Abstract
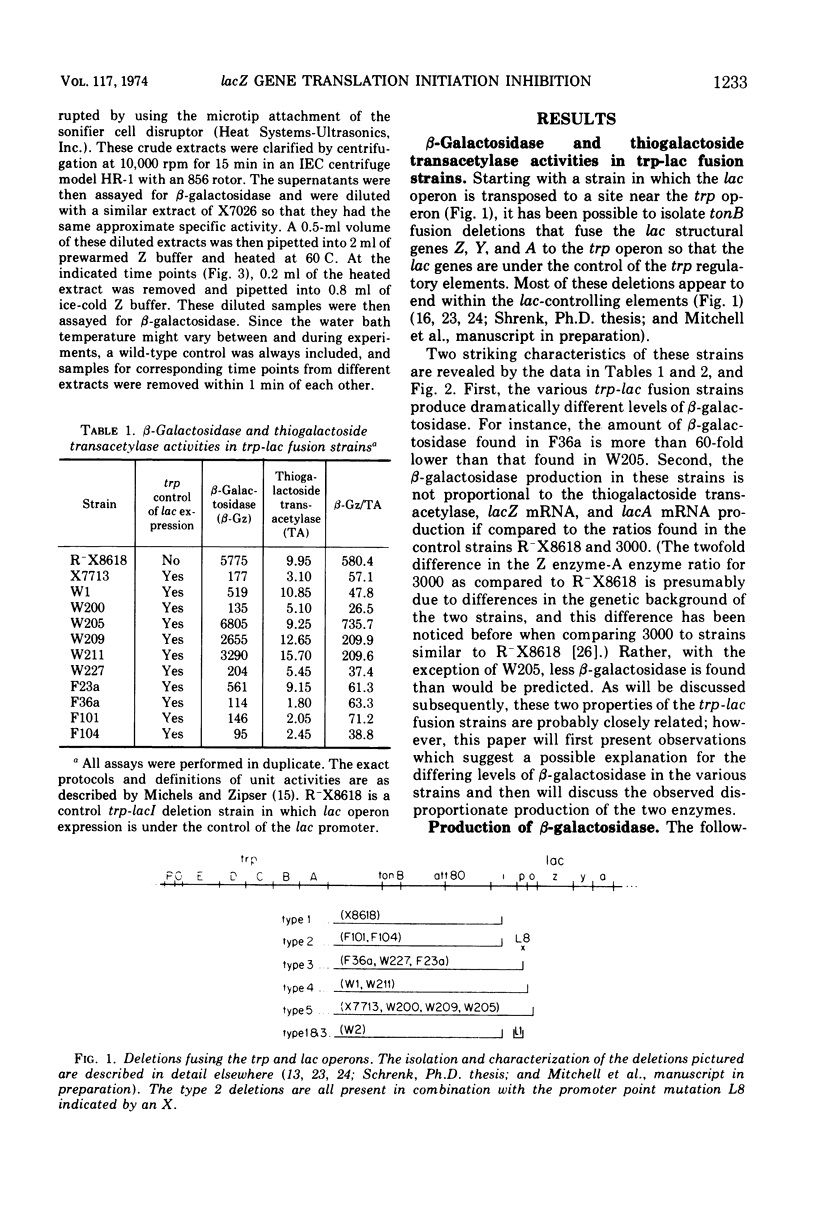
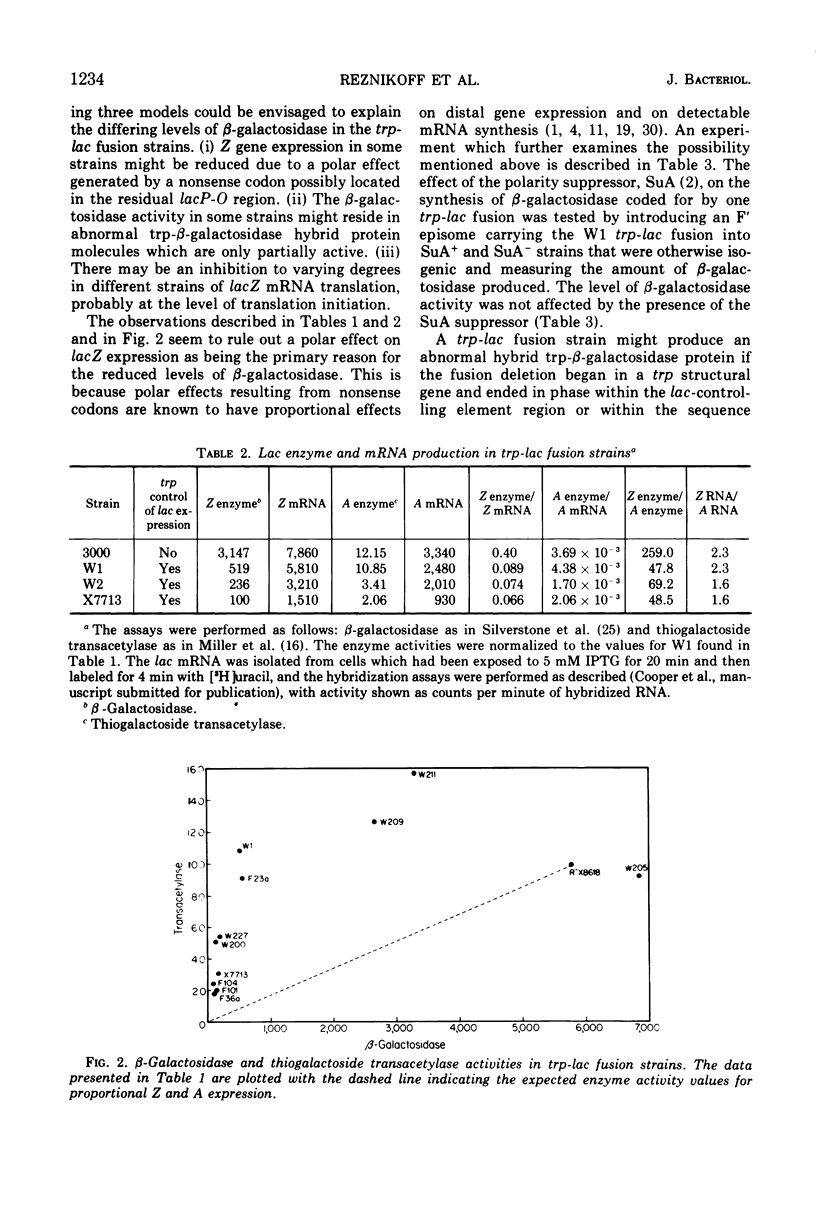
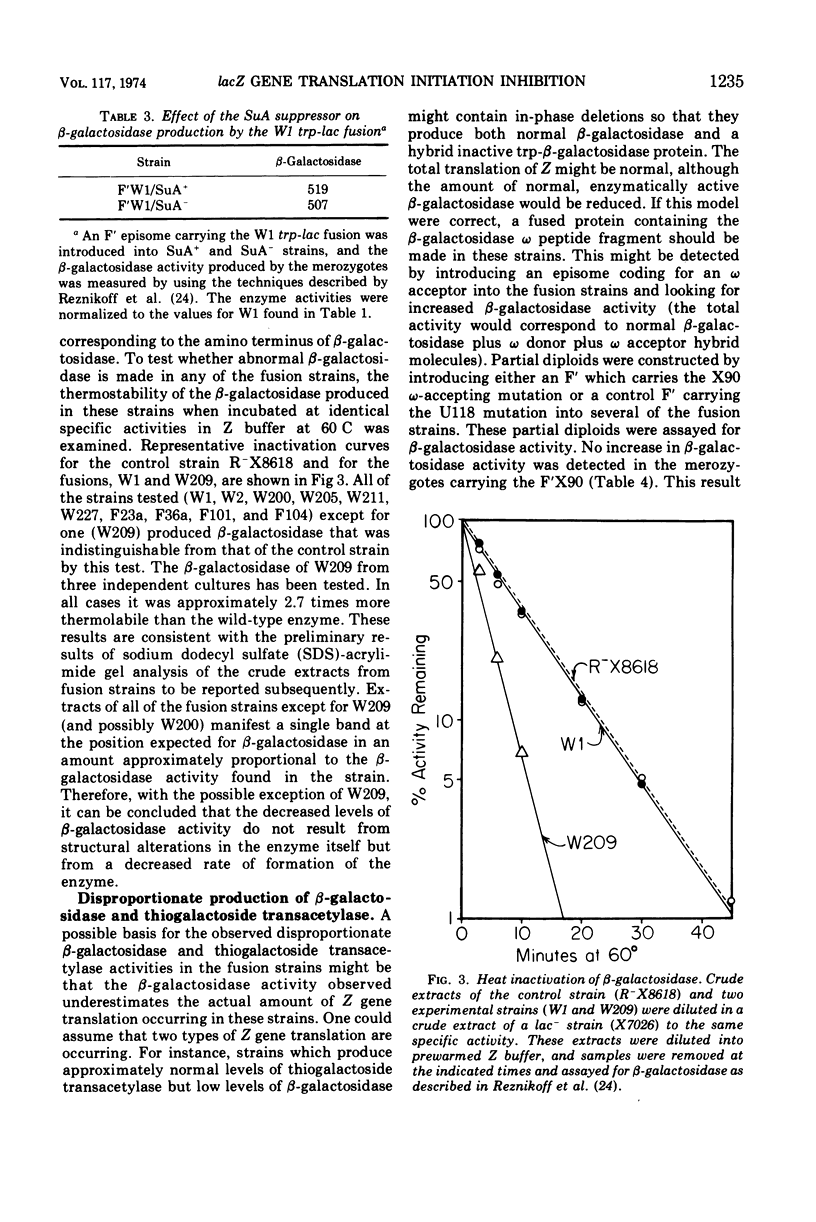
Different levels of β-galactosidase are found in various trp-lac fusion strains. These levels of β-galactosidase fall within a 60-fold range. The amount of thiogalactoside transacetylase activity detected in these same strains only varies 10-fold and is found in amounts greater than those predicted from the β-galactosidase levels. The observation that the β-galactosidase and thiogalactoside transacetylase levels are not directly proportional, that the lacZ messenger ribonucleic acid (mRNA) levels are not proportional to the β-galactosidase activity, that, at least for the one fusion strain tested, the SuA polarity suppressor does not affect the β-galactosidase level, and that, in all but one strain, the β-galactosidase activity appears to reside in normal β-galactosidase molecules suggests that the disproportionately low production of β-galactosidase is due to a decrease in the frequency of translation initiation of lacZ mRNA in these strains. Several mechanisms are proposed to explain this decrease. Some possible bases for the disproportional production of β-galactosidase and thiogalactoside transacetylase are also described. The preferred explanation for these disproportional enzyme levels is that only a fraction of the full complement of ribosomes need initiate translation at lacZ for the functional synthesis of lac mRNA to occur and that once the lac ribonucleic acid is made a full complement of ribosomes can bind at internal translation initiation sites at Y and A.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECKWITH J. RESTORATION OF OPERON ACTIVITY BY SUPPRESSORS. Biochim Biophys Acta. 1963 Sep 17;76:162–164. [PubMed] [Google Scholar]
- Beckwith J. R., Signer E. R. Transposition of the lac region of Escherichia coli. I. Inversion of the lac operon and transduction of lac by phi80. J Mol Biol. 1966 Aug;19(2):254–265. doi: 10.1016/s0022-2836(66)80003-7. [DOI] [PubMed] [Google Scholar]
- Contesse G., Naono S., Gros F. Effet des mutations polaires sur la transcription de l'opéron lactose chez Escherichia coli. C R Acad Sci Hebd Seances Acad Sci D. 1966 Oct 10;263(15):1007–1010. [PubMed] [Google Scholar]
- Engelhardt D. L., Webster R. E., Zinder N. D. Amber mutants and polarity in vitro. J Mol Biol. 1967 Oct 14;29(1):45–58. doi: 10.1016/0022-2836(67)90180-5. [DOI] [PubMed] [Google Scholar]
- Imamoto F. Diversity of regulation of genetic transcription. I. Effect of antibiotics which inhibit the process of translation on RNA metabolism in Escherichia coli. J Mol Biol. 1973 Feb 25;74(2):113–136. doi: 10.1016/0022-2836(73)90102-2. [DOI] [PubMed] [Google Scholar]
- Imamoto F. Evidence for premature termination of transcription of the tryptophan operon in polarity mutants of Escherichia coli. Nature. 1970 Oct 17;228(5268):232–235. doi: 10.1038/228232a0. [DOI] [PubMed] [Google Scholar]
- Imamoto F., Kano Y. Inhibition of transcription of the tryptophan operon in Escherichia coli by a block in initiation of translation. Nat New Biol. 1971 Aug 11;232(2):169–173. doi: 10.1038/newbio232169a0. [DOI] [PubMed] [Google Scholar]
- Imamoto F., Yanofsky C. Transcription of the tryptophan operon in polarity mutants of Escherichia coli. II. Evidence for normal production of tryp-mRNA molecules and for premature termination of transcription. J Mol Biol. 1967 Aug 28;28(1):25–35. doi: 10.1016/s0022-2836(67)80074-3. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Independent translation of the genes of bacteriophage f2 RNA. J Mol Biol. 1968 Mar 28;32(3):681–685. doi: 10.1016/0022-2836(68)90351-3. [DOI] [PubMed] [Google Scholar]
- Mackie G., Wilson D. B. Polarity and transcription in the galactose operon of E. coli. Biochem Biophys Res Commun. 1972 Jul 11;48(1):226–234. doi: 10.1016/0006-291x(72)90367-1. [DOI] [PubMed] [Google Scholar]
- Michels C. A., Reznikoff W. S. The gradient of polarity of z gene nonsense mutations in trp-lac fusion strains of Escherichia coli. J Mol Biol. 1971 Jan 14;55(1):119–122. doi: 10.1016/0022-2836(71)90286-5. [DOI] [PubMed] [Google Scholar]
- Michels C. A., Zipser D. The non-linear relationship between the enzyme activity and structural protein concentration of thiogalactoside transacetylase of E. coli. Biochem Biophys Res Commun. 1969 Feb 21;34(4):522–527. doi: 10.1016/0006-291x(69)90413-6. [DOI] [PubMed] [Google Scholar]
- Miller J. H., Reznikoff W. S., Silverstone A. E., Ippen K., Signer E. R., Beckwith J. R. Fusions of the lac and trp Regions of the Escherichia coli Chromosome. J Bacteriol. 1970 Dec;104(3):1273–1279. doi: 10.1128/jb.104.3.1273-1279.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse D. E., Primakoff P. Relief of polarity in E. coli by "suA". Nature. 1970 Apr 4;226(5240):28–31. doi: 10.1038/226028a0. [DOI] [PubMed] [Google Scholar]
- Morse D. E., Yanofsky C. Polarity and the degradation of mRNA. Nature. 1969 Oct 25;224(5217):329–331. doi: 10.1038/224329a0. [DOI] [PubMed] [Google Scholar]
- Newton W. A., Beckwith J. R., Zipser D., Brenner S. Nonsense mutants and polarity in the lac operon of Escherichia coli. J Mol Biol. 1965 Nov;14(1):290–296. doi: 10.1016/s0022-2836(65)80250-9. [DOI] [PubMed] [Google Scholar]
- Platt T., Weber K., Ganem D., Miller J. H. Translational restarts: AUG reinitiation of a lac repressor fragment. Proc Natl Acad Sci U S A. 1972 Apr;69(4):897–901. doi: 10.1073/pnas.69.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechler M. M., Bruni C. B., Martin R. G., Terry W. An intercistronic region in the histidine operon of Salmonella typhimurium. J Mol Biol. 1972 Aug 28;69(3):427–452. doi: 10.1016/0022-2836(72)90256-2. [DOI] [PubMed] [Google Scholar]
- Rechler M. M., Martin R. G. The intercistronic divide: translation of an intercistronic region in the histidine operon of Salmonella typhimurium. Nature. 1970 Jun 6;226(5249):908–911. doi: 10.1038/226908a0. [DOI] [PubMed] [Google Scholar]
- Reznikoff W. S., Miller J. H., Scaife J. G., Beckwith J. R. A mechanism for repressor action. J Mol Biol. 1969 Jul 14;43(1):201–213. doi: 10.1016/0022-2836(69)90089-8. [DOI] [PubMed] [Google Scholar]
- Silverstone A. E., Goman M., Scaife J. G. ALT: a new factor involved in the synthesis of RNA by Escherichia coli. Mol Gen Genet. 1972;118(3):223–234. doi: 10.1007/BF00333459. [DOI] [PubMed] [Google Scholar]
- Silverstone A. E., Magasanik B. Polycistronic effects of catabolite repression on the lac operon. J Bacteriol. 1972 Dec;112(3):1184–1192. doi: 10.1128/jb.112.3.1184-1192.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullmann A., Perrin D., Jacob F., Monod J. Identification par complémentation in vitro et purification d'un segment peptidique de la beta-galatosidase d'escherichia coli. J Mol Biol. 1965 Jul;12(3):918–923. doi: 10.1016/s0022-2836(65)80338-2. [DOI] [PubMed] [Google Scholar]
- Voll M. J. Translation and polarity in the histidine operon. 3. The isolation of prototrophic polar mutations. J Mol Biol. 1967 Nov 28;30(1):109–124. doi: 10.1016/0022-2836(67)90247-1. [DOI] [PubMed] [Google Scholar]
- Yanofsky C., Ito J. Nonsense codons and polarity in the tryptophan operon. J Mol Biol. 1966 Nov 14;21(2):313–334. doi: 10.1016/0022-2836(66)90102-1. [DOI] [PubMed] [Google Scholar]
- Zinder N. D., Engelhardt D. L., Webster R. E. Punctuation in the genetic code. Cold Spring Harb Symp Quant Biol. 1966;31:251–256. doi: 10.1101/sqb.1966.031.01.033. [DOI] [PubMed] [Google Scholar]



