Abstract
A mutant strain of Escherichia coli capable of growth on l-1,2-propanediol was isolated previously. The mutant is characterized by constitutive production of a propanediol:nicotinamide adenenine dinucleotide (NAD) oxidoreductase which is essential for the new growth property. In the present study, it is shown that phage P1 cotransduces the genetic locus conferring this property and the genes for the utilization of l-fucose. A further indication of a relationship between these two growth properties is provided by the observation that wild-type E. coli excretes propanediol during fermentation of l-fucose. Under these conditions, a propanediol dehydrogenase (lactaldehyde reductase) is induced. This enzyme migrates on diethylaminoethyl-cellulose with the propanediol dehydrogenase produced constitutively by the mutant strain. A key event in the establishment of the ability to grow on propanediol is evidently a shift in the expression and function of propanediol dehydrogenase; an enzyme catalyzing formation of a reduced fermentation product anaerobically in wild-type cells functions aerobically to oxidize this same product in the mutant. l-Lactaldehyde, which is thus derived from propanediol, is converted to l-lactate by another dehydrogenase (l-lactaldehyde:NAD oxidoreductase) which is constitutively produced by both wild-type and mutant cells. The normal function of this enzyme is not yet established. l-Lactate is converted to pyruvate by an inducible NAD-independent l-lactate dehydrogenase. Thus, the carbons of propanediol are brought into the central metabolic network of the cell.
Full text
PDF


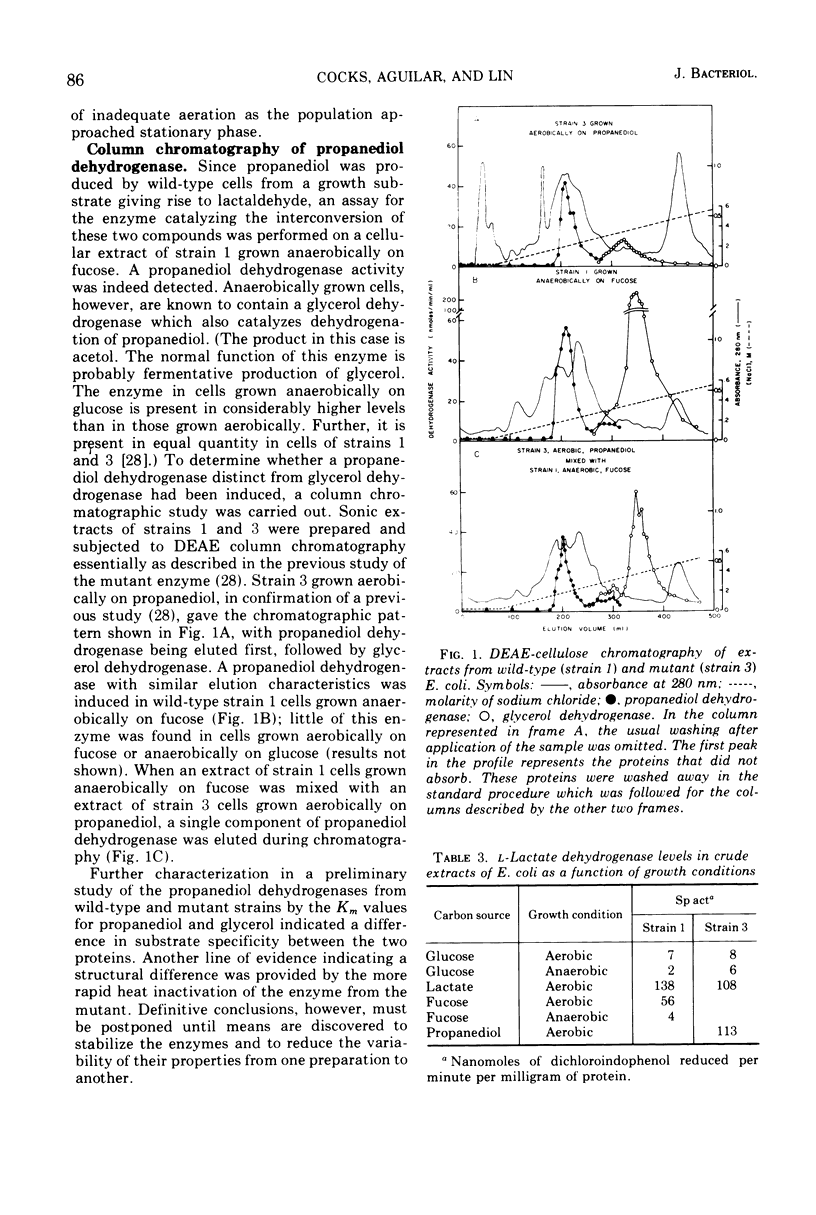
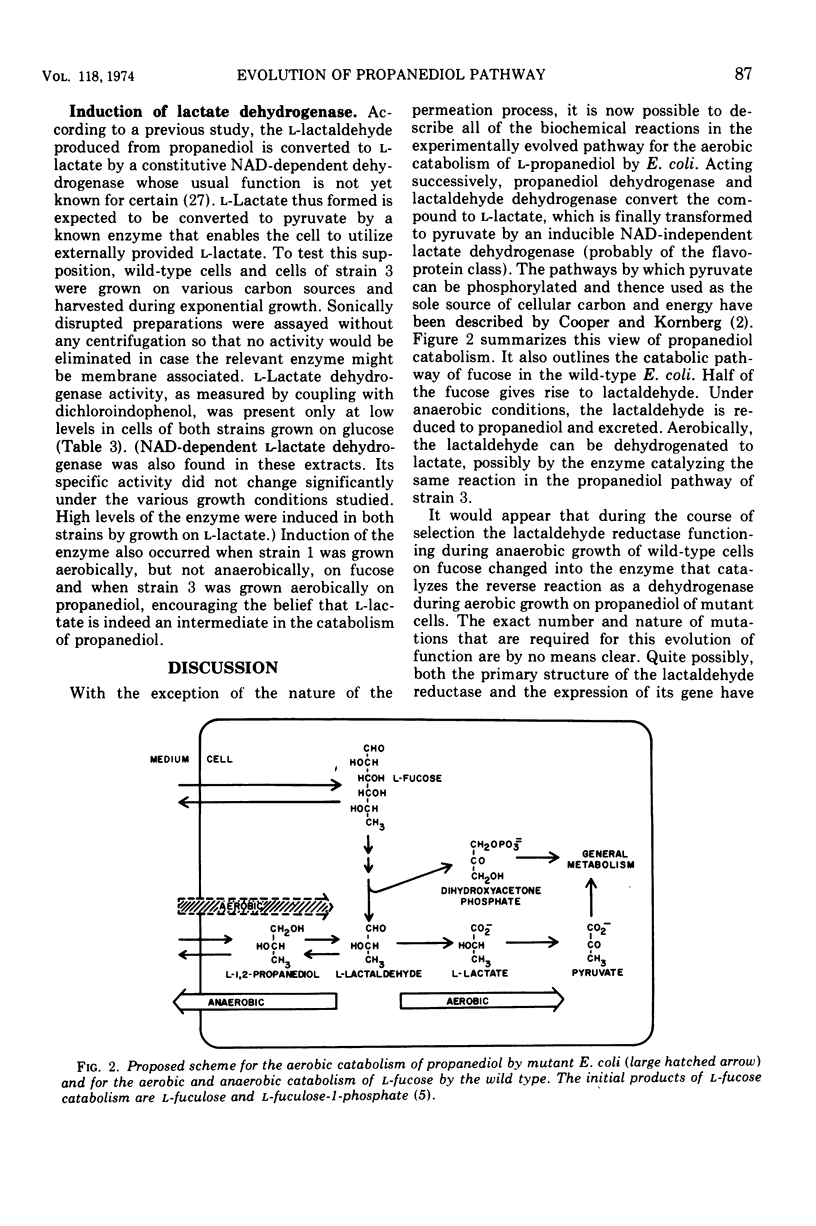

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown J. E., Brown P. R., Clarke P. H. Butyramide-utilizing mutants of Pseudomonas aeruginosa 8602 which produce an amidase with altered substrate specificity. J Gen Microbiol. 1969 Aug;57(2):273–285. doi: 10.1099/00221287-57-2-273. [DOI] [PubMed] [Google Scholar]
- Cooper R. A., Kornberg H. L. Net formation of phosphoenolpyruvate from pyruvate by Escherichia coli. Biochim Biophys Acta. 1965 Jul 8;104(2):618–620. doi: 10.1016/0304-4165(65)90374-0. [DOI] [PubMed] [Google Scholar]
- Dover S., Halpern Y. S. Utilization of -aminobutyric acid as the sole carbon and nitrogen source by Escherichia coli K-12 mutants. J Bacteriol. 1972 Feb;109(2):835–843. doi: 10.1128/jb.109.2.835-843.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGLESBERG E. Physiological basis for rhamnose utilization by a mutant of Pasteurella pestis. II. A single mutational event leading to the production of two enzymes. Arch Biochem Biophys. 1957 Sep;71(1):179–193. doi: 10.1016/0003-9861(57)90020-6. [DOI] [PubMed] [Google Scholar]
- GHALAMBOR M. A., HEATH E. C. The metabolism of L-fucose. II. The enzymatic cleavage of L-fuculose 1-phosphate. J Biol Chem. 1962 Aug;237:2427–2433. [PubMed] [Google Scholar]
- HALPERN Y. S., UMBARGER H. E. Utilization of L-glutamic and 2-oxoglutaric acid as sole sources of carbon by Escherichia coli. J Gen Microbiol. 1961 Oct;26:175–183. doi: 10.1099/00221287-26-2-175. [DOI] [PubMed] [Google Scholar]
- Hegeman G. D., Rosenberg S. L. The evolution of bacterial enzyme systems. Annu Rev Microbiol. 1970;24:429–462. doi: 10.1146/annurev.mi.24.100170.002241. [DOI] [PubMed] [Google Scholar]
- KIM K. H. ISOLATION AND PROPERTIES OF A PUTRESCINE-DEGRADING MUTANT OF ESCHERICHIA COLI. J Bacteriol. 1963 Aug;86:320–323. doi: 10.1128/jb.86.2.320-323.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEIN H. P., DOUDOROFF M. The mutation of Pseudomonas putrefaciens to glucose utilization and its enzymatic basis. J Bacteriol. 1950 Jun;59(6):739–750. doi: 10.1128/jb.59.6.739-750.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline E. S., Mahler H. R. The lactic dehydrogenases of E. coli. Ann N Y Acad Sci. 1965 Jul 31;119(3):905–919. doi: 10.1111/j.1749-6632.1965.tb47451.x. [DOI] [PubMed] [Google Scholar]
- LESTER G., BONNER D. M. Genetic control of raffinose utilization in Escherichia coli. J Bacteriol. 1957 Apr;73(4):544–552. doi: 10.1128/jb.73.4.544-552.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LURIA S. E., ADAMS J. N., TING R. C. Transduction of lactose-utilizing ability among strains of E. coli and S. dysenteriae and the properties of the transducing phage particles. Virology. 1960 Nov;12:348–390. doi: 10.1016/0042-6822(60)90161-6. [DOI] [PubMed] [Google Scholar]
- Langridge J. Mutations conferring quantitative and qualitative increases in beta-galactosidase activity in Escherichia coli. Mol Gen Genet. 1969;105(1):74–83. doi: 10.1007/BF00750315. [DOI] [PubMed] [Google Scholar]
- LeBlanc D. J., Mortlock R. P. Metabolism of D-arabinose: a new pathway in Escherichia coli. J Bacteriol. 1971 Apr;106(1):90–96. doi: 10.1128/jb.106.1.90-96.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiss H. K., Brill W. J., Magasanik B. Genetic control of histidine degradation in Salmonella typhimurium, strain LT-2. J Biol Chem. 1969 Oct 10;244(19):5382–5391. [PubMed] [Google Scholar]
- Mortlock R. P., Fossitt D. D., Wood W. A. A basis for utlization of unnatural pentoses and pentitols by Aerobacter aerogenes. Proc Natl Acad Sci U S A. 1965 Aug;54(2):572–579. doi: 10.1073/pnas.54.2.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver E. J., Mortlock R. P. Growth of Aerobacter aerogenes on D-arabinose: origin of the enzyme activities. J Bacteriol. 1971 Oct;108(1):287–292. doi: 10.1128/jb.108.1.287-292.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overath P., Pauli G., Schairer H. U. Fatty acid degradation in Escherichia coli. An inducible acyl-CoA synthetase, the mapping of old-mutations, and the isolation of regulatory mutants. Eur J Biochem. 1969 Feb;7(4):559–574. [PubMed] [Google Scholar]
- SAWADA H., TAKAGI Y. THE METABOLISM OF L-RHAMNOSE IN ESCHERICHIA COLI. 3. L-RHAMULOSE-PHOSPHATE ALDOLASE. Biochim Biophys Acta. 1964 Oct 23;92:26–32. doi: 10.1016/0926-6569(64)90265-2. [DOI] [PubMed] [Google Scholar]
- Salanitro J. P., Wegener W. S. Growth of Escherichia coli on short-chain fatty acids: growth characteristics of mutants. J Bacteriol. 1971 Nov;108(2):885–892. doi: 10.1128/jb.108.2.885-892.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefler S. Inducible system for the utilization of beta-glucosides in Escherichia coli. I. Active transport and utilization of beta-glucosides. J Bacteriol. 1967 Jan;93(1):254–263. doi: 10.1128/jb.93.1.254-263.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhara S., Wu T. T., Chused T. M., Lin E. C. Ferrous-activated nicotinamide adenine dinucleotide-linked dehydrogenase from a mutant of Escherichia coli capable of growth on 1, 2-propanediol. J Bacteriol. 1969 Apr;98(1):87–95. doi: 10.1128/jb.98.1.87-95.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridhara S., Wu T. T. Purification and properties of lactaldehyde dehydrogenase from Escherichia coli. J Biol Chem. 1969 Oct 10;244(19):5233–5238. [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwinkel E., Furmanski P., Reeves H. C., Ajl S. J. Growth of Escherichia coli on fatty acids: requirement for coenzyme A transferase activity. Biochem Biophys Res Commun. 1968 Dec 30;33(6):902–908. doi: 10.1016/0006-291x(68)90397-5. [DOI] [PubMed] [Google Scholar]
- Weeks G., Shapiro M., Burns R. O., Wakil S. J. Control of fatty acid metabolism. I. Induction of the enzymes of fatty acid oxidation in Escherichia coli. J Bacteriol. 1969 Feb;97(2):827–836. doi: 10.1128/jb.97.2.827-836.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T. T., Lin E. C., Tanaka S. Mutants of Aerobacter aerogenes capable of utilizing xylitol as a novel carbon. J Bacteriol. 1968 Aug;96(2):447–456. doi: 10.1128/jb.96.2.447-456.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]



