Abstract
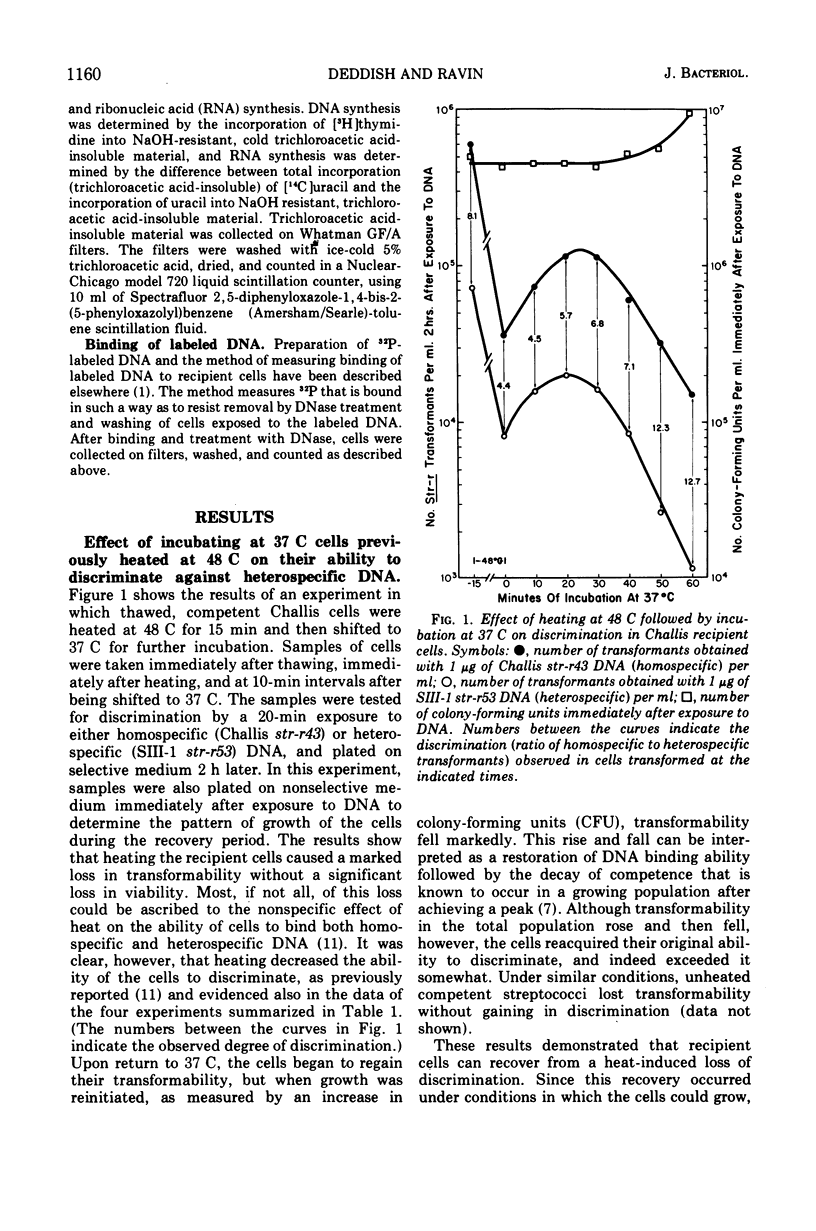
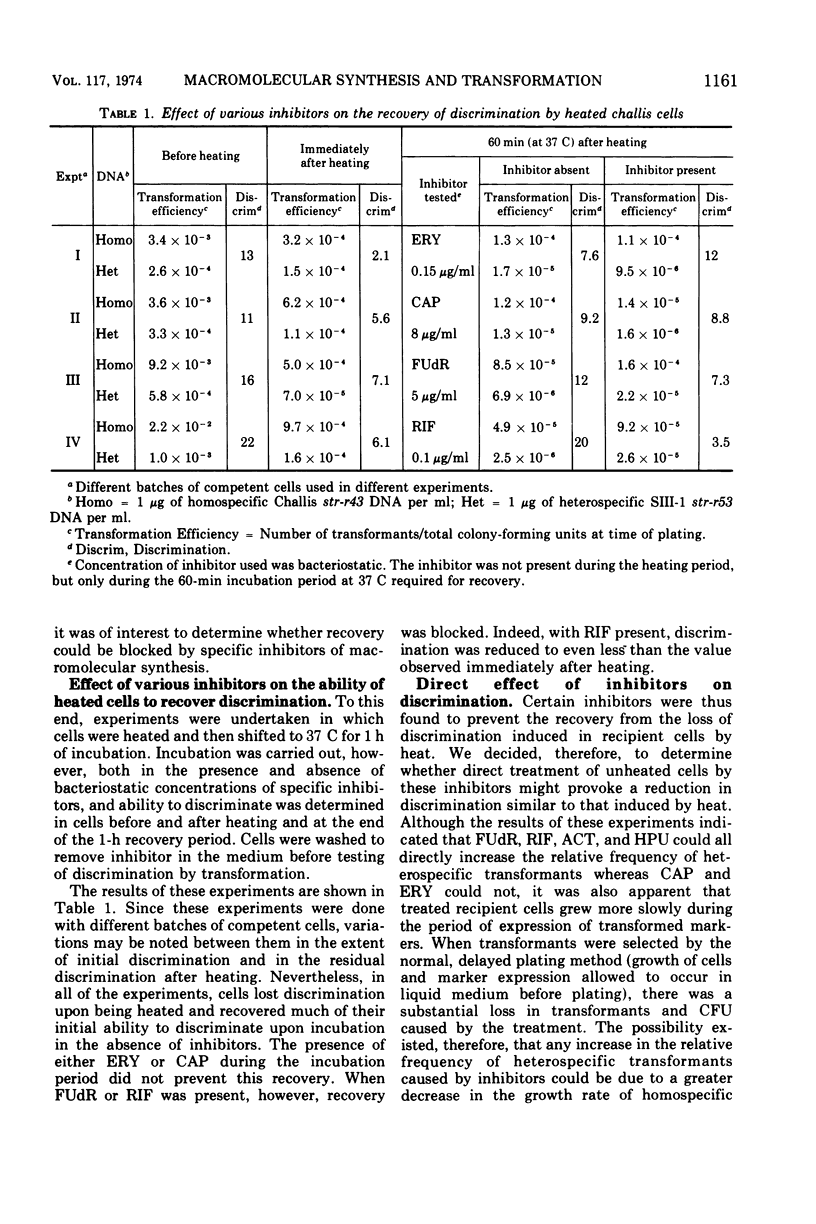
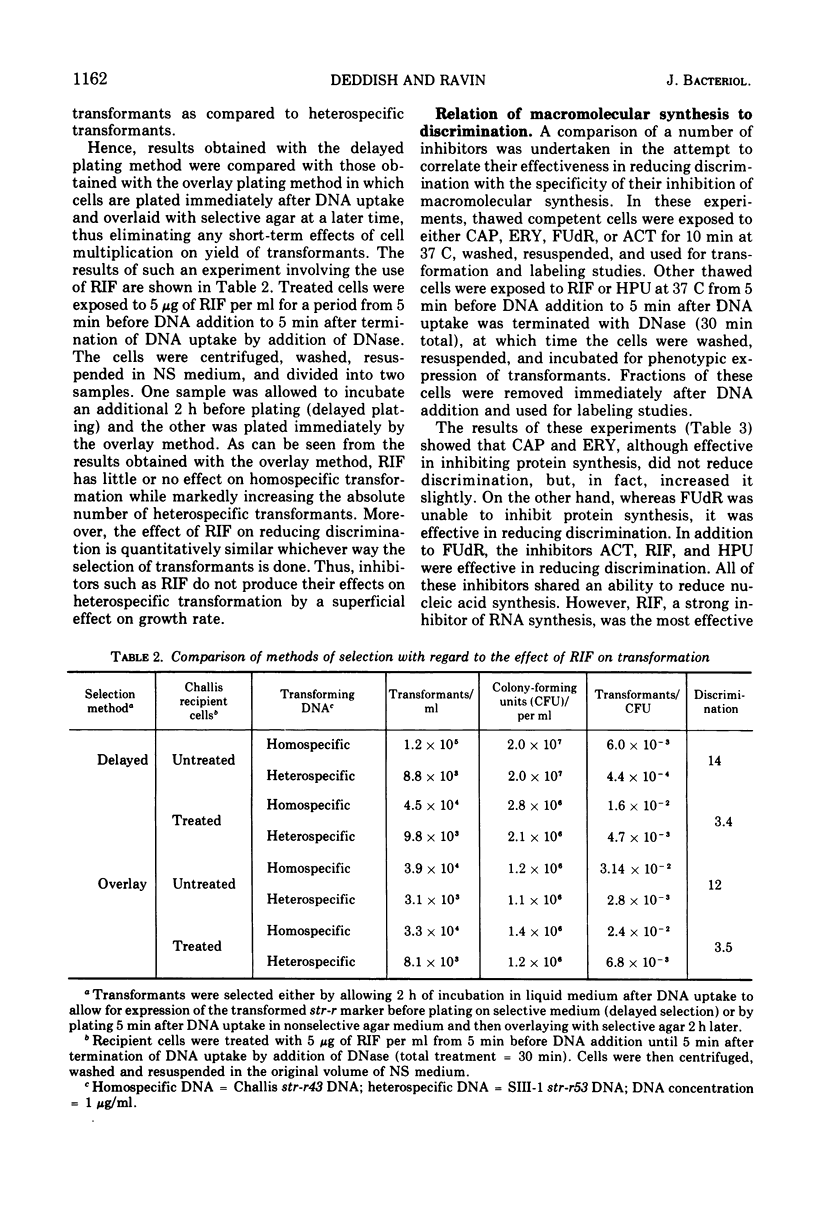
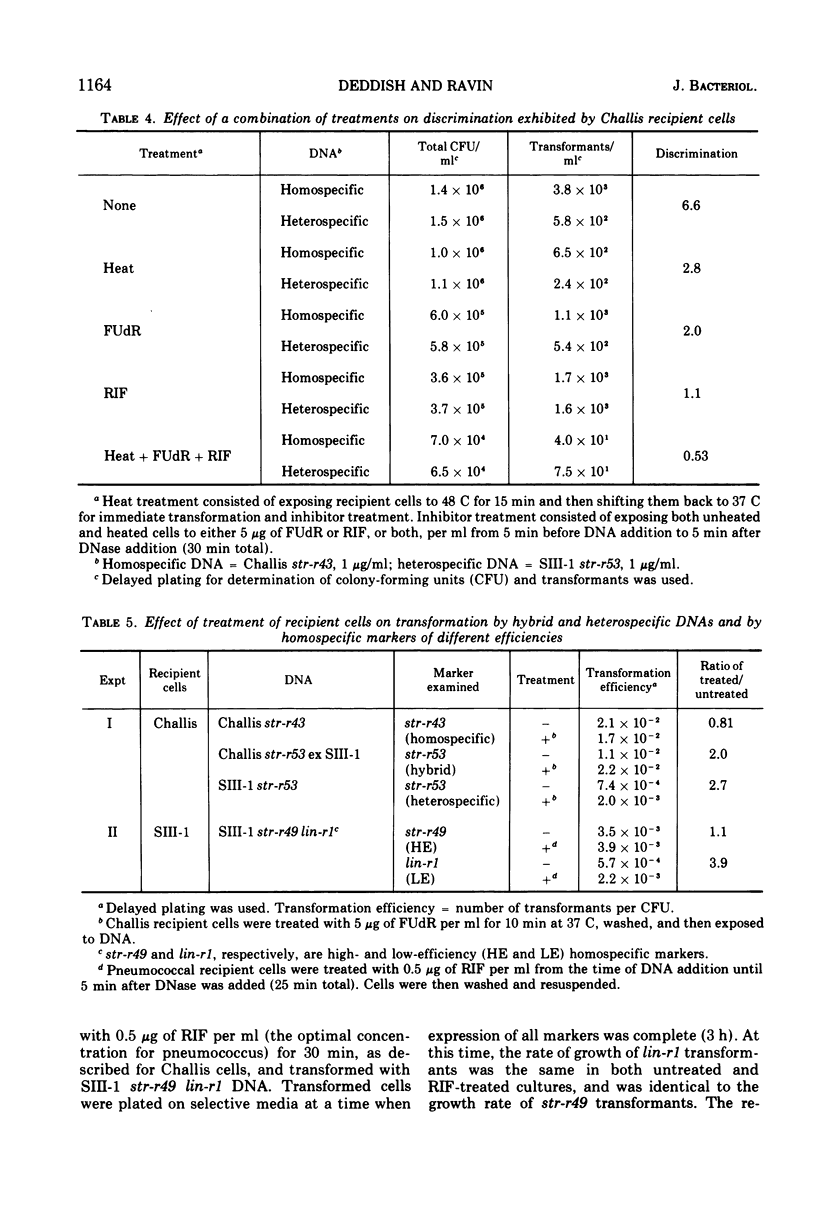
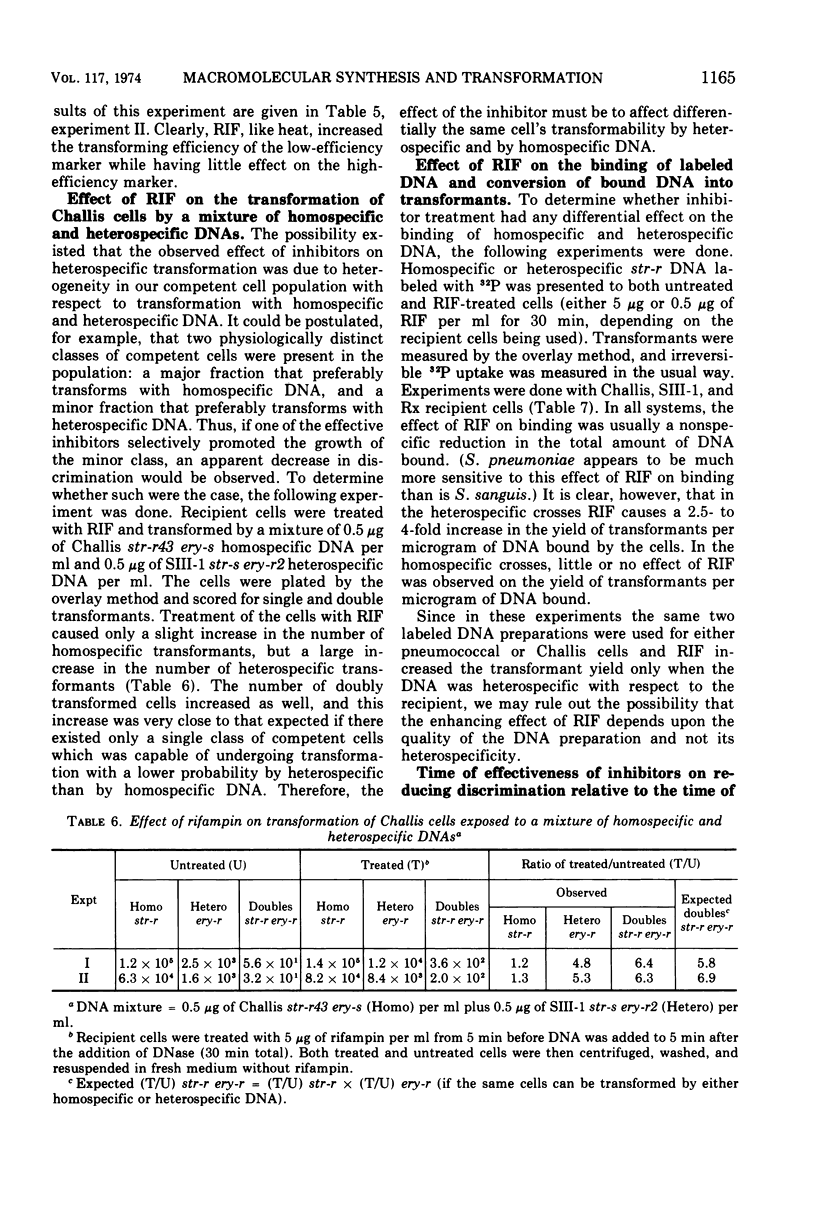
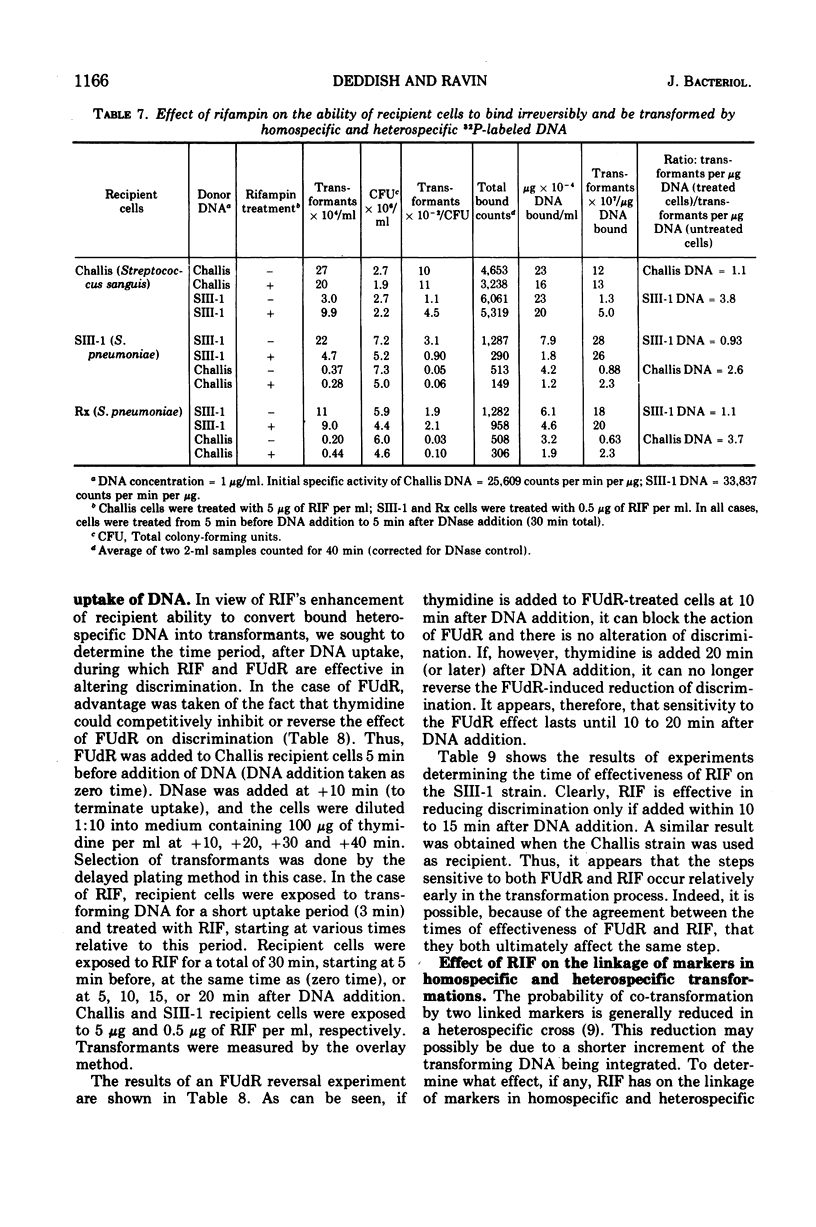
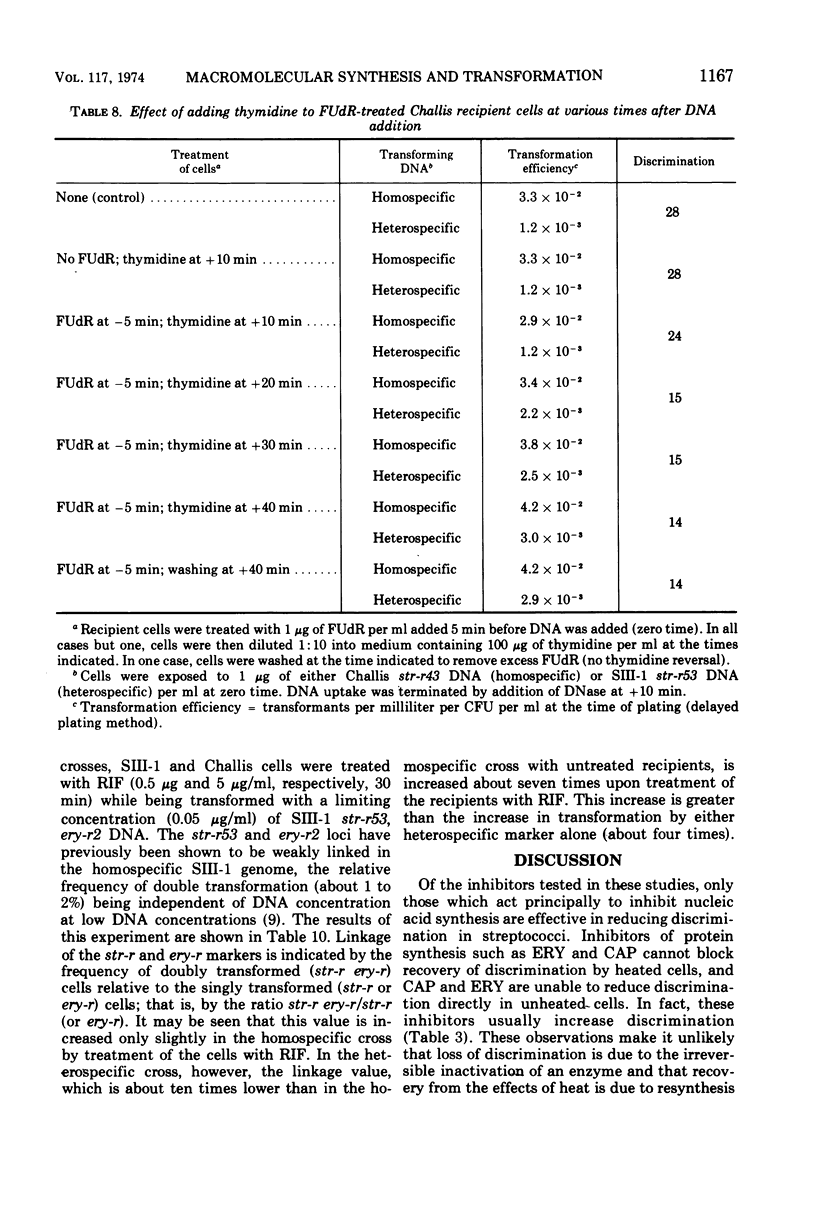
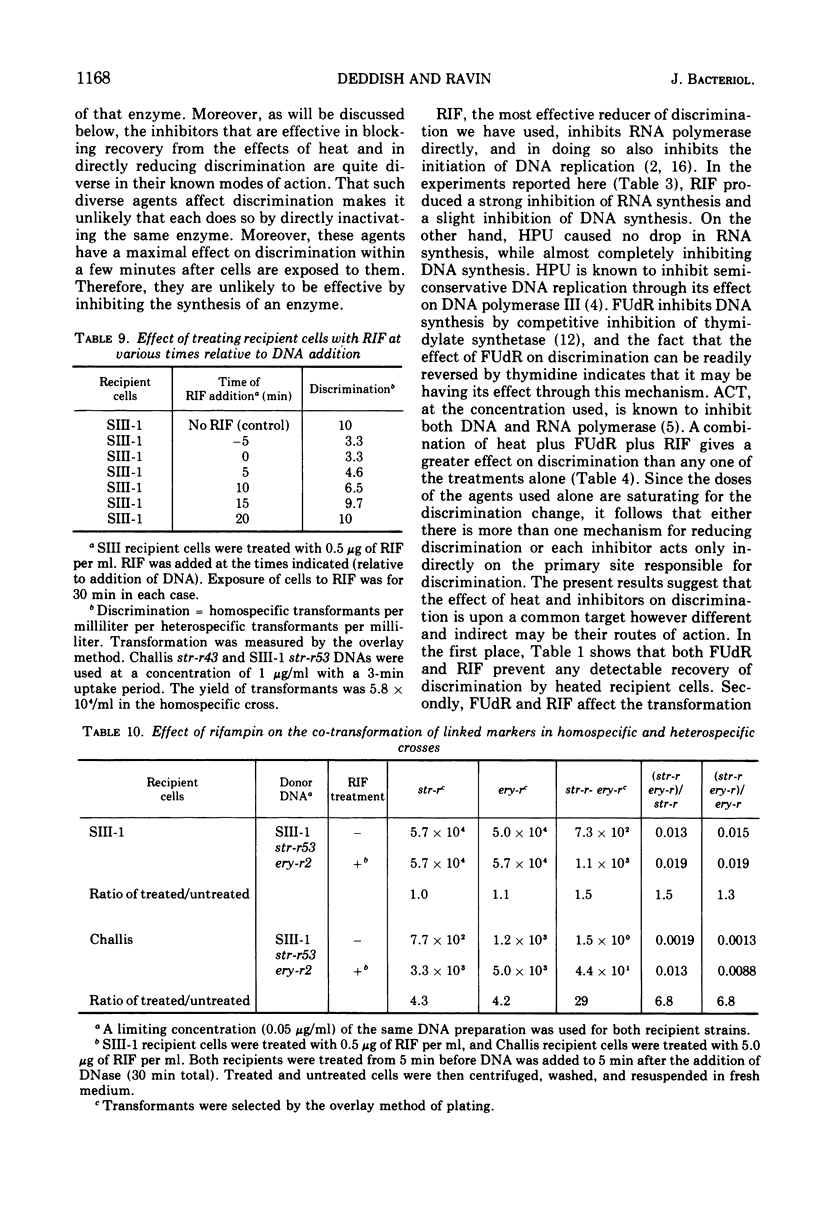
In previous studies with Streptococcus sanguis and S. pneumoniae as recipients and donors of transforming deoxyribonucleic acid (DNA), it was found that heating recipients just prior to exposure to DNA caused an increase in the number of transformants induced by heterospecific DNA relative to that induced by homospecific DNA. In the present studies, S. sanguis recipients were found to recover from this effect of heat (48 C, 15 min) when incubated at 37 C before exposure to DNA. Inhibitors of nucleic acid synthesis, such as rifampin, 5-fluorodeoxyuridine, actinomycin, and p-hydroxyphenylazo-uracil, but not inhibitors of protein synthesis, such as chloramphenicol and erythromycin, prevented recovery from the effect of heat. Inhibitors of nucleic acid synthesis caused changes in unheated cells similar to those observed with heat treatment; these changes included increased transformability by genetically hybrid DNA and by low-efficiency markers in homospecific DNA. The effect of a combination of heat and inhibitors on transformation by heterospecific DNA was greater than when single treatments were used. The most effective inhibitor used alone was rifampin: in treated recipient cells, the yield of transformants produced by a given amount of irreversibly bound heterospecific DNA was increased without a significant change in the yield of transformants produced by bound homospecific DNA. A cell being doubly transformed by homospecific and heterospecific DNA was enhanced specifically in its transformability with the latter as a consequence of rifampin treatment. Treatment with rifampin also increased co-transformation by linked heterospecific markers. The period during which recipient cells were sensitive to the effects induced by rifampin and fluorodeoxyuridine lasted from 10 to 20 min after DNA uptake.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brutlag D., Schekman R., Kornberg A. A possible role for RNA polymerase in the initiation of M13 DNA synthesis. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2826–2829. doi: 10.1073/pnas.68.11.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzarelli N. R., Low R. L. Mutational alteration of Bacillus subtilis DNA polymerase 3 to hydroxyphenylazopyrimidine resistance: polymerase 3 is necessary for DNA replication. Biochem Biophys Res Commun. 1973 Mar 5;51(1):151–157. doi: 10.1016/0006-291x(73)90521-4. [DOI] [PubMed] [Google Scholar]
- GOLDBERG I. H., REICH E. ACTINOMYCIN INHIBITION OF RNA SYNTHESIS DIRECTED BY DNA. Fed Proc. 1964 Sep-Oct;23:958–964. [PubMed] [Google Scholar]
- Gurney T., Jr, Fox M. S. Physical and genetic hybrids formed in bacterial transformation. J Mol Biol. 1968 Feb 28;32(1):83–100. doi: 10.1016/0022-2836(68)90147-2. [DOI] [PubMed] [Google Scholar]
- Hotchkiss R. D. CYCLICAL BEHAVIOR IN PNEUMOCOCCAL GROWTH AND TRANSFORMABILITY OCCASIONED BY ENVIRONMENTAL CHANGES. Proc Natl Acad Sci U S A. 1954 Feb;40(2):49–55. doi: 10.1073/pnas.40.2.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRK J. M. The mode of action of actinomycin D. Biochim Biophys Acta. 1960 Jul 29;42:167–169. doi: 10.1016/0006-3002(60)90769-1. [DOI] [PubMed] [Google Scholar]
- RAVIN A. W., DESA J. H. GENETIC LINKAGE OF MUTATIONAL SITES AFFECTING SIMILAR CHARACTERS IN PNEUMOCOCCUS AND STREPTOCOCCUS. J Bacteriol. 1964 Jan;87:86–96. doi: 10.1128/jb.87.1.86-96.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYES P., HEIDELBERGER C. FLUORINATED PYRIMIDINES. XXV. THE INHIBITION OF THYMIDYLATE SYNTHETASE FROM EHRLICH ASCITES CARCINOMA CELLS BY PYRIMIDINE ANALOGS. Biochim Biophys Acta. 1965 May 11;103:177–179. [PubMed] [Google Scholar]
- ROODYN D. B., MANDEL H. G. A simple membrane fractionation method for determining the distribution of radioactivity in chemical fractions of Bacillus cereus. Biochim Biophys Acta. 1960 Jun 17;41:80–88. doi: 10.1016/0006-3002(60)90371-1. [DOI] [PubMed] [Google Scholar]
- ROTHEIM M. B., RAVIN A. W. The mapping of genetic loci affecting streptomycin resistance in Pneumococcus. Genetics. 1961 Dec;46:1619–1634. doi: 10.1093/genetics/46.12.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravin A. W., Chen K. C. Heterospecific transformation of pneumococcus and streptococcus. 3. Reduction of linkage. Genetics. 1967 Dec;57(4):851–864. doi: 10.1093/genetics/57.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravin A. W., Ma M. Specific effects of heating of transformable streptococci on their ability to discriminate between homospecific, heterospecific, and hybrid deoxyribonucleic acid. J Bacteriol. 1972 Feb;109(2):616–625. doi: 10.1128/jb.109.2.616-625.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow J. K., Boling M. E., Allison D. P., Beattie K. L. Relationship between prophage induction and transformation in Haemophilus influenzae. J Bacteriol. 1973 Jul;115(1):153–161. doi: 10.1128/jb.115.1.153-161.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W., Brutlag D., Schekman R., Kornberg A. RNA synthesis initiates in vitro conversion of M13 DNA to its replicative form. Proc Natl Acad Sci U S A. 1972 Apr;69(4):965–969. doi: 10.1073/pnas.69.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]



