Abstract
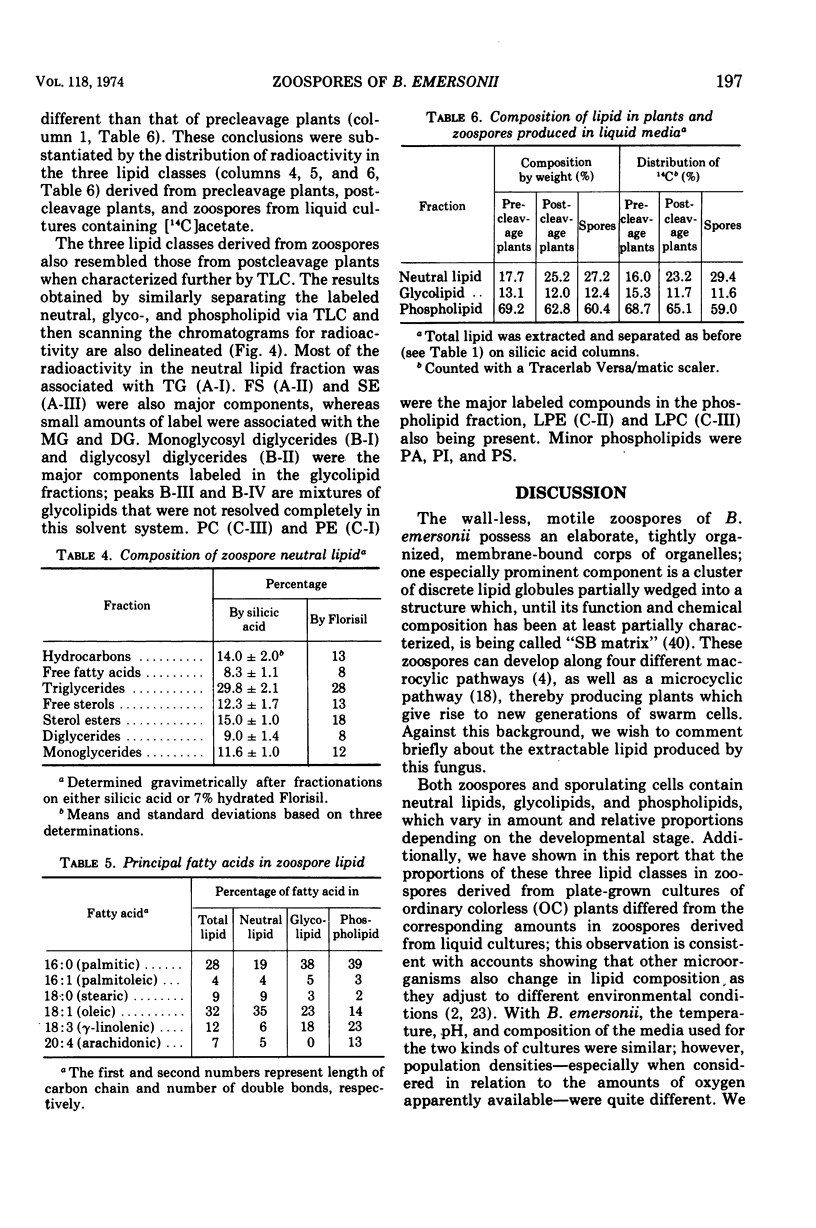
The zoospores of Blastocladiella emersonii, when derived from cultures grown on solid media, contain about 11% total lipid. This lipid was separated chromatographically on silicic acid into neutral lipid (46.6%), glycolipid (15.8%), and phospholipid (37.6%). Each class was fractionated further on columns of silicic acid, Florisil, or diethylaminoethyl-cellulose, and monitored by thin-layer chromatography. Triglycerides were the major neutral lipids, mono- and diglycosyldiglycerides were the major glycolipids, and phosphatidylcholine and phosphatidylethanolamine were the major phospholipids. Other neutral lipids and phospholipids detected were: hydrocarbons, free fatty acids, free sterols, sterol esters, diglycerides, monoglycerides, lysophosphatidylcholine, lysophosphatidylethanolamine, phosphatidic acid, phosphatidylserine, and phosphatidylinositol. Palmitic, palmitoleic, stearic, oleic, γ-linolenic, and arachidonic acids were the most frequently occurring fatty acids. When B. emersonii was grown in 14C-labeled liquid media, lipid again accounted for 11% of both mature plants and zoospores released from them. The composition of the lipid extracted from such plants and spores was also the same; however, it differed markedly from that of the lipid in spores harvested from solid media, consisting of 28.3% neutral lipid, 12.0% glycolipid, and 59.7% phospholipid. The major lipids were again triglycerides for neutral lipids, mono- and diglycosyldiglycerides for glycolipids, and phosphatidyl choline and phosphatidylethanolamine for phospholipids.
Full text
PDF

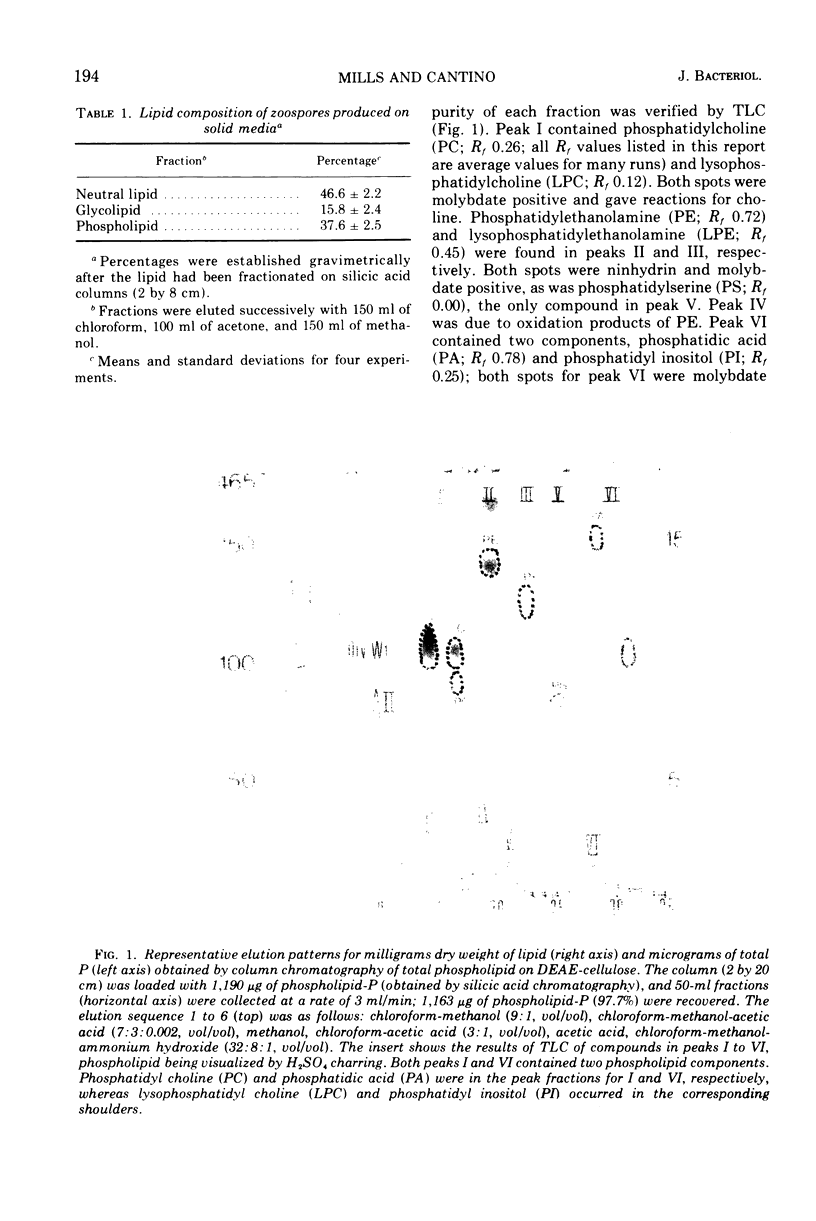
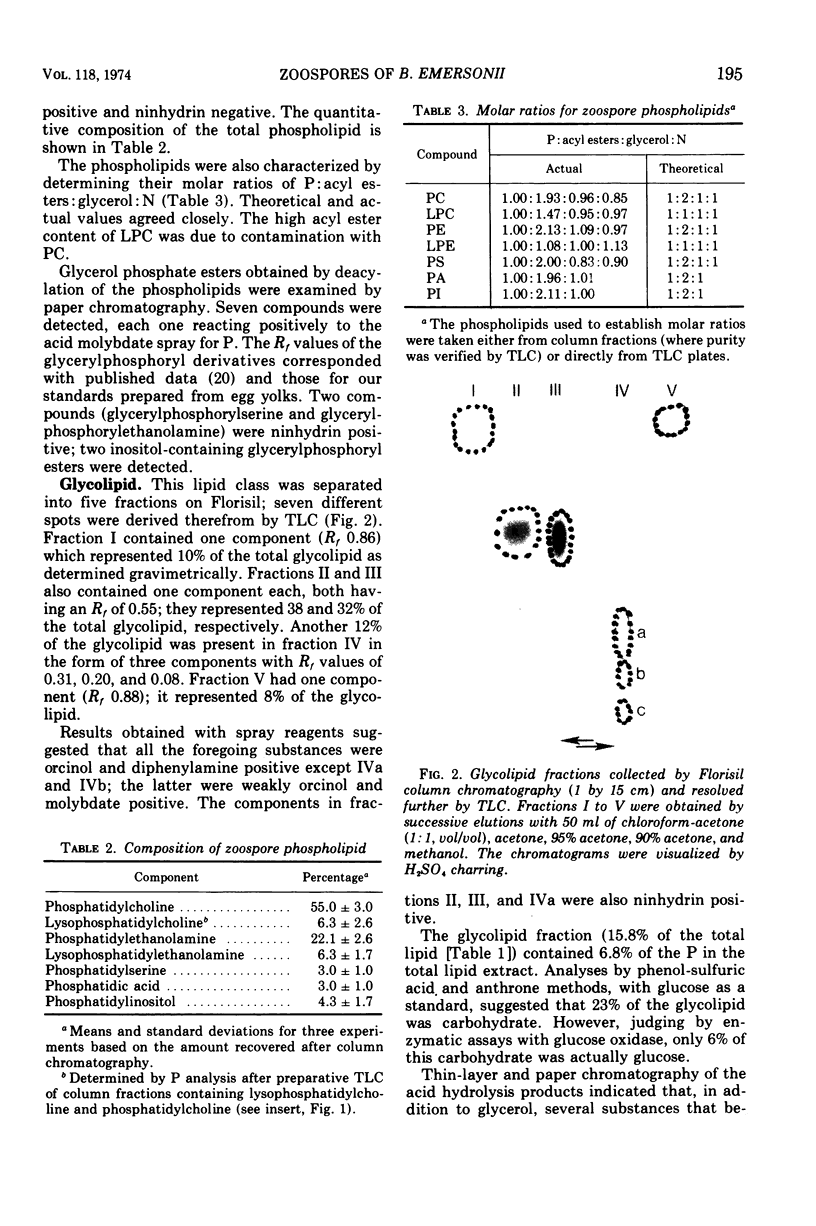





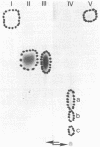
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Bowman R. D., Mumma R. O. The lipids of Phythium ultimum. Biochim Biophys Acta. 1967 Dec 5;144(3):501–510. doi: 10.1016/0005-2760(67)90038-0. [DOI] [PubMed] [Google Scholar]
- CANTINO E. C., HYATT M. T. Phenotypic sex determination in the life history of a new species of Blastocladiella, B. emersonii. Antonie Van Leeuwenhoek. 1953;19(1):25–70. doi: 10.1007/BF02594831. [DOI] [PubMed] [Google Scholar]
- CANTINO E. C. INTRACELLULAR DISTRIBUTION OF 14-C DURING SPOROGENESIS IN BLASTOCLADIELLA EMERSONII. EFFECT OF LIGHT ON HEMOPROTEIN. Arch Mikrobiol. 1965 May 28;51:42–59. doi: 10.1007/BF00406849. [DOI] [PubMed] [Google Scholar]
- DITTMER J. C., LESTER R. L. A SIMPLE, SPECIFIC SPRAY FOR THE DETECTION OF PHOSPHOLIPIDS ON THIN-LAYER CHROMATOGRAMS. J Lipid Res. 1964 Jan;5:126–127. [PubMed] [Google Scholar]
- GOLDSTEIN A., CANTINO E. C. Light-stimulated polysaccharide and protein synthesis by synchronized, single generations of Blastocladiella emersonii. J Gen Microbiol. 1962 Sep;28:689–699. doi: 10.1099/00221287-28-4-689. [DOI] [PubMed] [Google Scholar]
- Gordon P. A., Stewart P. R., Clark-Walker G. D. Fatty acid and sterol composition of Mucor genevensis in relation to dimorphism and anaerobic growth. J Bacteriol. 1971 Jul;107(1):114–120. doi: 10.1128/jb.107.1.114-120.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. M. A comparison of the glycolipids found in different strains of ascites tumour cells in mice. Nature. 1965 Jul 31;207(996):505–507. doi: 10.1038/207505a0. [DOI] [PubMed] [Google Scholar]
- HANAHAN D. J., OLLEY J. N. Chemical nature of monophosphoinositides. J Biol Chem. 1958 Apr;231(2):813–828. [PubMed] [Google Scholar]
- HANES C. S., ISHERWOOD F. A. Separation of the phosphoric esters on the filter paper chromatogram. Nature. 1949 Dec 31;164(4183):1107-12, illust. doi: 10.1038/1641107a0. [DOI] [PubMed] [Google Scholar]
- ISHERWOOD F. A., JERMYN M. A. Relationship between the structure of the simple sugars and their behaviour on the paper chromatogram. Biochem J. 1951 May;48(5):515–524. doi: 10.1042/bj0480515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khouw B. T., McCurdy H. D. Tricarboxylic acid cycle enzymes and morphogenesis in Blastocladiella emersonii. J Bacteriol. 1969 Jul;99(1):197–205. doi: 10.1128/jb.99.1.197-205.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostiw L. L., Boylen C. W., Tyson B. J. Lipid composition of growing and starving cells of Arthrobacter crystallopoietes. J Bacteriol. 1972 Jul;111(1):103–111. doi: 10.1128/jb.111.1.103-111.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall M. O., Kates M. Biosynthesis of phosphatidylglycerol by cell-free preparations from spinach leaves. Biochim Biophys Acta. 1972 Apr 18;260(4):558–570. doi: 10.1016/0005-2760(72)90005-7. [DOI] [PubMed] [Google Scholar]
- McCurdy H. D., Cantino E. C. Isocitritase, Glycine-Alanine Transaminase, and Development in Blastocladiella Emersonii. Plant Physiol. 1960 Jul;35(4):463–476. doi: 10.1104/pp.35.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R. B., Cantino E. C. DNA profile of the spore of Blastocladiella emersonii: evidence for -particle DNA. Arch Mikrobiol. 1971;78(3):252–267. doi: 10.1007/BF00424898. [DOI] [PubMed] [Google Scholar]
- RAPPORT M. M., ALONZO N. Photometric determination of fatty acid ester groups in phospholipides. J Biol Chem. 1955 Nov;217(1):193–198. [PubMed] [Google Scholar]
- RHODES D. N., LEA C. H. Phospholipids. IV. On the composition of hen's egg phospholipids. Biochem J. 1957 Mar;65(3):526–533. doi: 10.1042/bj0650526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safe S., Brewer D. Lipid composition of Chaetomium cochliodes: effect of media. Lipids. 1973 May;8(5):311–314. doi: 10.1007/BF02531910. [DOI] [PubMed] [Google Scholar]
- Shaw R. The fatty acids of phycomycete fungi, and the significance of the gamma-linolenic acid component. Comp Biochem Physiol. 1966 Jun;18(2):325–331. doi: 10.1016/0010-406x(66)90190-3. [DOI] [PubMed] [Google Scholar]
- Sloane-Stanley G. H. A simple procedure for the estimation of very small amounts of nitrogen in lipids. Biochem J. 1967 Jul;104(1):293–295. doi: 10.1042/bj1040293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. D., Silverman P. M. Lipid turnover during morphogenesis in the water mold Blastocladiella emersonii. Biochem Biophys Res Commun. 1973 Oct 1;54(3):1191–1197. doi: 10.1016/0006-291x(73)90818-8. [DOI] [PubMed] [Google Scholar]
- Sumner J. L., Morgan E. D. The fatty acid composition of sporangiospores and vegetative mycelium of temperature-adapted fungi in the order mucorales. J Gen Microbiol. 1969 Dec;59(2):215–221. doi: 10.1099/00221287-59-2-215. [DOI] [PubMed] [Google Scholar]
- Sumner J. L. The fatty acid composition of Blastocladiella emersonii. Can J Microbiol. 1970 Dec;16(12):1161–1164. doi: 10.1139/m70-196. [DOI] [PubMed] [Google Scholar]
- Truesdell L. C., Cantino E. C. The induction and early events of germination in the zoospore of Blastocladiella emersonii. Curr Top Dev Biol. 1971;6(6):1–44. doi: 10.1016/s0070-2153(08)60636-5. [DOI] [PubMed] [Google Scholar]
- WELLS M. A., DITTMER J. C. THE USE OF SEPHADEX FOR THE REMOVAL OF NONLIPID CONTAMINANTS FROM LIPID EXTRACTS. Biochemistry. 1963 Nov-Dec;2:1259–1263. doi: 10.1021/bi00906a015. [DOI] [PubMed] [Google Scholar]
- White D. C., Frerman F. E. Extraction, characterization, and cellular localization of the lipids of Staphylococcus aureus. J Bacteriol. 1967 Dec;94(6):1854–1867. doi: 10.1128/jb.94.6.1854-1867.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]