Abstract
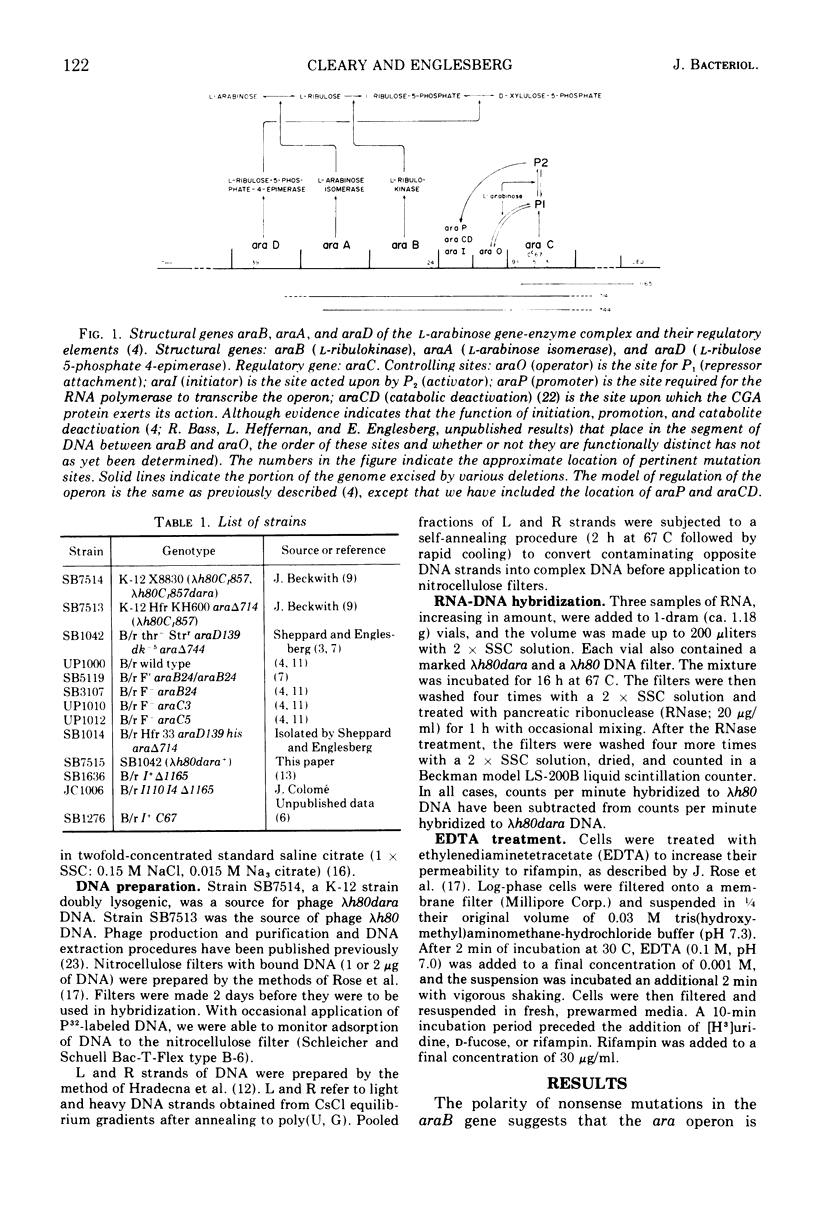
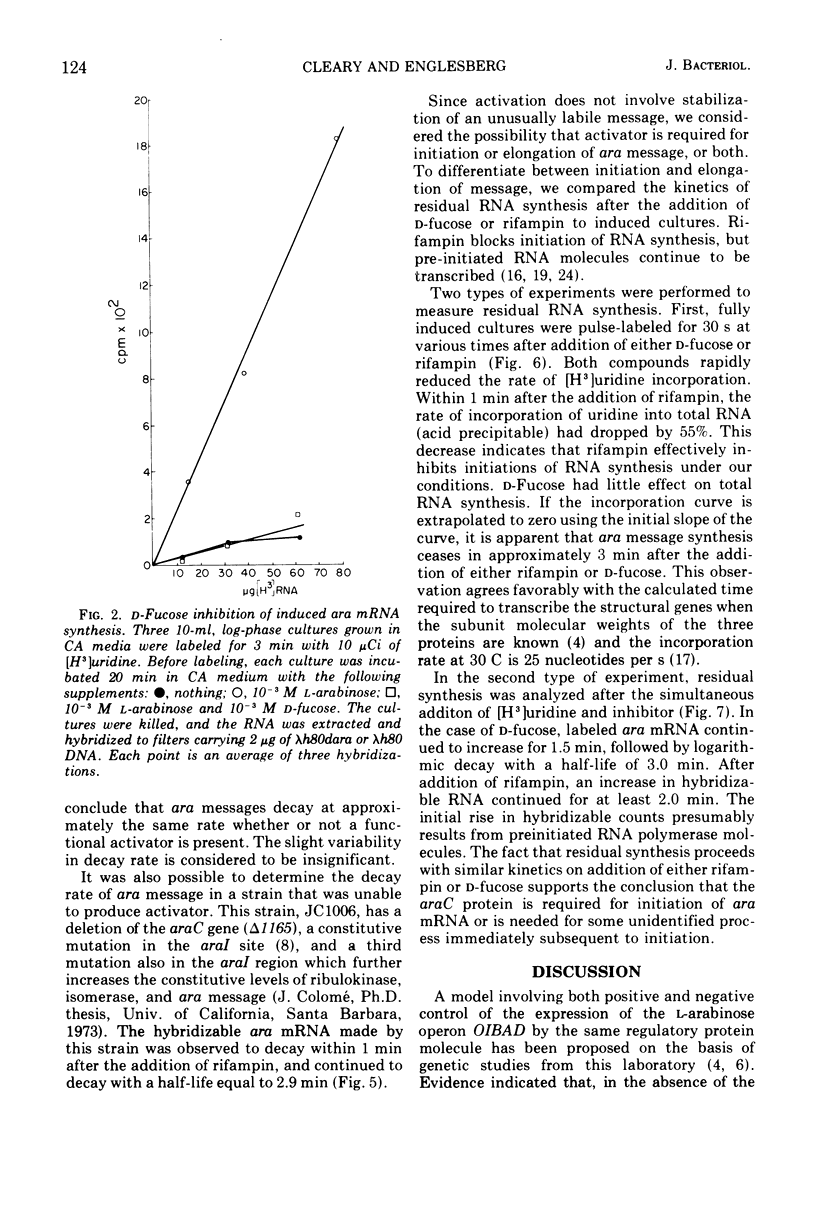
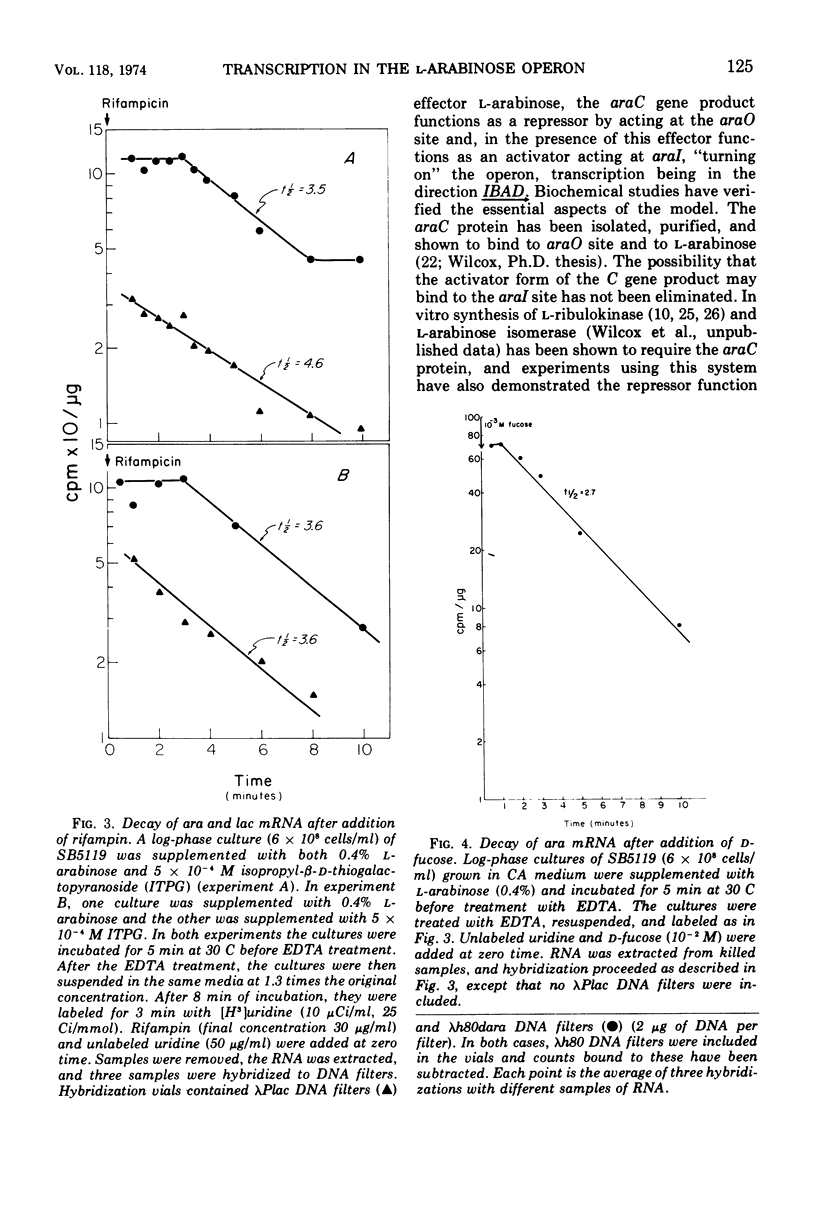
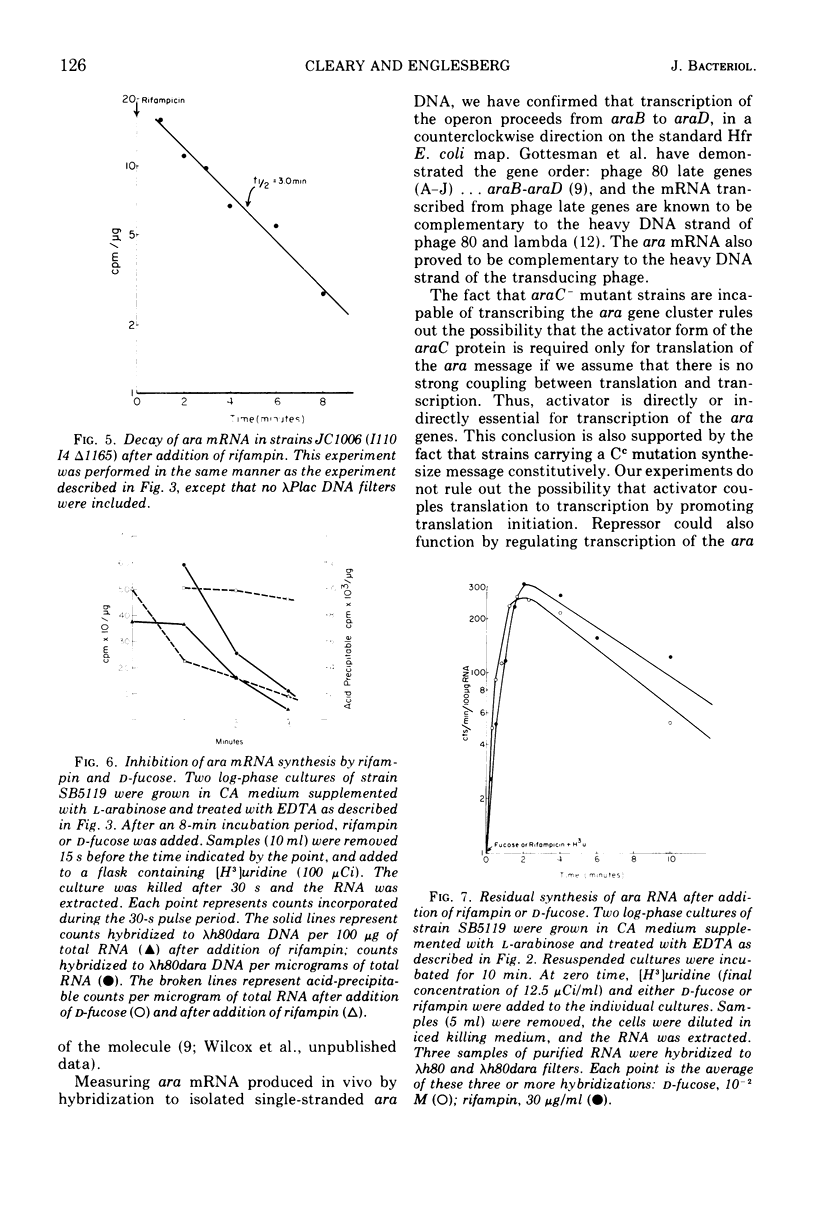
The structural genes involved in l-arabinose metabolism are regulated by the protein product of the araC gene. This protein functions as both an activator and repressor of enzyme synthesis in this gene complex. Using λh80dara deoxyribonucleic acid in hybridization studies, we have shown that the ara operon, including structural genes araB, araA, and araD, is transcribed in the direction araB to araD and that initiation of transcription of these genes requires an active araC gene. The half-life of this message, approximately 3 min at 30 C, is the same in the presence or absence of the araC protein in the activator state. However, an unexplained 2-min lag in decay of ara messenger ribonucleic acid that does not occur in decay of lac messenger ribonucleic acid is observed. This lag period requires activated araC protein.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beverin S., Sheppard D. E., Park S. S. D-Fucose as a gratuitous inducer of the L-arabinose operon in strains of Escherichia coli B-r mutant in gene araC. J Bacteriol. 1971 Jul;107(1):79–86. doi: 10.1128/jb.107.1.79-86.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGLESBERG E., ANDERSON R. L., WEINBERG R., LEE N., HOFFEE P., HUTTENHAUER G., BOYER H. L-Arabinose-sensitive, L-ribulose 5-phosphate 4-epimerase-deficient mutants of Escherichia coli. J Bacteriol. 1962 Jul;84:137–146. doi: 10.1128/jb.84.1.137-146.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGLESBERG E. Enzymatic characterization of 17 L-arabinose negative mutants of Escherichia coli. J Bacteriol. 1961 Jun;81:996–1006. doi: 10.1128/jb.81.6.996-1006.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englesberg E., Irr J., Power J., Lee N. Positive control of enzyme synthesis by gene C in the L-arabinose system. J Bacteriol. 1965 Oct;90(4):946–957. doi: 10.1128/jb.90.4.946-957.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englesberg E., Squires C., Meronk F., Jr The L-arabinose operon in Escherichia coli B-r: a genetic demonstration of two functional states of the product of a regulator gene. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1100–1107. doi: 10.1073/pnas.62.4.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS J., ENGLESBERG E. Determination of the order of mutational sites governing L-arabinose utilization in Escherichia coli B/r bv transduction with phage Plbt. Virology. 1959 Nov;9:314–331. doi: 10.1016/0042-6822(59)90125-4. [DOI] [PubMed] [Google Scholar]
- Gielow L., Largen M., Englesberg E. Initiator constitutive mutants of the L-arabinose operon (OIBAD) of Escherichia coli B/r. Genetics. 1971 Nov;69(3):289–302. doi: 10.1093/genetics/69.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt J., Schleif R. Arabinose C protein: regulation of the arabinose operon in vitro. Nat New Biol. 1971 Oct 6;233(40):166–170. doi: 10.1038/newbio233166a0. [DOI] [PubMed] [Google Scholar]
- Kessler D. P., Englesberg E. Arabinose-leucine deletion mutants of Escherichia coli B-r. J Bacteriol. 1969 Jun;98(3):1159–1169. doi: 10.1128/jb.98.3.1159-1169.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs B. L., Yanofsky C. Host and bacteriophage specific messenger RNA degradation in T7-infected Escherichia coli. Nat New Biol. 1971 Dec 8;234(49):168–170. doi: 10.1038/newbio234168a0. [DOI] [PubMed] [Google Scholar]
- Morse D. E., Yanofsky C. A transcription-initiating mutation within a structural gene of the tryptophan operon. J Mol Biol. 1969 May 14;41(3):317–328. doi: 10.1016/0022-2836(69)90278-2. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Mosteller R. D., Yanofsky C. Tryptophan messenger ribonucleic acid elongation rates and steady-state levels of tryptophan operon enzymes under various growth conditions. J Mol Biol. 1970 Aug;51(3):541–550. doi: 10.1016/0022-2836(70)90007-0. [DOI] [PubMed] [Google Scholar]
- Schleif R. F. L-arabinose operon messenger of Escherichia coli. Its inducibility and translation efficiency relative to lactose operon messenger. J Mol Biol. 1971 Oct 14;61(1):275–279. doi: 10.1016/0022-2836(71)90226-9. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Maizel J. V., Jr T7-directed protein synthesis. Virology. 1969 Nov;39(3):575–586. doi: 10.1016/0042-6822(69)90105-6. [DOI] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Perlman R. L., Pastan I. Regulation of lac transcription in antibiotic-treated E. coli. Nat New Biol. 1971 Mar 10;230(10):41–44. doi: 10.1038/newbio230041a0. [DOI] [PubMed] [Google Scholar]
- Wilcox G., Clemetson K. J., Santi D. V., Englesberg E. Purification of the araC protein. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2145–2148. doi: 10.1073/pnas.68.9.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox G., Singer J., Heffernan L. Deoxyribonucleic acid-ribonucleic acid hybridization studies on the L-Arabinose operon of Escherichia coli B-r. J Bacteriol. 1971 Oct;108(1):1–4. doi: 10.1128/jb.108.1.1-4.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A. M., Ghosh S., Echols H., Spiegelman W. G. Repression by the cI protein of phage lambda: in vitro inhibition of RNA synthesis. J Mol Biol. 1972 Jun 28;67(3):407–421. doi: 10.1016/0022-2836(72)90459-7. [DOI] [PubMed] [Google Scholar]
- Yang H. L., Zubay G. Synthesis of the arabinose operon regulator protein in a cell-free system. Mol Gen Genet. 1973 Apr 12;122(2):131–136. doi: 10.1007/BF00435186. [DOI] [PubMed] [Google Scholar]
- Zubay G., Gielow L., Englesberg E. Cell-free studies on the regulation of the arabinose operon. Nat New Biol. 1971 Oct 6;233(40):164–165. doi: 10.1038/newbio233164a0. [DOI] [PubMed] [Google Scholar]



