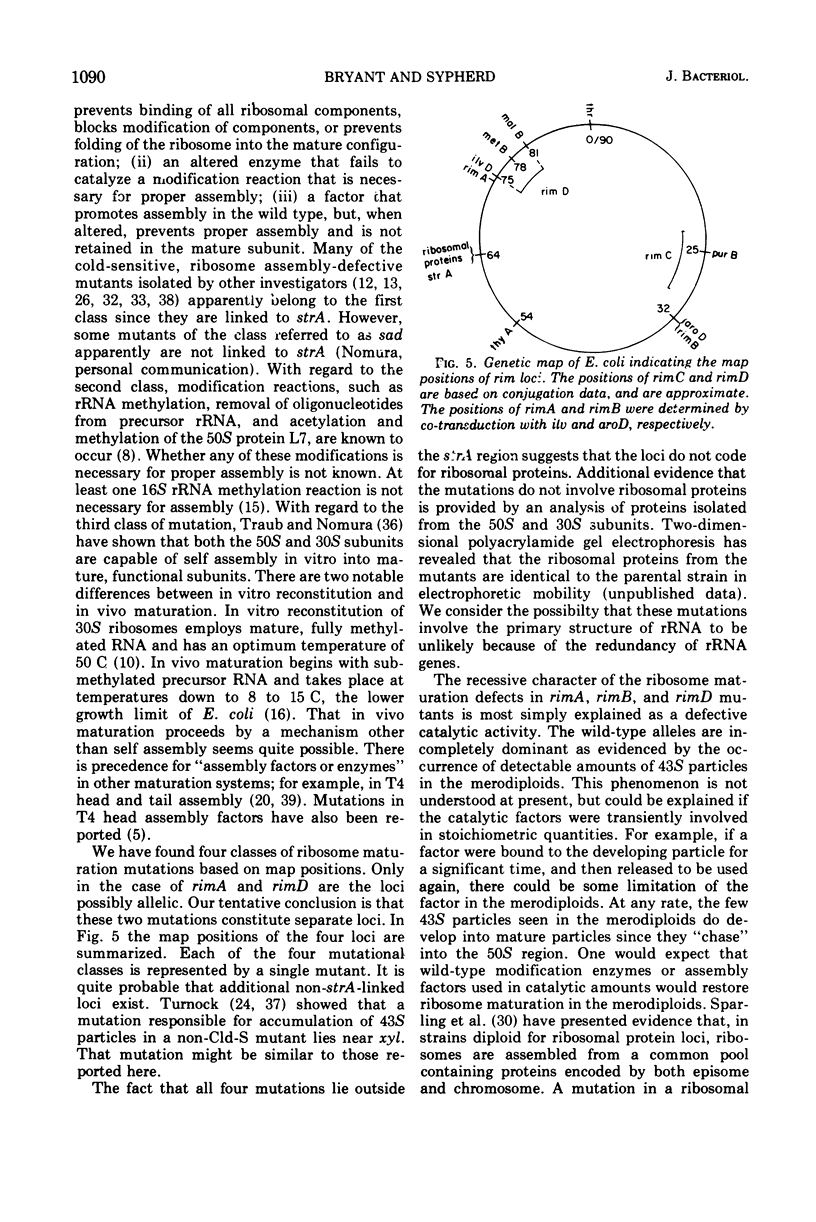
Four cold-sensitive mutants of Escherichia coli, which have defects in the maturation of the 50S ribosomal subunit, were isolated. Each of the mutations was shown to map at a different locus. The loci were assigned the name rim (ribosome maturation) and were shown to map as follows: rimA is co-transduced with ilvD and with pyrE; rimB is co-transduced with aroD; conjugation experiments limited rimD to a region between ilv and malB, and conjugation experiments limited rimC to the 22 to 30 min region of the chromosome. In merodiploids heterozygous for rimA, rimB, or rimD, the wild-type allele was shown to be dominant to the mutant allele. The observation that the rim loci lie outside the strA region and separate from each other, as well as the recessive character of the rim loci, suggests that the mutants may be defective in ribosome maturation factors rather than being defective in ribosomal structural proteins.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bollen A., Faelen M., Lecocq J. P., Herzog A., Zengel J., Kahan L., Nomura M. The structural gene for the ribosomal protein S18 in Escherichia coli. I. Genetic studies on a mutant having an alteration in the protein S18. J Mol Biol. 1973 Jun 5;76(4):463–472. doi: 10.1016/0022-2836(73)90485-3. [DOI] [PubMed] [Google Scholar]
- CLARK A. J., MARGULIES A. D. ISOLATION AND CHARACTERIZATION OF RECOMBINATION-DEFICIENT MUTANTS OF ESCHERICHIA COLI K12. Proc Natl Acad Sci U S A. 1965 Feb;53:451–459. doi: 10.1073/pnas.53.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppo A., Manzi A., Pulitzer J. F., Takahashi H. Abortive bacteriophage T4 head assembly in mutants of Escherichia coli. J Mol Biol. 1973 May 5;76(1):61–87. doi: 10.1016/0022-2836(73)90081-8. [DOI] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Caro L. G., Allison D. P., Stallions D. R. Early stages of conjugation in Escherichia coli. J Bacteriol. 1969 Nov;100(2):1091–1104. doi: 10.1128/jb.100.2.1091-1104.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Charamella L. J., Berg C. M., Harris P. E. Kinetic and genetic analyses of D-cycloserine inhibition and resistance in Escherichia coli. J Bacteriol. 1965 Nov;90(5):1238–1250. doi: 10.1128/jb.90.5.1238-1250.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Nomura M. The genetics of bacterial ribosomes. Annu Rev Genet. 1972;6:203–234. doi: 10.1146/annurev.ge.06.120172.001223. [DOI] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egberts E., Traub P., Herrlich P., Schweiger M. Functional integrity of Escherichia coli 30-S ribosomes reconstituted from RNA and protein: in vitro synthesis of S-adenosylmethionine cleaving enzyme. Biochim Biophys Acta. 1972 Sep 14;277(3):681–684. doi: 10.1016/0005-2787(72)90116-5. [DOI] [PubMed] [Google Scholar]
- Gesteland R. F. Isolation and characterization of ribonuclease I mutants of Escherichia coli. J Mol Biol. 1966 Mar;16(1):67–84. doi: 10.1016/s0022-2836(66)80263-2. [DOI] [PubMed] [Google Scholar]
- Guthrie C., Nashimoto H., Nomura M. Structure and function of E. coli ribosomes. 8. Cold-sensitive mutants defective in ribosome assembly. Proc Natl Acad Sci U S A. 1969 Jun;63(2):384–391. doi: 10.1073/pnas.63.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie C., Nashimoto H., Nomura M. Studies on the assembly of ribosomes in vivo. Cold Spring Harb Symp Quant Biol. 1969;34:69–75. doi: 10.1101/sqb.1969.034.01.011. [DOI] [PubMed] [Google Scholar]
- Hashimoto K. Streptomycin Resistance in Escherichia Coli Analyzed by Transduction. Genetics. 1960 Jan;45(1):49–62. doi: 10.1093/genetics/45.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helser T. L., Davies J. E., Dahlberg J. E. Mechanism of kasugamycin resistance in Escherichia coli. Nat New Biol. 1972 Jan 5;235(53):6–9. doi: 10.1038/newbio235006a0. [DOI] [PubMed] [Google Scholar]
- INGRAHAM J. L. Growth of psychrophilic bacteria. J Bacteriol. 1958 Jul;76(1):75–80. doi: 10.1128/jb.76.1.75-80.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahan L., Zengel J., Nomura M., Bollen A., Herzog A. The structural gene for the ribosomal protein S18 in Escherichia coli. II. Chemical studies on the protein S18 having an altered electrophoretic mobility. J Mol Biol. 1973 Jun 5;76(4):473–483. doi: 10.1016/0022-2836(73)90486-5. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt E., Wittmann H. G. Ribosomal proteins. XII. Number of proteins in small and large ribosomal subunits of Escherichia coli as determined by two-dimensional gel electrophoresis. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1276–1282. doi: 10.1073/pnas.67.3.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurland C. G. Structure and function of the bacterial ribosome. Annu Rev Biochem. 1972;41(10):377–408. doi: 10.1146/annurev.bi.41.070172.002113. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- LIN E. C., LERNER S. A., JORGENSEN S. E. A method for isolating constitutive mutants for carbohydrate-catabolizing enzymes. Biochim Biophys Acta. 1962 Jul 2;60:422–424. doi: 10.1016/0006-3002(62)90423-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Low B. Rapid mapping of conditional and auxotrophic mutations in Escherichia coli K-12. J Bacteriol. 1973 Feb;113(2):798–812. doi: 10.1128/jb.113.2.798-812.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald R. E., Turnock G., Forchhammer J. The synthesis and function of ribosomes in a new mutant of Escherichia coli. Proc Natl Acad Sci U S A. 1967 Jan;57(1):141–147. doi: 10.1073/pnas.57.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M. Bacterial ribosome. Bacteriol Rev. 1970 Sep;34(3):228–277. doi: 10.1128/br.34.3.228-277.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKADA T., YANAGISAWA K., RYAN F. J. A method for securing thymineless mutants from strains of E. coli. Z Vererbungsl. 1961;92:403–412. doi: 10.1007/BF00890061. [DOI] [PubMed] [Google Scholar]
- Pace B., Pace N. R. Gene dosage for 5S ribosomal ribonucleic acid in Escherichia coli ad Bacillus megaterium. J Bacteriol. 1971 Jan;105(1):142–149. doi: 10.1128/jb.105.1.142-149.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparling P. F., Modolell J., Takeda Y., Davis B. D. Ribosomes from Escherichia coli merodiplods heterozygous for resistance to streptomycin and to spectinomycin. J Mol Biol. 1968 Nov 14;37(3):407–421. doi: 10.1016/0022-2836(68)90111-3. [DOI] [PubMed] [Google Scholar]
- Sypherd P. S., Strauss N. CHLORAMPHENICOL-PROMOTED REPRESSION OF beta-GALACTOSIDASE SYNTHESIS IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1963 Mar;49(3):400–407. doi: 10.1073/pnas.49.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai P. C., Ingraham J. L. A ribosome-like particle accumulated at low temperature by a cold-sensitive mutant of Salmonella typhimurium. Biochim Biophys Acta. 1971 Feb 25;232(1):151–166. doi: 10.1016/0005-2787(71)90499-0. [DOI] [PubMed] [Google Scholar]
- Tai P. C., Kessler D. P., Ingraham J. Cold-sensitive mutations in Salmonella typhimurium which affect ribosome synthesis. J Bacteriol. 1969 Mar;97(3):1298–1304. doi: 10.1128/jb.97.3.1298-1304.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata R. Genetic studies of the ribosomal proteins in Escherichia coli. 8. Mapping of ribosomal protein components by intergeneric mating experiments between Serratia marcescens and Escherichia coli. Mol Gen Genet. 1972;118(4):363–371. doi: 10.1007/BF00333571. [DOI] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub P., Nomura M. Structure and function of E. coli ribosomes. V. Reconstitution of functionally active 30S ribosomal particles from RNA and proteins. Proc Natl Acad Sci U S A. 1968 Mar;59(3):777–784. doi: 10.1073/pnas.59.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnock G. A genetic analysis of a mutant of Escherichia coli with a defect in the assembly of ribosomes. Mol Gen Genet. 1969 Aug 15;104(4):295–312. doi: 10.1007/BF00334229. [DOI] [PubMed] [Google Scholar]
- Tyler B., Ingraham J. L. Studies on ribosomal mutants of Salmonella typhimurium LT-2. Mol Gen Genet. 1973 May 9;122(3):197–214. doi: 10.1007/BF00278597. [DOI] [PubMed] [Google Scholar]
- Wood W. B., Henninger M. Attachment of tail fibers in bacteriophage T4 assembly: some properties of the reaction in vitro and its genetic control. J Mol Biol. 1969 Feb 14;39(3):603–618. doi: 10.1016/0022-2836(69)90148-x. [DOI] [PubMed] [Google Scholar]
- YANKOFSKY S. A., SPIEGELMAN S. The identification of the ribosomal RNA cistron by sequence complementarity. II. Saturation of and competitive interaction at the RNA cistron. Proc Natl Acad Sci U S A. 1962 Aug;48:1466–1472. doi: 10.1073/pnas.48.8.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]



