Abstract
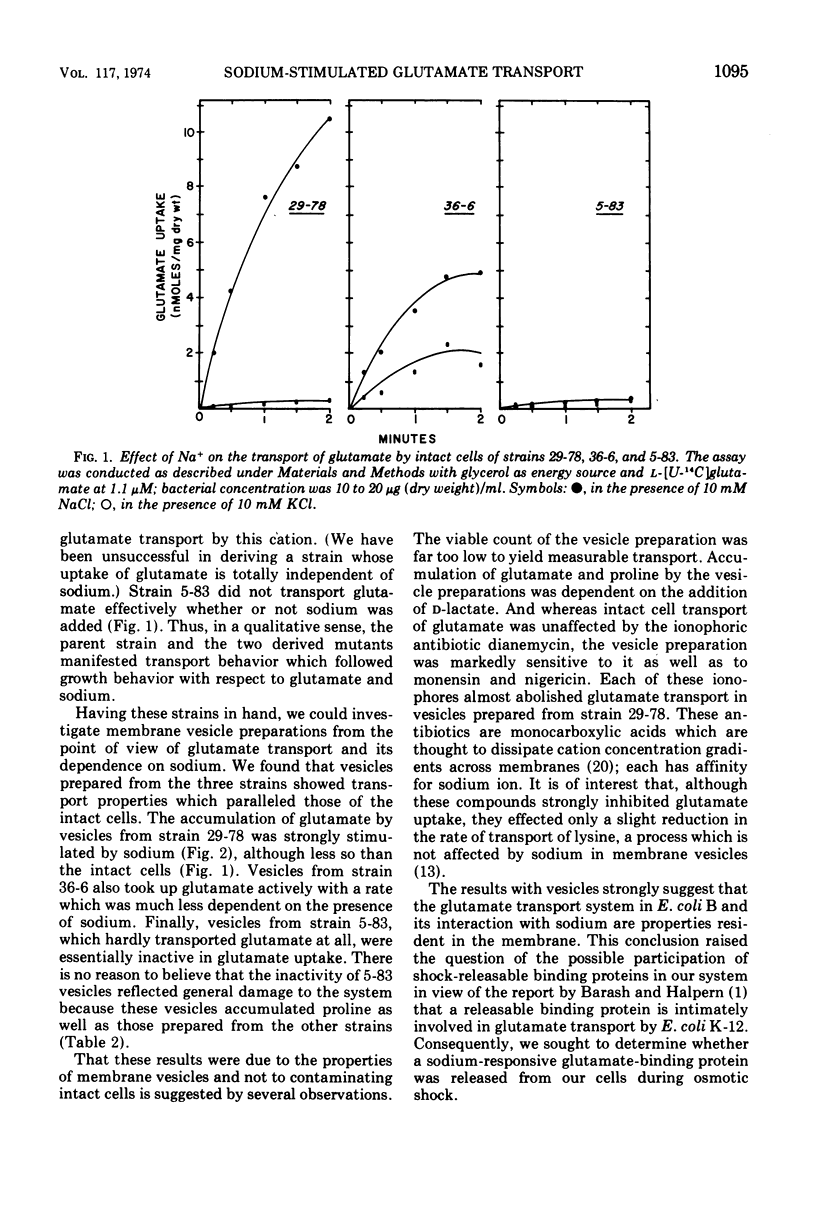
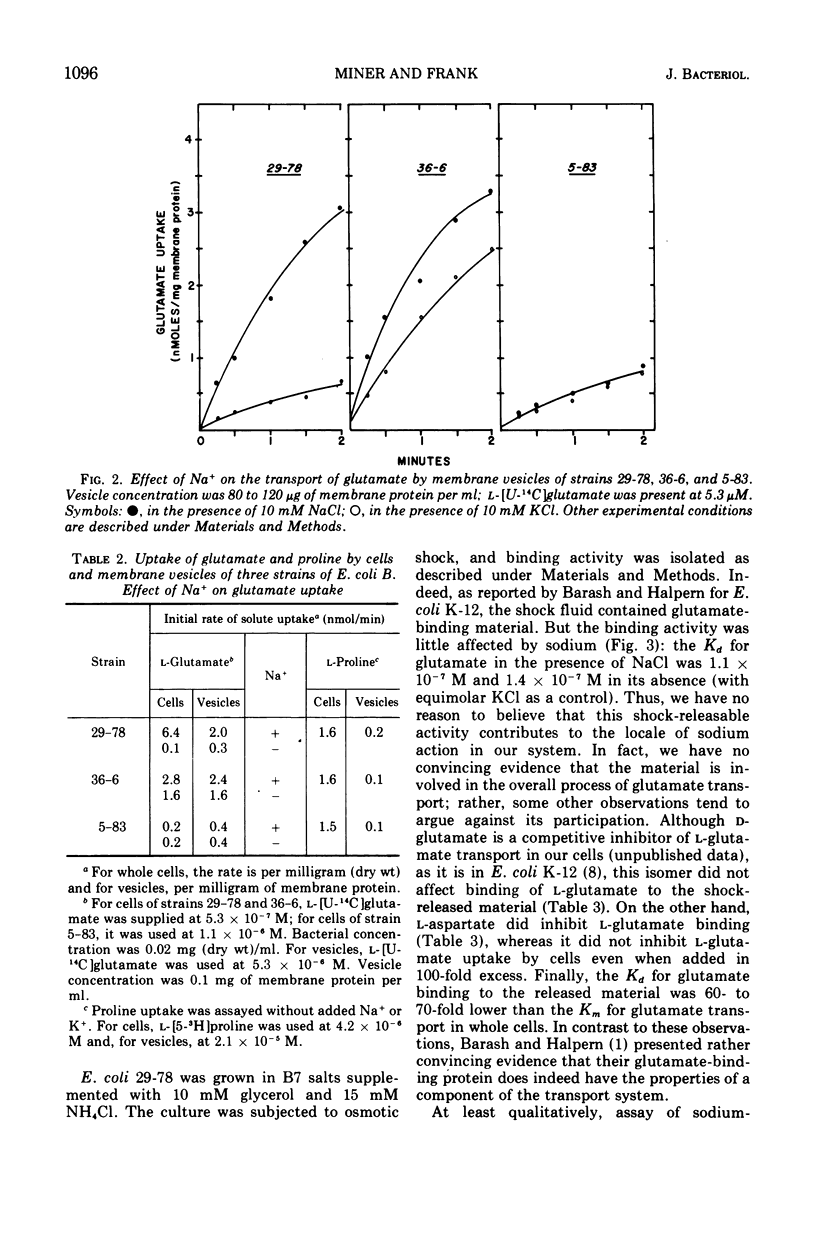
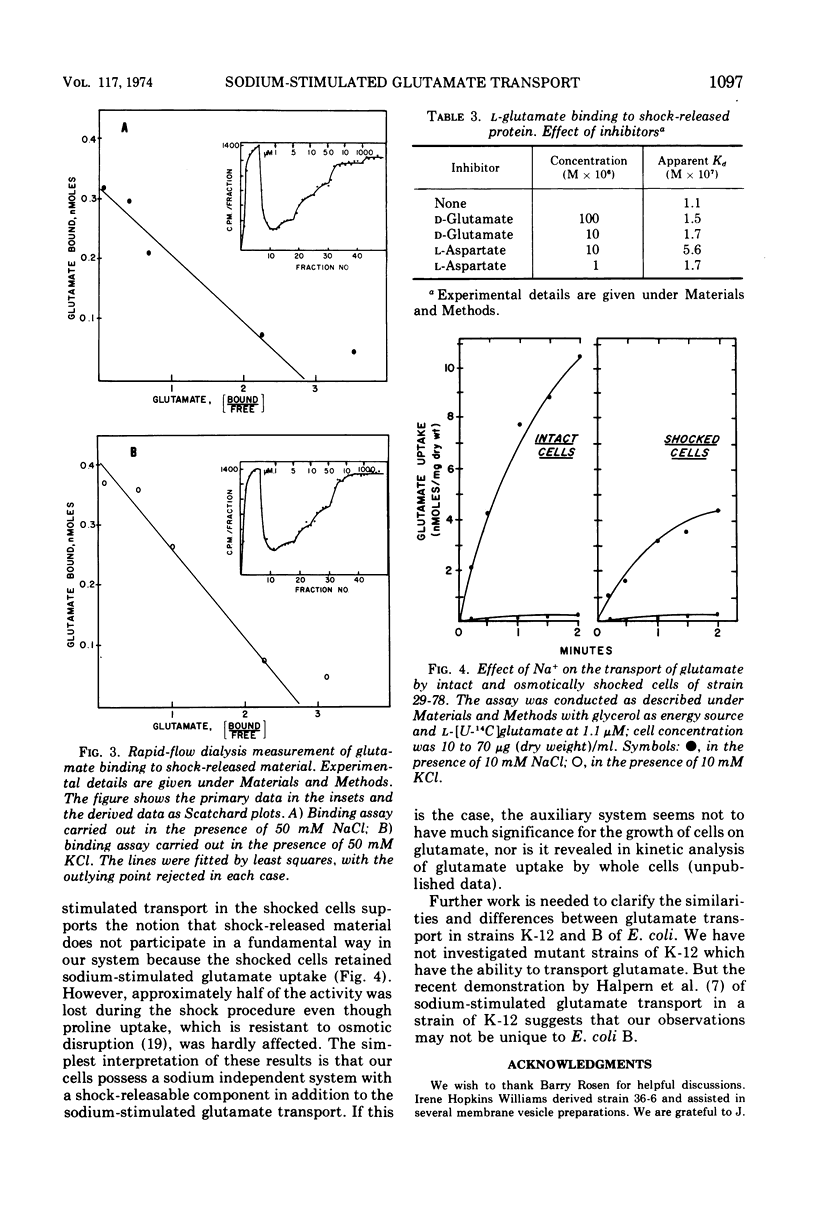
Three phenotypically distinct strains of Escherichia coli B were studied: one in which the transport of glutamate was strongly stimulated by sodium, one in which the transport was relatively independent of sodium, and one which did not transport glutamate. Membrane vesicle preparations from the three strains followed the behavior of whole cells with respect to sodium-stimulated transport. Although glutamate-binding material could be released from cells by osmotic shock, its affinity for glutamate was not significantly influenced by sodium. Furthermore, the shocked cells retained sodium-stimulated transport. The accumulated results suggest that the sodium-activated glutamate transport system resides in the cytoplasmic membrane and that releasable binding protein(s) is not intimately involved in its function.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barash H., Halpern Y. S. Glutamate-binding protein and its relation to glutamate transport in Escherichia coli K-12. Biochem Biophys Res Commun. 1971 Nov 5;45(3):681–688. doi: 10.1016/0006-291x(71)90470-0. [DOI] [PubMed] [Google Scholar]
- Colowick S. P., Womack F. C. Binding of diffusible molecules by macromolecules: rapid measurement by rate of dialysis. J Biol Chem. 1969 Feb 25;244(4):774–777. [PubMed] [Google Scholar]
- Drapeau G. R., Matula T. I., MacLeod R. A. Nutrition and metabolism of marine bacteria. XV. Relation of Na+-activated transport to the Na+ requirement of a marine pseudomonad for growth. J Bacteriol. 1966 Jul;92(1):63–71. doi: 10.1128/jb.92.1.63-71.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank L., Hopkins I. Sodium-stimulated transport of glutamate in Escherichia coli. J Bacteriol. 1969 Oct;100(1):329–336. doi: 10.1128/jb.100.1.329-336.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank L. Proline metabolism in Escherichia coli. IV. Preparation of proline-2- and proline-5- 3H and location of 3H in proline. Anal Biochem. 1966 Dec;17(3):423–433. doi: 10.1016/0003-2697(66)90178-3. [DOI] [PubMed] [Google Scholar]
- HALPERN Y. S., UMBARGER H. E. Utilization of L-glutamic and 2-oxoglutaric acid as sole sources of carbon by Escherichia coli. J Gen Microbiol. 1961 Oct;26:175–183. doi: 10.1099/00221287-26-2-175. [DOI] [PubMed] [Google Scholar]
- Halpern Y. S., Barash H., Dover S., Druck K. Sodium and potassium requirements for active transport of glutamate by Escherichia coli K-12. J Bacteriol. 1973 Apr;114(1):53–58. doi: 10.1128/jb.114.1.53-58.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern Y. S., Even-Shoshan A. Properties of the glutamate transport system in Escherichia coli. J Bacteriol. 1967 Mar;93(3):1009–1016. doi: 10.1128/jb.93.3.1009-1016.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H., Altendorf K., Harold F. M. Role of an electrical potential in the coupling of metabolic energy to active transport by membrane vesicles of Escherichia coli. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1804–1808. doi: 10.1073/pnas.70.6.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback H. R., Stadtman E. R. Proline uptake by an isolated cytoplasmic membrane preparation of Escherichia coli. Proc Natl Acad Sci U S A. 1966 Apr;55(4):920–927. doi: 10.1073/pnas.55.4.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lombardi F. J., Kaback H. R. Mechanisms of active transport in isolated bacterial membrane vesicles. 8. The transport of amino acids by membranes prepared from Escherichia coli. J Biol Chem. 1972 Dec 25;247(24):7844–7857. [PubMed] [Google Scholar]
- MacLeod R. A., Thurman P., Rogers H. J. Comparative transport activity of intact cells, membrane vesicles, and mesosomes of Bacillus licheniformis. J Bacteriol. 1973 Jan;113(1):329–340. doi: 10.1128/jb.113.1.329-340.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Pavlasova E., Harold F. M. Energy coupling in the transport of beta-galactosides by Escherichia coli: effect of proton conductors. J Bacteriol. 1969 Apr;98(1):198–204. doi: 10.1128/jb.98.1.198-204.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno J. R., Oxender D. L. Amino-acid-binding protein released from Escherichia coli by osmotic shock. J Biol Chem. 1966 Dec 10;241(23):5732–5734. [PubMed] [Google Scholar]
- Pressman B. C., Harris E. J., Jagger W. S., Johnson J. H. Antibiotic-mediated transport of alkali ions across lipid barriers. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1949–1956. doi: 10.1073/pnas.58.5.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayman M. K., Lo T. C., Sanwal B. D. Transport of succinate in Escherichia coli. II. Characteristics of uptake and energy coupling with transport in membrane preparations. J Biol Chem. 1972 Oct 10;247(19):6332–6339. [PubMed] [Google Scholar]
- Rosen B. P. Basic amino acid transport in Escherichia coli. II. Purification and properties of an arginine-specific binding protein. J Biol Chem. 1973 Feb 25;248(4):1211–1218. [PubMed] [Google Scholar]
- Rosen B. P. Basic amino acid transport in Escherichia coli. J Biol Chem. 1971 Jun 10;246(11):3653–3662. [PubMed] [Google Scholar]
- Rosen B. P. Beta-galactoside transport and proton movements in an adenosine triphosphatase-deficient mutant of Escherichia coli. Biochem Biophys Res Commun. 1973 Aug 21;53(4):1289–1296. doi: 10.1016/0006-291x(73)90605-0. [DOI] [PubMed] [Google Scholar]
- Sachan D. S., Stern J. R. Studies of citrate transport in Aerobacter aerogenes: binding of citrate by a membrane bound oxalacetate decarboxylase. Biochem Biophys Res Commun. 1971 Oct 15;45(2):402–408. doi: 10.1016/0006-291x(71)90833-3. [DOI] [PubMed] [Google Scholar]
- Schultz S. G., Curran P. F. Coupled transport of sodium and organic solutes. Physiol Rev. 1970 Oct;50(4):637–718. doi: 10.1152/physrev.1970.50.4.637. [DOI] [PubMed] [Google Scholar]
- Stock J., Roseman S. A sodium-dependent sugar co-transport system in bacteria. Biochem Biophys Res Commun. 1971 Jul 2;44(1):132–138. doi: 10.1016/s0006-291x(71)80168-7. [DOI] [PubMed] [Google Scholar]
- West I. C. Lactose transport coupled to proton movements in Escherichia coli. Biochem Biophys Res Commun. 1970 Nov 9;41(3):655–661. doi: 10.1016/0006-291x(70)90063-x. [DOI] [PubMed] [Google Scholar]
- West I., Mitchell P. Proton-coupled beta-galactoside translocation in non-metabolizing Escherichia coli. J Bioenerg. 1972 Aug;3(5):445–462. doi: 10.1007/BF01516082. [DOI] [PubMed] [Google Scholar]
- Wong P. T., Thompson J., MacLeod R. A. Nutrition and metabolism of marine bacteria. XVII. Ion-dependent retention of alpha-aminoisobutyric acid and its relation to Na+ dependent transport in a marine pseudomonad. J Biol Chem. 1969 Feb 10;244(3):1016–1025. [PubMed] [Google Scholar]


