Abstract
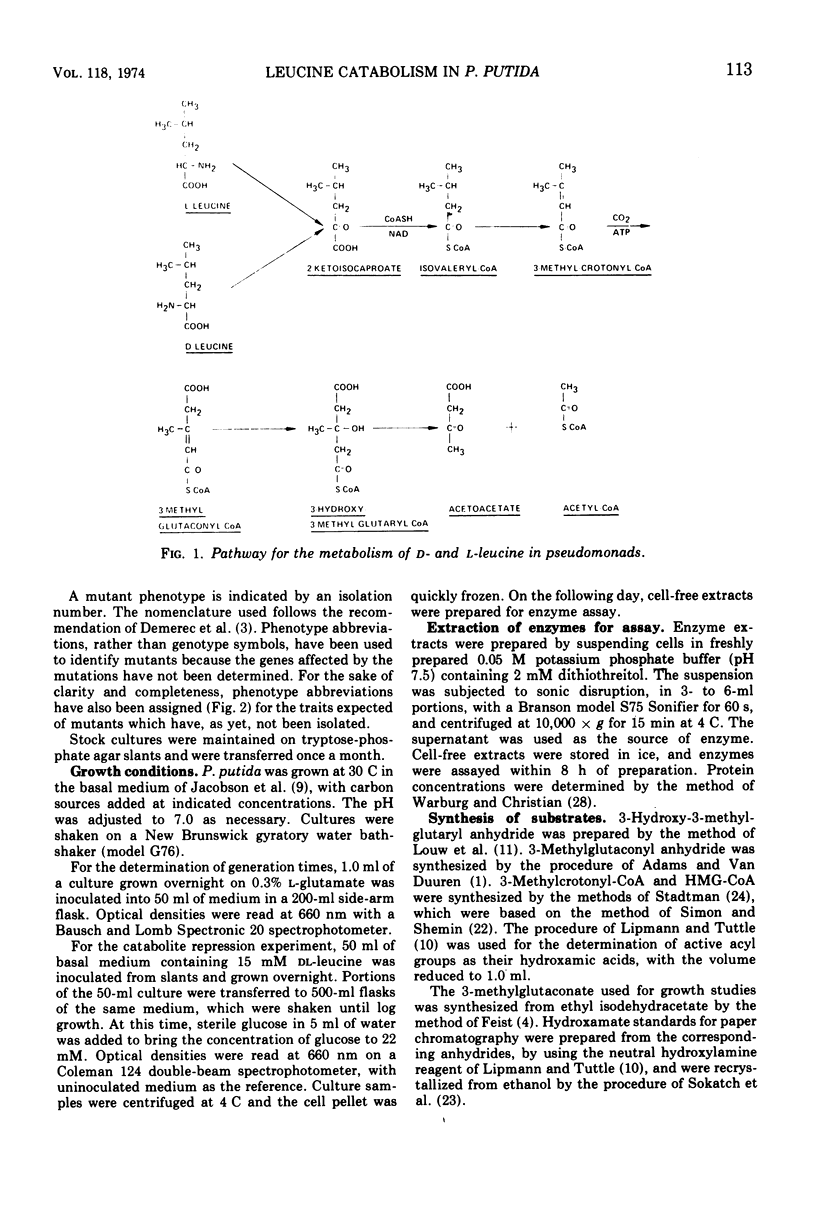
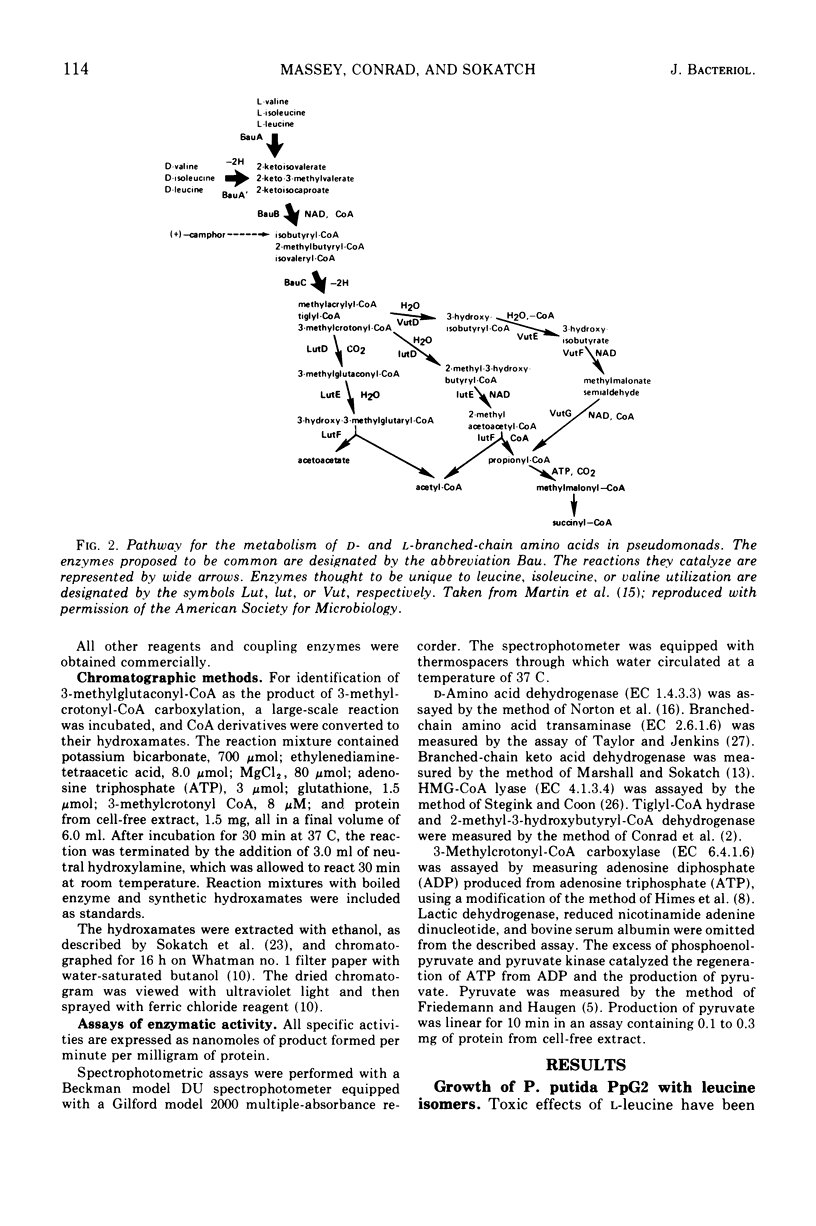
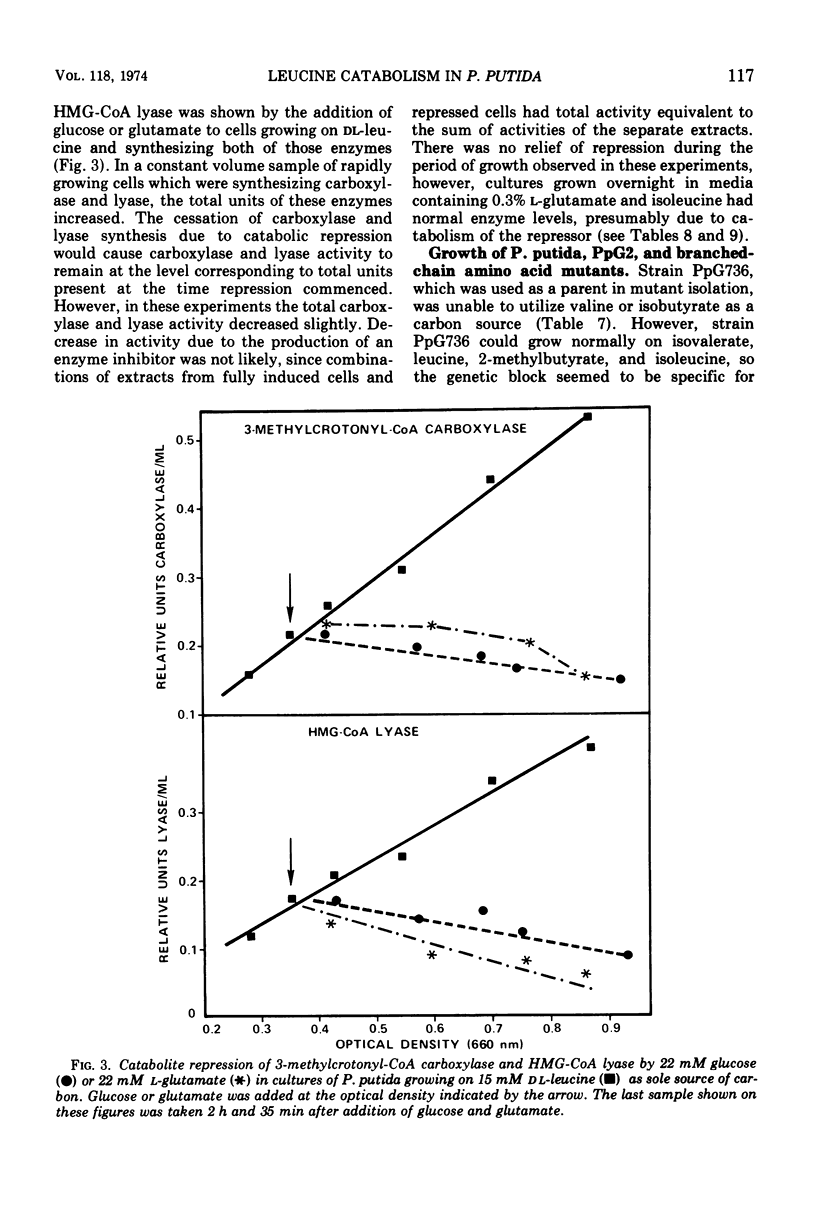
The generation time of Pseudomonas putida with l-leucine was 20 h in synthetic media but only 3 h with d-leucine. Slow growth in the presence of l-leucine was partially overcome by addition of 0.1 mM amounts of either d-valine, l-valine, or 2-ketoisovalerate. The activities of five enzymes which take part in the oxidation of leucine by P. putida were measured under various conditions of growth. Four enzymes were induced by growth with dl-leucine as sole source of carbon: d-amino acid dehydrogenase, branched-chain keto acid dehydrogenase, 3-methylcrotonyl-coenzyme A carboxylase, and 3-hydroxy-3-methylglutaryl-coenzyme A lyase. The segment of the pathway required for oxidation of 3-methylcrotonate was induced by growth on isovalerate or 3-methylcrotonate without formation of the preceding enzymes. The synthesis of carboxylase and lyase appeared to have been repressed by the addition of l-glutamate or glucose to cells growing on dl-leucine as the sole carbon source. Mutants unable to grow at the expense of isovalerate had reduced levels of carboxylase and lyase, whereas the levels of three enzymes common to the catabolism of all three branched-chain amino acids and those of two isoleucine catabolic enzymes were normal.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Conrad R. S., Massey L. K., Sokatch J. R. D- and L-isoleucine metabolism and regulation of their pathways in Pseudomonas putida. J Bacteriol. 1974 Apr;118(1):103–111. doi: 10.1128/jb.118.1.103-111.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILZ H., KNAPPE J., RINGELMANN E., LYNEN F. Methylglutaconase, eine neue Hydratase, die am Stoffwechsel verzweigter Carbonsäuren beteiligt ist. Biochem Z. 1958;329(6):476–489. [PubMed] [Google Scholar]
- HIMES R. H., YOUNG D. L., RINGELMANN E., LYNEN F. The biochemical function of biotin. V. Further studies on beta-methylcrotonyl CoA carboxylase. Biochem Z. 1963;337:48–61. [PubMed] [Google Scholar]
- Hill F., Schlegel H. G. Die alpha-Isopropylmalat-Synthetase bei Hydrogenomonas H 16. Arch Mikrobiol. 1969;68(1):1–17. doi: 10.1007/BF00408442. [DOI] [PubMed] [Google Scholar]
- Jacobson L. A., Bartholomaus R. C., Gunsalus I. C. Repression of malic enzyme by acetate in Pseudomonas. Biochem Biophys Res Commun. 1966 Sep 22;24(6):955–960. doi: 10.1016/0006-291x(66)90343-3. [DOI] [PubMed] [Google Scholar]
- LYNEN F., KNAPPE J., LORCH E., JUETTING G., RINGELMANN E., LACHANCE J. P. [On the biochemical function of biotin. II. Purification and mode of action of beta-methyl-crotonyl-carboxylase]. Biochem Z. 1961;335:123–167. [PubMed] [Google Scholar]
- Louw A. I., Bekersky I., Mosbach E. H. Improved synthesis of 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA). J Lipid Res. 1969 Nov;10(6):683–686. [PubMed] [Google Scholar]
- Marshall V. D., Sokatch J. R. Regulation of valine catabolism in Pseudomonas putida. J Bacteriol. 1972 Jun;110(3):1073–1081. doi: 10.1128/jb.110.3.1073-1081.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall V. P., Sokatch J. R. Oxidation of D-amino acids by a particulate enzyme from Pseudomonas aeruginosa. J Bacteriol. 1968 Apr;95(4):1419–1424. doi: 10.1128/jb.95.4.1419-1424.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. R., Marshall V. D., Sokatch J. R., Unger L. Common enzymes of branched-chain amino acid catabolism in Pseudomonas putida. J Bacteriol. 1973 Jul;115(1):198–204. doi: 10.1128/jb.115.1.198-204.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORTON J. E., BULMER G. S., SOKATCH J. R. THE OXIDATION OF D-ALANINE BY CELL MEMBRANES OF PSEUDOMONAS AERUGINOSA. Biochim Biophys Acta. 1963 Oct 8;78:136–147. doi: 10.1016/0006-3002(63)91619-6. [DOI] [PubMed] [Google Scholar]
- Norton J. E., Sokatch J. R. Purification and partial characterization of the branched chain amino acid transaminase of Pseudomonas aeruginosa. Biochim Biophys Acta. 1970 May 13;206(2):261–269. doi: 10.1016/0005-2744(70)90109-9. [DOI] [PubMed] [Google Scholar]
- Ornston L. N., Ornston M. K., Chou G. Isolation of spontaneous mutant strains of Pseudomonas putida. Biochem Biophys Res Commun. 1969 Jul 7;36(1):179–184. doi: 10.1016/0006-291x(69)90666-4. [DOI] [PubMed] [Google Scholar]
- RILLING H. C., COON M. J. The enzymatic isomerization of alpha-methylvinylacetyl coenzyme A and the specificity of a bacterial alpha-methylcrotonyl coenzyme A carboxylase. J Biol Chem. 1960 Nov;235:3087–3092. [PubMed] [Google Scholar]
- Siddiqi M. A., Rodwell V. W. Bacterial metabolism of mevalonic acid. J Bacteriol. 1967 Jan;93(1):207–214. doi: 10.1128/jb.93.1.207-214.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokatch J. R., Sanders L. E., Marshall V. P. Oxidation of methylmalonate semialdehyde to propionyl coenzyme A in Pseudomonas aeruginosa grown on valine. J Biol Chem. 1968 May 25;243(10):2500–2506. [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Stegink L. D., Coon M. J. Stereospecificity and other properties of highly purified beta-hydroxy-beta-methylglutaryl coenzyme A cleavage enzyme from bovine liver. J Biol Chem. 1968 Oct 25;243(20):5272–5279. [PubMed] [Google Scholar]
- Taylor R. T., Jenkins W. T. Leucine aminotransferase. I. Colorimetric assays. J Biol Chem. 1966 Oct 10;241(19):4391–4395. [PubMed] [Google Scholar]



