Abstract
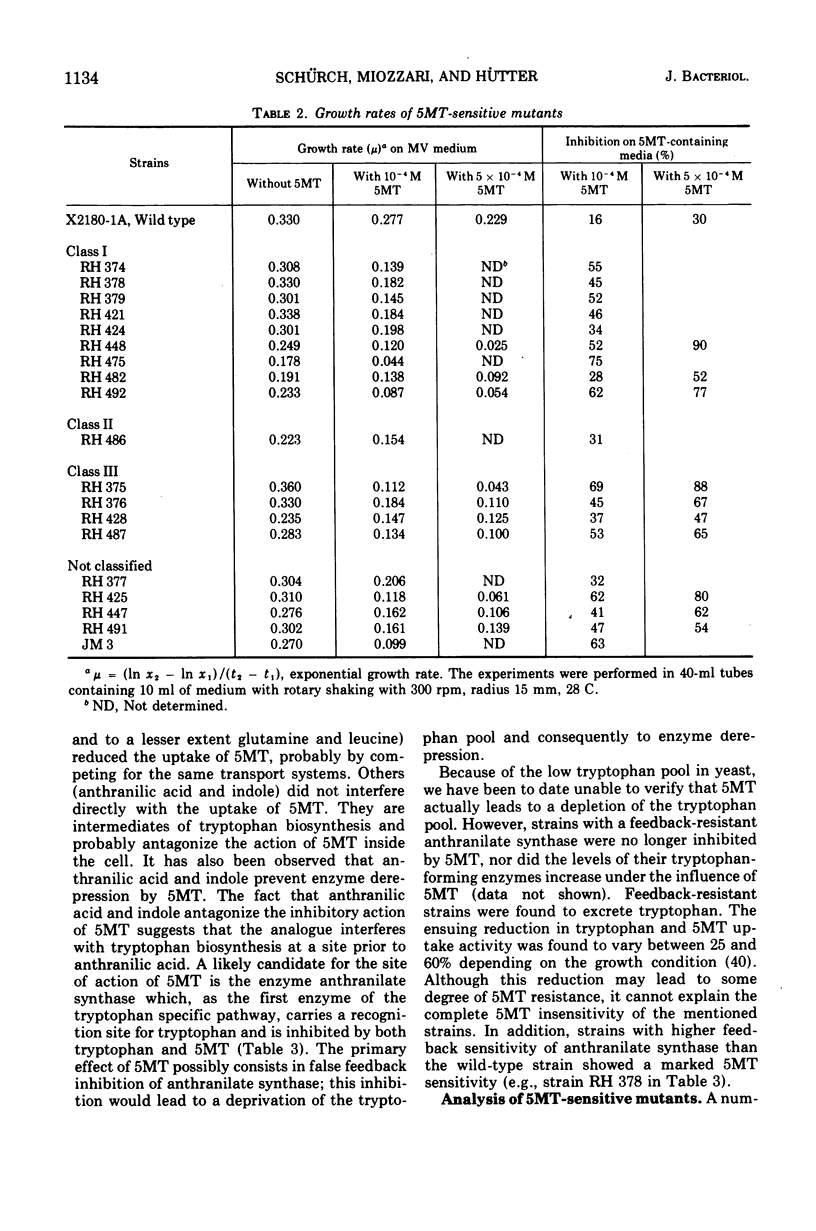
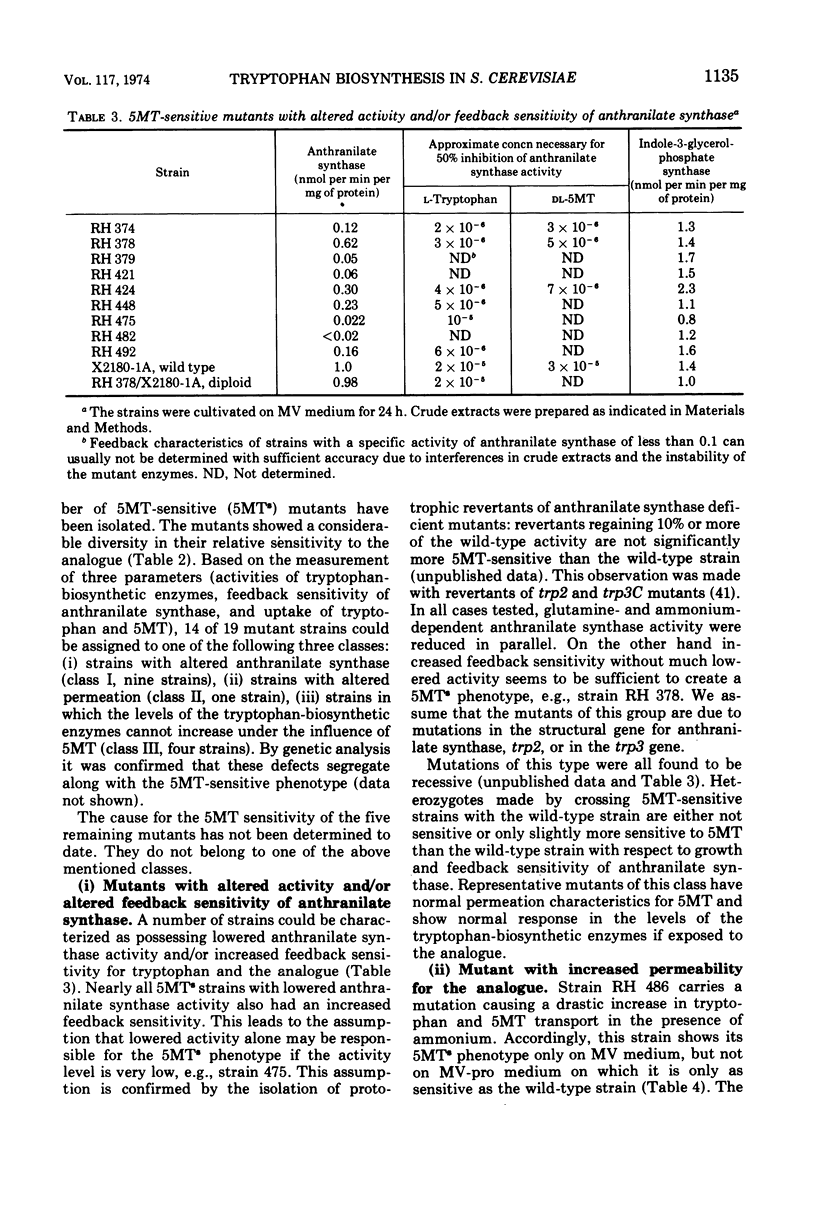
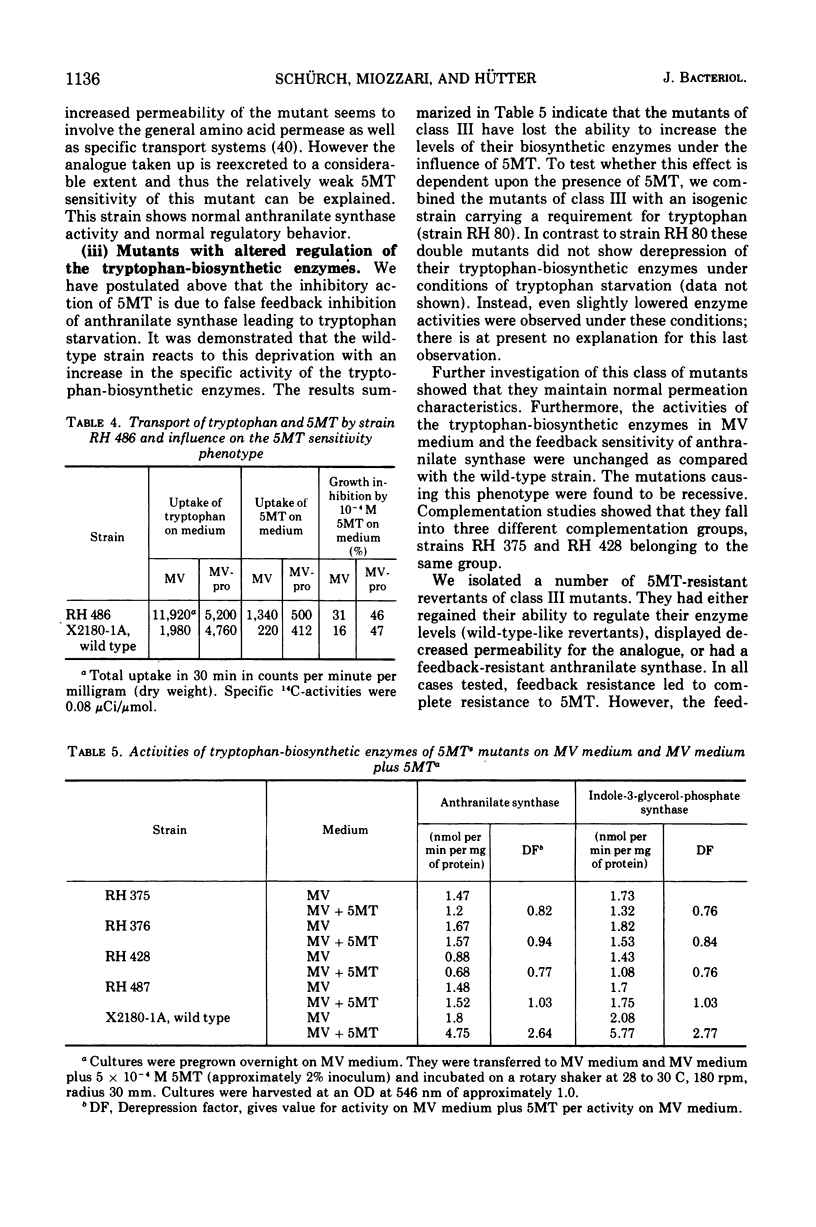
In a wild-type strain of Saccharomyces cerevisiae the tryptophan analogue dl-5-methyl-tryptophan (5MT) causes only a slight reduction of the growth rate. Uptake experiments indicate that the limited inhibition is partly due to low levels of 5MT inside the cell. On the other hand, this low concentration of 5MT leads to an increase in the activity of the tryptophan-biosynthetic enzymes. Evidence is presented that suggests that 5MT acts primarily through feedback inhibition of anthranilate synthase, the first enzyme of the pathway. A number of 5MT-sensitive mutants have been isolated, characterized, and assigned to one of the following three classes: class I, strains with altered activity and/or feedback sensitivity of anthranilate synthase; class II, strains with elevated uptake of 5MT; class III, mutants with altered regulation of the tryptophan-biosynthetic enzymes, which do not exhibit increases in activity in the presence of 5MT. This failure to exhibit increased enzyme activities in mutants of class III can also be observed after tryptophan starvation. Two mutants of class III show high sensitivity towards 3-amino-1,2,4-triazole. They can not exhibit derepression of some histidine- and arginine-biosynthetic enzymes under conditions that lead to an increase in these same enzymes in the wild-type strain.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bechet J., Greenson M., Wiame J. M. Mutations affecting the repressibility of arginine biosynthetic enzymes in Saccharomyces cerevisiae. Eur J Biochem. 1970 Jan;12(1):31–39. doi: 10.1111/j.1432-1033.1970.tb00817.x. [DOI] [PubMed] [Google Scholar]
- Bechet J., Wiame J. M. Indication of a specific regulatory binding protein for ornithinetranscarbamylase in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1965 Nov 8;21(3):226–234. doi: 10.1016/0006-291x(65)90276-7. [DOI] [PubMed] [Google Scholar]
- Brooks C. J., DeBusk B. G., DeBusk A. G., Catcheside D. E. A new class of p-fluorophenylalanine-resistant mutants in Neurospora crassa. Biochem Genet. 1972 Jun;6(4):239–254. doi: 10.1007/BF00486118. [DOI] [PubMed] [Google Scholar]
- Bussey H., Umbarger H. E. Biosynthesis of the branched-chain amino acids in yeast: a trifluoroleucine-resistant mutant with altered regulation of leucine uptake. J Bacteriol. 1970 Aug;103(2):286–294. doi: 10.1128/jb.103.2.286-294.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARSIOTIS M., LACY A. M. INCREASED ACTIVITY OF TRYPTOPHAN BIOSYNTHETIC ENZYMES IN HISTIDINE MUTANTS OF NEUROSPORA CRASSA. J Bacteriol. 1965 Jun;89:1472–1477. doi: 10.1128/jb.89.6.1472-1477.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carsiotis M., Jones R. F., Lacy A. M., Cleary T. J., Fankhauser D. B. Histidine-mediated control of tryptophan biosynthetic enzymes in Neurospora crassa. J Bacteriol. 1970 Oct;104(1):98–106. doi: 10.1128/jb.104.1.98-106.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherest H., Surdin-Kerjan Y., Robichon-Szulmajster H. Methionine-mediated repression in Saccharomyces cerevisiae: a pleiotropic regulatory system involving methionyl transfer ribonucleic acid and the product of gene eth2. J Bacteriol. 1971 Jun;106(3):758–772. doi: 10.1128/jb.106.3.758-772.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMoss J. A., Jackson R. W., Chalmers J. H., Jr Genetic control of the structure and activity of an enzyme aggregate in the tryptophan pathway of Neurospora crassa. Genetics. 1967 Jul;56(3):413–424. doi: 10.1093/genetics/56.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doy C. H., Cooper J. M. Aromatic biosynthesis in yeast. I. The synthesis of tryptophan and the regulation of this pathway. Biochim Biophys Acta. 1966 Oct 31;127(2):302–316. [PubMed] [Google Scholar]
- Giles N. H., Case M. E., Partridge C. W., Ahmed S. I. A gene cluster in Nuerospora crassa coding for an aggregate of five aromatic synthetic enzymes. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1453–1460. doi: 10.1073/pnas.58.4.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenson M., Hou C., Crabeel M. Multiplicity of the amino acid permeases in Saccharomyces cerevisiae. IV. Evidence for a general amino acid permease. J Bacteriol. 1970 Sep;103(3):770–777. doi: 10.1128/jb.103.3.770-777.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenson M., Mousset M., Wiame J. M., Bechet J. Multiplicity of the amino acid permeases in Saccharomyces cerevisiae. I. Evidence for a specific arginine-transporting system. Biochim Biophys Acta. 1966 Oct 31;127(2):325–338. doi: 10.1016/0304-4165(66)90387-4. [DOI] [PubMed] [Google Scholar]
- Gross S. R. The regulation of synthesis of leucine biosynthetic enzymes in Neurospora. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1538–1546. doi: 10.1073/pnas.54.6.1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAWTHORNE D. C., MORTIMER R. K. Super-suppressors in yeast. Genetics. 1963 Apr;48:617–620. doi: 10.1093/genetics/48.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga S., Ito K., Matsuyama T., Ozaki H., Yura T. 5-methyltryptophan-resistant mutations lniked with the arginine G marker in Escherichia coli. J Bacteriol. 1968 Nov;96(5):1880–1881. doi: 10.1128/jb.96.5.1880-1881.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch S. O., Roth C. W., Crawford I. P., Nester E. W. Control of tryptophan biosynthesis by the methyltryptophan resistance gene in Bacillus subtilis. J Bacteriol. 1971 Jan;105(1):38–45. doi: 10.1128/jb.105.1.38-45.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSTON J. R., MORTIMER R. K. Use of snail digestive juice in isolation of yeast spore tetrads. J Bacteriol. 1959 Aug;78:292–292. doi: 10.1128/jb.78.2.292-292.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane J. F., Jensen R. A. The molecular aggregation of anthranilate synthase in Bacillus subtilis. Biochem Biophys Res Commun. 1970 Oct 23;41(2):328–333. doi: 10.1016/0006-291x(70)90507-3. [DOI] [PubMed] [Google Scholar]
- Kashmiri S. V., Gross S. R. Mutations affecting the regulation of production of the enzymes of leucine synthesis in Neurospora. Genetics. 1970 Mar-Apr;64(3):423–440. [PMC free article] [PubMed] [Google Scholar]
- Kuhn J. C., Pabst M. J., Somerville R. L. Mutant strains of Escherichia coli K-12 exhibiting enhanced sensitivity to 5-methyltryptophan. J Bacteriol. 1972 Oct;112(1):93–101. doi: 10.1128/jb.112.1.93-101.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lester G. Genetic control of amino acid permeability in Neurospora crassa. J Bacteriol. 1966 Feb;91(2):677–684. doi: 10.1128/jb.91.2.677-684.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester G. In vivo regulation of intermediate reactions in the pathway of tryptophan biosynthesis in Neurospora crassa. J Bacteriol. 1968 Nov;96(5):1768–1773. doi: 10.1128/jb.96.5.1768-1773.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester G. Regulation of tryptophan biosynthetic enzymes in Neurospora crassa. J Bacteriol. 1971 Jul;107(1):193–202. doi: 10.1128/jb.107.1.193-202.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingens F., Goebel W., Uesseler H. Regulation der Biosynthese der aromatischen Aminosäuren in Saccharomyces cerevisiae. 2. Repression, Induktion und Aktivierung. Eur J Biochem. 1967 May;1(3):363–374. doi: 10.1111/j.1432-1033.1967.tb00083.x. [DOI] [PubMed] [Google Scholar]
- Lomax C. A., Woods R. A. A complex genetic locus controlling purine nucleotide biosynthesis in yeast. Mol Gen Genet. 1973 Jan 24;120(2):139–149. doi: 10.1007/BF00267242. [DOI] [PubMed] [Google Scholar]
- SOMERVILLE R. L., YANOFSKY C. STUDIES ON THE REGULATION OF TRYPTOPHAN BIOSYNTHESIS IN ESCHERICHIA COLI. J Mol Biol. 1965 Apr;11:747–759. doi: 10.1016/s0022-2836(65)80032-8. [DOI] [PubMed] [Google Scholar]
- Satyanarayana T., Umbarger H. E., Lindegren G. Biosynthesis of branched-chain amino acids in yeast: regulation of leucine biosynthesis in prototrophic and leucine auxotrophic strains. J Bacteriol. 1968 Dec;96(6):2018–2024. doi: 10.1128/jb.96.6.2018-2024.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schürch-Rathgeb Y. Der trp3-Locus von Saccharomyces cerevisiae. Arch Genet (Zur) 1972;45(3):129–159. [PubMed] [Google Scholar]
- Turner J. R., Matchett W. H. Alteration of tryptophan-mediated regulation in Neurospora crassa by indoleglycerol phosphate. J Bacteriol. 1968 May;95(5):1608–1614. doi: 10.1128/jb.95.5.1608-1614.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbarger H. E. Regulation of amino acid metabolism. Annu Rev Biochem. 1969;38:323–370. doi: 10.1146/annurev.bi.38.070169.001543. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Wegman J., DeMoss J. A. The enzymatic conversion of anthranilate to indolylglycerol phosphate in Neurospora crassa. J Biol Chem. 1965 Oct;240(10):3781–3788. [PubMed] [Google Scholar]
- Whitt D. D., Carlton B. C. Non-coordinate regulation in 5-methyl tryptophan-resistant mutants of Bacillus subtilis. Biochem Biophys Res Commun. 1968 Nov 25;33(4):636–642. doi: 10.1016/0006-291x(68)90343-4. [DOI] [PubMed] [Google Scholar]
- Wiater A., Hulanicka D., Klopotowski T. Structural requirements for inhibition of yeast imidazoleglycerol phosphate dehydratase by triazole and anion inhibitors. Acta Biochim Pol. 1971;18(3):289–297. [PubMed] [Google Scholar]



