Abstract
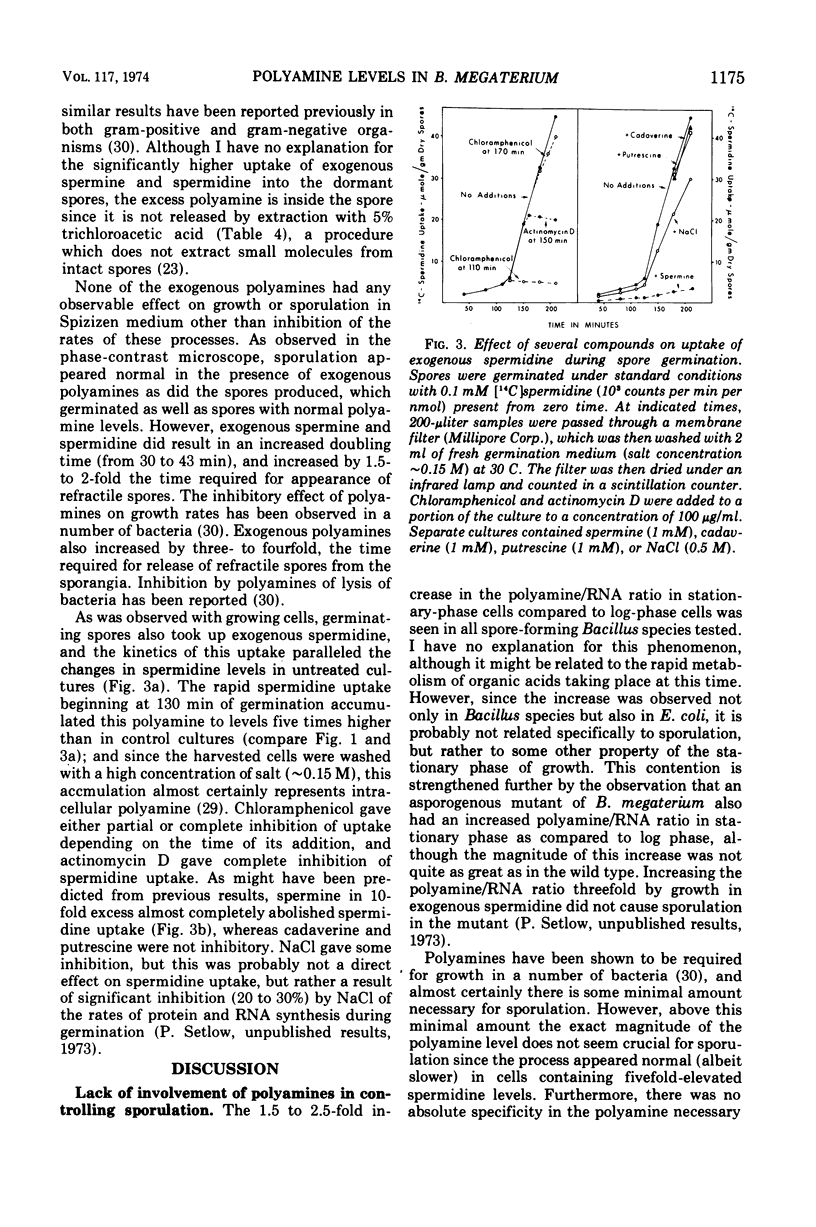
Spermidine was the major (>95%) polyamine of Bacillus megaterium in all stages of growth, although it could be replaced completely by spermine. Log-phase cells had 40 to 50% as much spermidine, based on ribonucleic acid (RNA) content, as did either stationary-phase cells or dormant spores; similar results were obtained in three other bacilli including an asporogenous mutant. Polyamine levels were essentially the same in B. megaterium grown in rich or poor media, or in media of high or low ionic strength. Polyamine levels were elevated three- to sixfold by exogenous spermidine without a major effect on growth, sporulation, or subsequent spore germination. During germination, the absolute amount of spermidine remained constant for almost 2 h until net RNA synthesis had lowered the polyamine/RNA ratio to a value close to that in log-phase cells. At this time, the spermidine level began to rise, and thereafter spermidine and RNA increased in parallel. This parallel relationship between the spermidine and RNA levels was abolished by actinomycin D, but not by chloramphenicol.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyle S. M., Cohen P. S., Morse J. W., Selden M. Relations between spermidine content and RNA stability in rifampicin treated Escherichia coli. Biochem Biophys Res Commun. 1972 Apr 28;47(2):451–458. doi: 10.1016/0006-291x(72)90735-8. [DOI] [PubMed] [Google Scholar]
- Changchien L. M., Aronson J. N. Spermidine requirement for Bacillus thuringiensis ribosomes in cell-free phenylalanine incorporation. J Bacteriol. 1970 Sep;103(3):734–740. doi: 10.1128/jb.103.3.734-740.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. S., Hoffner N., Jansen M., Moore M., Raina A. POLYAMINES, RNA SYNTHESIS, AND STREPTOMYCIN LETHALITY IN A RELAXED MUTANT OF E. coli STRAIN 15 TAU. Proc Natl Acad Sci U S A. 1967 Mar;57(3):721–728. doi: 10.1073/pnas.57.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlin G., Broda P. Physiology and genetics of the "ribonucleic acid control" locus in escherichia coli. Bacteriol Rev. 1968 Sep;32(3):206–226. doi: 10.1128/br.32.3.206-226.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann V. A., Thomas G. A., Norton J. W., Herbst E. J. The effect of polyamones on the enzymatic degradation of ribosomes. Biochim Biophys Acta. 1968 Mar 18;157(1):43–51. doi: 10.1016/0005-2787(68)90262-1. [DOI] [PubMed] [Google Scholar]
- Gumport R. F., Weiss S. B. Transcriptional and sedimentation properties of ribonucleic acid polymerase from Micrococcus lysodeikticus. Biochemistry. 1969 Sep;8(9):3618–3628. doi: 10.1021/bi00837a019. [DOI] [PubMed] [Google Scholar]
- HERBST E. J., WEAVER R. H., KEISTER D. L. The gram reaction and cell composition: diamines and polyamines. Arch Biochem Biophys. 1958 May;75(1):171–177. doi: 10.1016/0003-9861(58)90407-7. [DOI] [PubMed] [Google Scholar]
- Hussey C., Losick R., Sonenshein A. L. Ribosomal RNA synthesis is turned off during sporulation of Bacillus subtilis. J Mol Biol. 1971 Apr 14;57(1):59–70. doi: 10.1016/0022-2836(71)90119-7. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y., Halvorson H. O. Evidence for a defective protein synthesizing system in dormant spores of Bacillus cereus. Arch Biochem Biophys. 1968 Mar 11;123(3):622–632. doi: 10.1016/0003-9861(68)90182-3. [DOI] [PubMed] [Google Scholar]
- Michaels R., Tchen T. T. Polyamine content of nucleated and enucleated Escherichia coli cells. J Bacteriol. 1968 May;95(5):1966–1967. doi: 10.1128/jb.95.5.1966-1967.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro G. F., Hercules K., Morgan J., Sauerbier W. Dependence of the putrescine content of Escherichia coli on the osmotic strength of the medium. J Biol Chem. 1972 Feb 25;247(4):1272–1280. [PubMed] [Google Scholar]
- Nelson D. L., Kornberg A. Biochemical studies of bacterial sporulation and germination. XIX. Phosphate metabolism during sporulation. J Biol Chem. 1970 Mar 10;245(5):1137–1145. [PubMed] [Google Scholar]
- Raina A., Cohen S. S. Polyamines and RNA synthesis in a polyauxotrophic strain of E. coli. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1587–1593. doi: 10.1073/pnas.55.6.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina A., Jansen M., Cohen S. S. Polyamines and the accumulation of ribonucleic acid in some polyauxotrophic strains of Escherichia coli. J Bacteriol. 1967 Nov;94(5):1684–1696. doi: 10.1128/jb.94.5.1684-1696.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. H., Medina V. J., Snyder S. H. The dynamics of synthesis and degradation of polyamines in normal and regenerating rat liver and brain. J Biol Chem. 1970 Dec 25;245(24):6732–6738. [PubMed] [Google Scholar]
- SACKS L. E., BAILEY G. F. DRY RUPTURE OF BACTERIAL SPORES. J Bacteriol. 1963 Mar;85:720–721. doi: 10.1128/jb.85.3.720-721.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLEPECKY R., FOSTER J. W. Alterations in metal content of spores of Bacillus megaterium and the effect on some spore properties. J Bacteriol. 1959 Jul;78(1):117–123. doi: 10.1128/jb.78.1.117-123.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P., Kornberg A. Biochemical studies of bacterial sporulation and germination. XVII. Sulfhydryl and disulfide levels in dormancy and germination. J Bacteriol. 1969 Dec;100(3):1155–1160. doi: 10.1128/jb.100.3.1155-1160.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P., Kornberg A. Biochemical studies of bacterial sporulation and germination. XXII. Energy metabolism in early stages of germination of Bacillus megaterium spores. J Biol Chem. 1970 Jul 25;245(14):3637–3644. [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. A., Kornberg A. Biochemical studies of bacterial sporulation and germaination. VII. Protein turnover during sporulation of Bacillus subtilis. J Biol Chem. 1968 Sep 10;243(17):4600–4605. [PubMed] [Google Scholar]
- Stevens L. The binding of spermine to the ribosomes and ribosomal ribonucleic acid from Bacillus stearothermophilus. Biochem J. 1969 Jun;113(1):117–121. doi: 10.1042/bj1130117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Transport systems for 1,4-diaminobutane, spermidine, and spermine in Escherichia coli. J Biol Chem. 1966 Aug 25;241(16):3714–3723. [PubMed] [Google Scholar]
- Tabor C. W. The effects of temperature on the acetylation of spermidine. Biochem Biophys Res Commun. 1968 Feb 26;30(4):339–342. doi: 10.1016/0006-291x(68)90747-x. [DOI] [PubMed] [Google Scholar]
- Tabor H., Tabor C. W. Biosynthesis and metabolism of 1,4-diaminobutane, spermidine, spermine, and related amines. Adv Enzymol Relat Areas Mol Biol. 1972;36:203–268. doi: 10.1002/9780470122815.ch7. [DOI] [PubMed] [Google Scholar]
- Witkin S. S., Rosenberg E. Induction of morphogenesis by methionine starvation in Myxococcus xanthus: polyamine control. J Bacteriol. 1970 Sep;103(3):641–649. doi: 10.1128/jb.103.3.641-649.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]



