Abstract
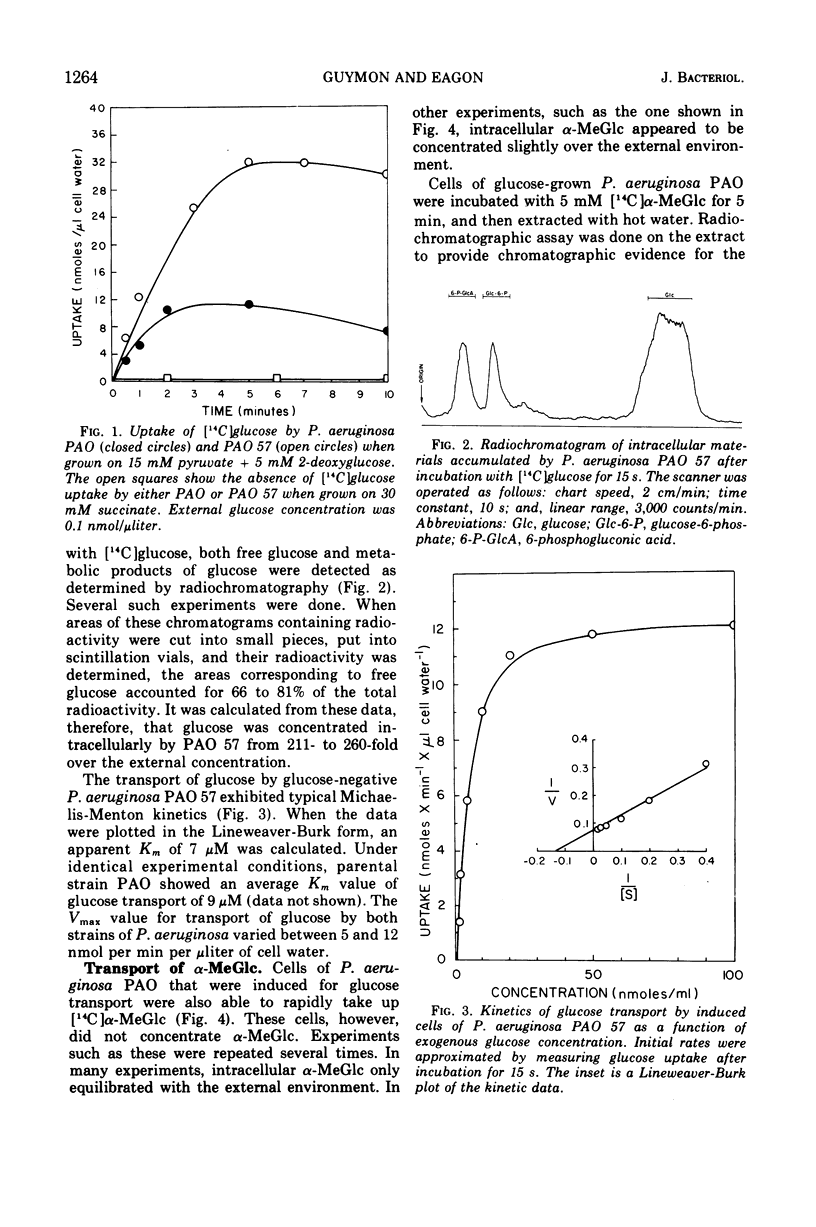
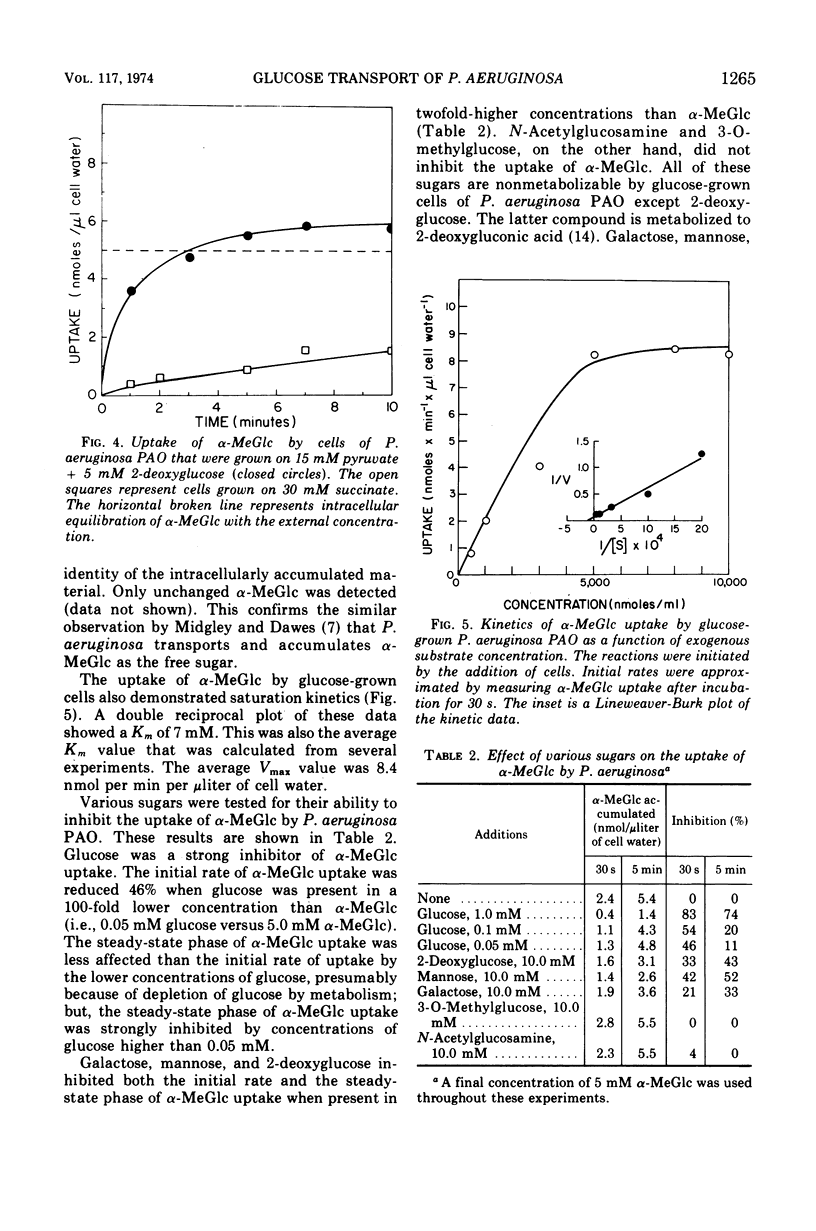
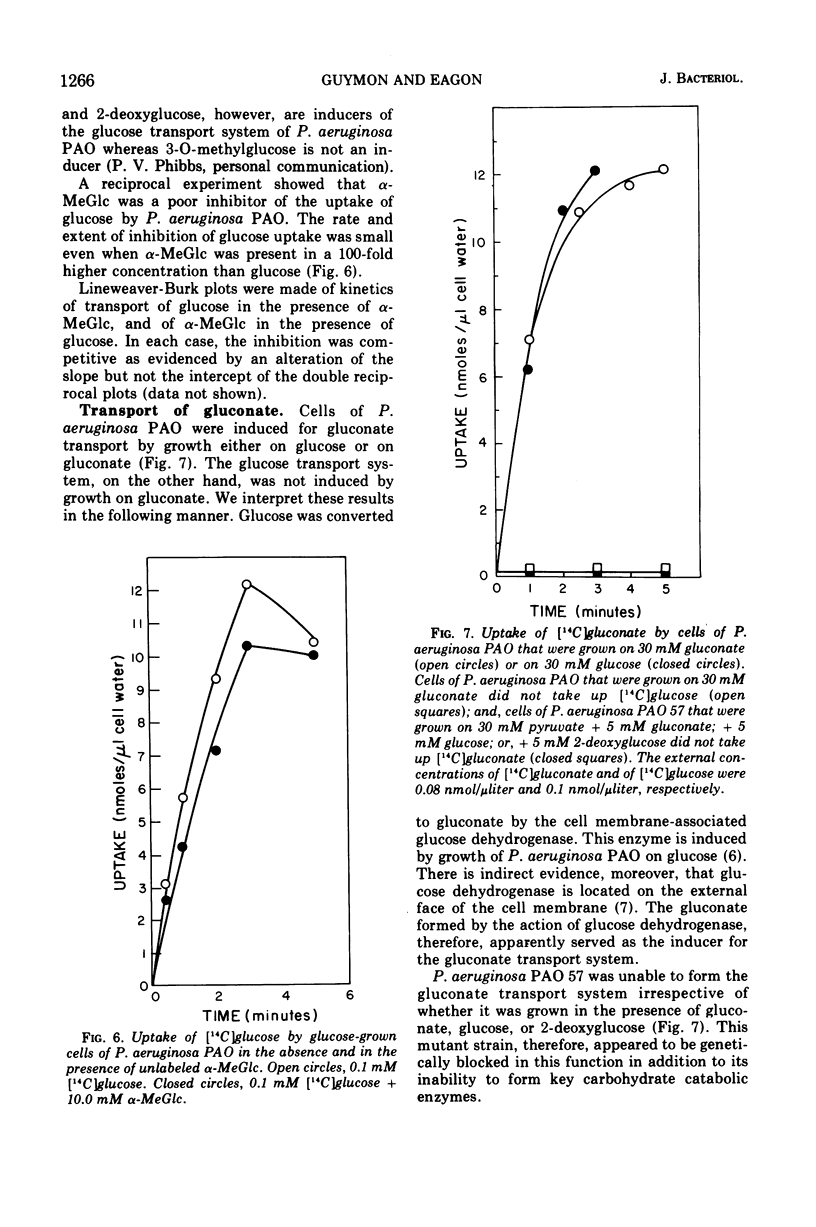
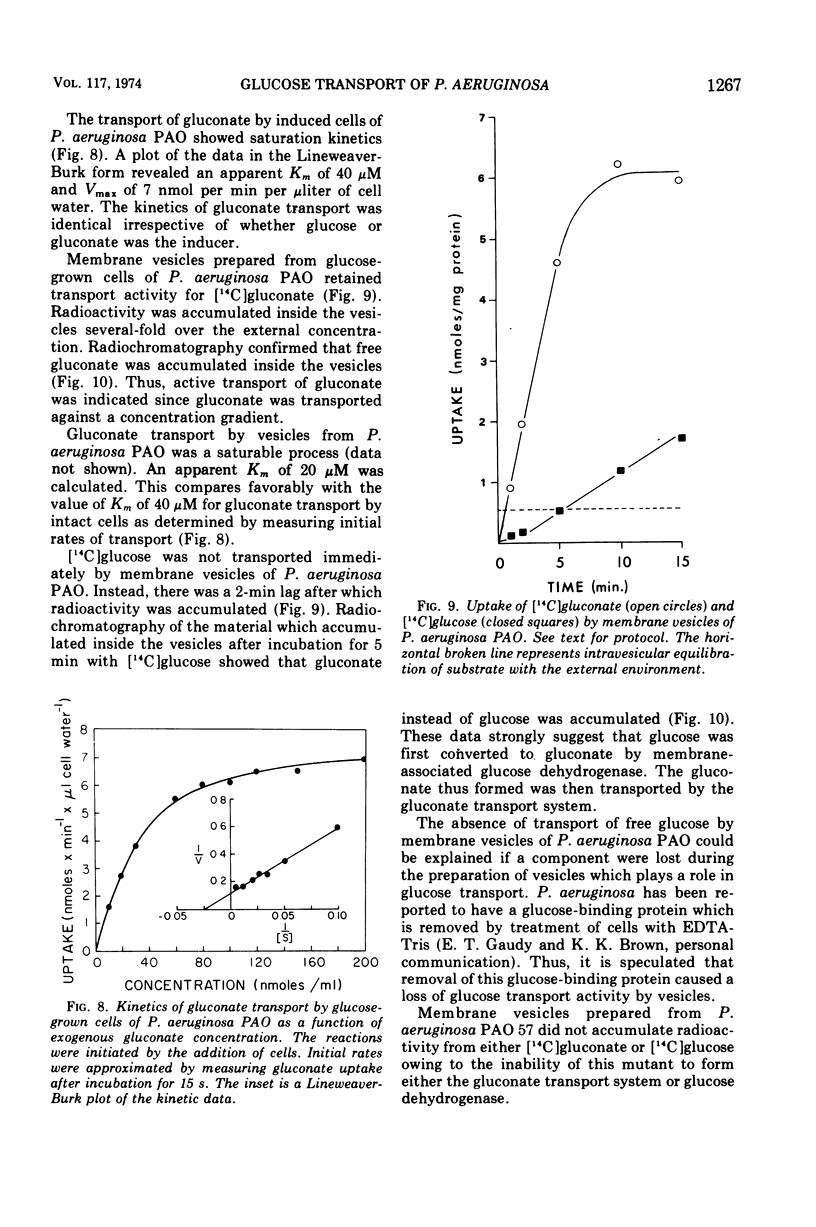
Glucose transport by Pseudomonas aeruginosa was studied. These studies were enhanced by the use of a mutant, strain PAO 57, which was unable to grow on glucose but which formed the inducible glucose transport system when grown in media containing glucose or other inducers such as 2-deoxy-d-glucose. Both PAO 57 and parental strain PAO transported glucose with an apparent Km of 7 μM. Free glucose was concentrated intracellularly by P. aeruginosa PAO 57 over 200-fold above the external level. These data constitute direct evidence that glucose is transported via active transport by P. aeruginosa. Various experimental data clearly indicated that P. aeruginosa PAO transported methyl α-d-glucose (α-MeGlc) via the glucose transport system. The apparent Km of α-MeGlc transport was 7 mM which indicated a 1,000-fold lower affinity of the glucose transport system for α-MeGlc than for glucose. While only unchanged α-MeGlc was detected intracellularly in P. aeruginosa, α-MeGlc was actually concentrated intracellularly less than 2-fold over the external level. Membrane vesicles of P. aeruginosa PAO retained transport activity for gluconate. This solute was concentrated intravesicularly several-fold over the external level. A component of the glucose transport system is believed to have been lost during vesicle preparation since glucose per se was not transported. Instead; glucose was converted to gluconate by membrane-associated glucose dehydrogenase and gluconate was then transported into the vesicles. Although this may constitute an alternate system for glucose transport, it is not a necessary prerequisite for glucose transport by intact cells since P. aeruginosa PAO 57, which lacks glucose dehydrogenase, was able to transport glucose at a rate equal to the parental strain.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes E. M., Jr Respiration-coupled glucose transport in membrane vesicles from Azotobacter vinelandii. Arch Biochem Biophys. 1972 Oct;152(2):795–799. doi: 10.1016/0003-9861(72)90275-5. [DOI] [PubMed] [Google Scholar]
- Eagon R. G. 2-Deoxyglucose transportation via passive diffusion and its oxidation, not phosphorylation, to 2-deoxygluconic acid by Pseudomonas aeruginosa. Can J Biochem. 1971 May;49(5):606–613. doi: 10.1139/o71-087. [DOI] [PubMed] [Google Scholar]
- Eagon R. G., Phibbs P. V., Jr Kinetics of transport of glucose, fructose, and mannitol by Pseudomonas aeruginosa. Can J Biochem. 1971 Sep;49(9):1031–1041. doi: 10.1139/o71-151. [DOI] [PubMed] [Google Scholar]
- FISCHER F. G., NEBEL H. J. Nachweis und Bestimmung von Glucosamin und Galaktosamin auf Papierchromatogrammen. Hoppe Seylers Z Physiol Chem. 1955 Sep 21;302(1):10–19. [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Hylemon P. B., Phibbs P. V., Jr Independent regulation of hexose catabolizing enzymes and glucose transport activity in Pseudomonas aeruginosa. Biochem Biophys Res Commun. 1972 Sep 5;48(5):1041–1048. doi: 10.1016/0006-291x(72)90813-3. [DOI] [PubMed] [Google Scholar]
- Midgley M., Dawes E. A. The regulation of transport of glucose and methyl alpha-glucoside in Pseudomonas aeruginosa. Biochem J. 1973 Feb;132(2):141–154. doi: 10.1042/bj1320141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukkada A. J., Long G. L., Romano A. H. The uptake of 2-deoxy-D-glucose by Pseudomonas aeruginosa and its regulation. Biochem J. 1973 Feb;132(2):155–162. doi: 10.1042/bj1320155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng F. M., Dawes E. A. Chemostat studies on the regulation of glucose metabolism in Pseudomonas aeruginosa by citrate. Biochem J. 1973 Feb;132(2):129–140. doi: 10.1042/bj1320129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phibbs P. V., Jr, Eagon R. G. Transport and phosphorylation of glucose, fructose, and mannitol by Pseudomonas aeruginosa. Arch Biochem Biophys. 1970 Jun;138(2):470–482. doi: 10.1016/0003-9861(70)90371-1. [DOI] [PubMed] [Google Scholar]
- Romano A. H., Eberhard S. J., Dingle S. L., McDowell T. D. Distribution of the phosphoenolpyruvate: glucose phosphotransferase system in bacteria. J Bacteriol. 1970 Nov;104(2):808–813. doi: 10.1128/jb.104.2.808-813.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinnett J. D., Guymon L. F., Eagon R. G. A novel technique for the preparation of transport-active membrane vesicles from Pseudomonas aeruginosa: observations on gluconate transport. Biochem Biophys Res Commun. 1973 May 1;52(1):285–290. doi: 10.1016/0006-291x(73)90985-6. [DOI] [PubMed] [Google Scholar]
- Tiwari N. P., Campbell J. J. Enzymatic control of the metabolic activity of Pseudomonas aeruginosa grown in glucose or succinate media. Biochim Biophys Acta. 1969 Dec 30;192(3):395–401. doi: 10.1016/0304-4165(69)90388-2. [DOI] [PubMed] [Google Scholar]
- WILLIAMS A. K., EAGON R. G. Oxidation of 2-deoxy-D-glucose to 2-deoxy-D-gluconic acid by extracts of pseudomonas aeruginosa. J Bacteriol. 1959 Feb;77(2):167–172. doi: 10.1128/jb.77.2.167-172.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H., Wilson T. H. The role of energy coupling in the transport of beta-galactosides by Escherichia coli. J Biol Chem. 1966 May 25;241(10):2200–2211. [PubMed] [Google Scholar]



