Abstract
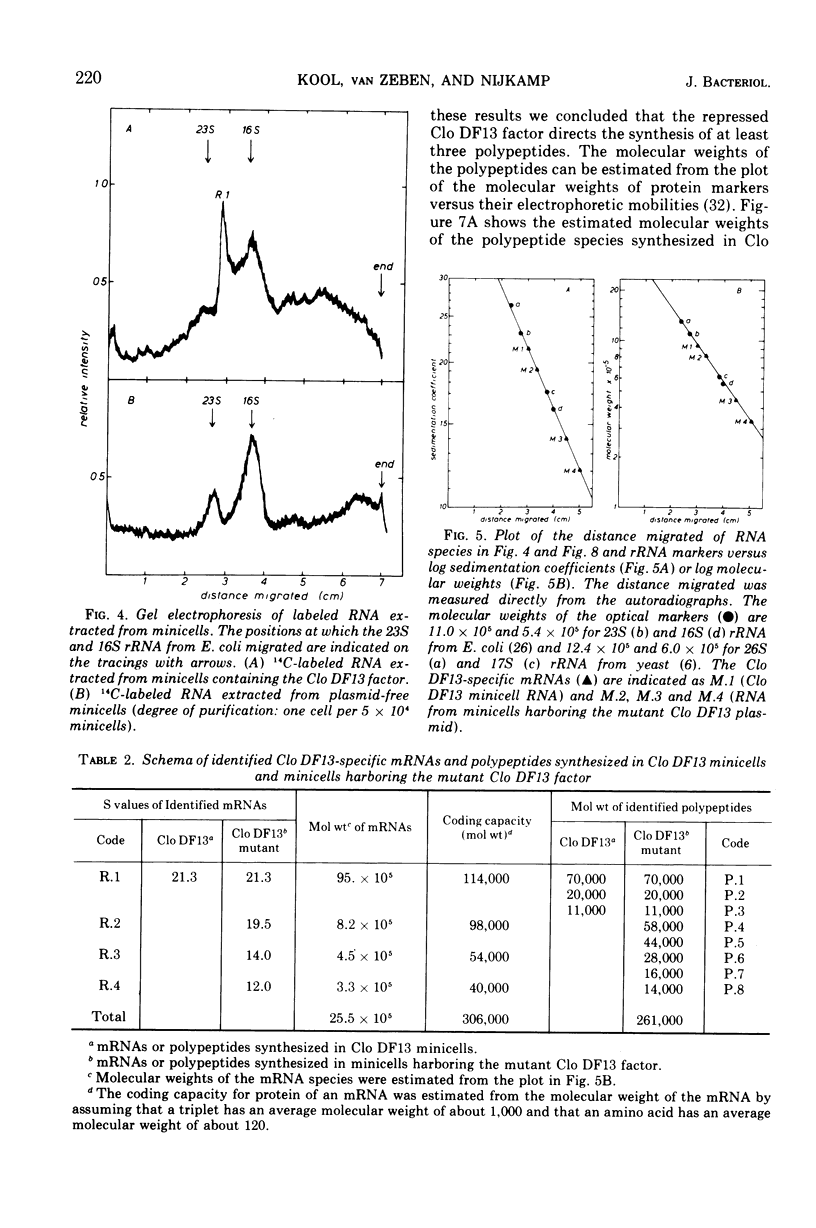
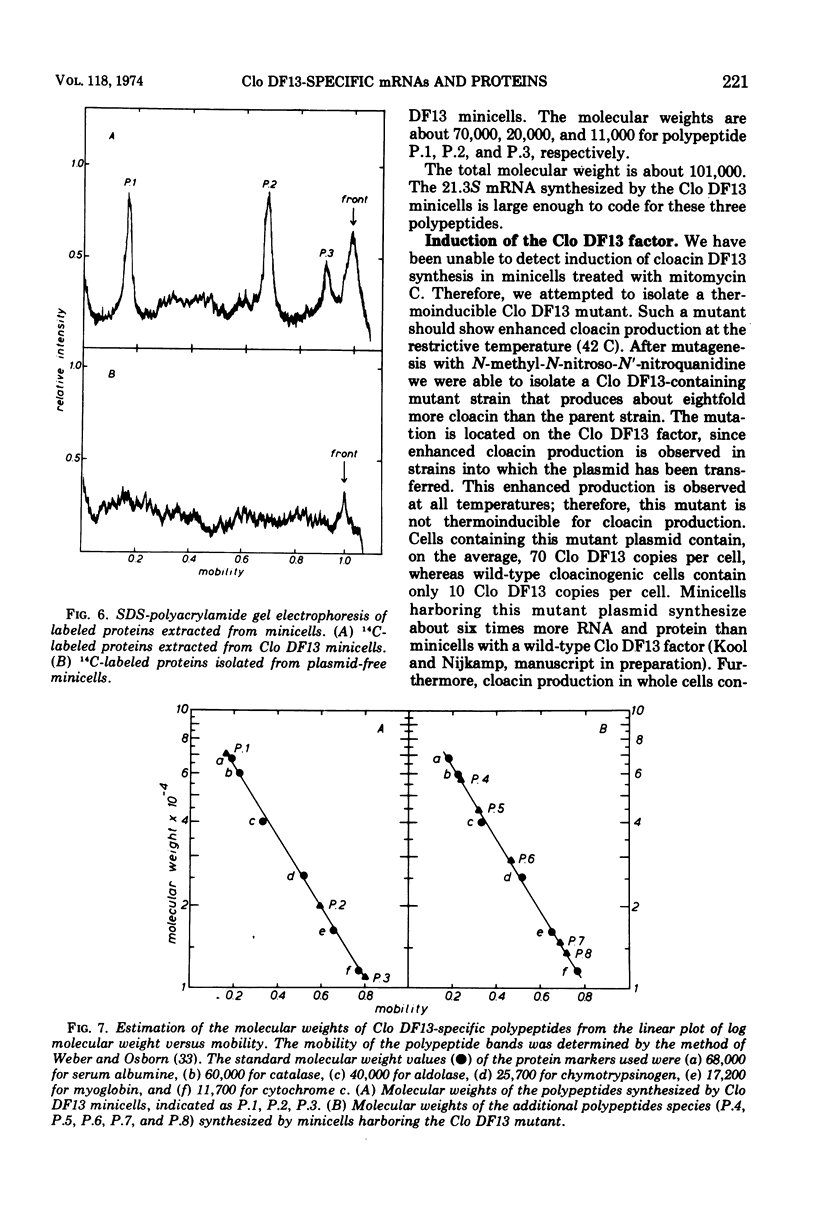
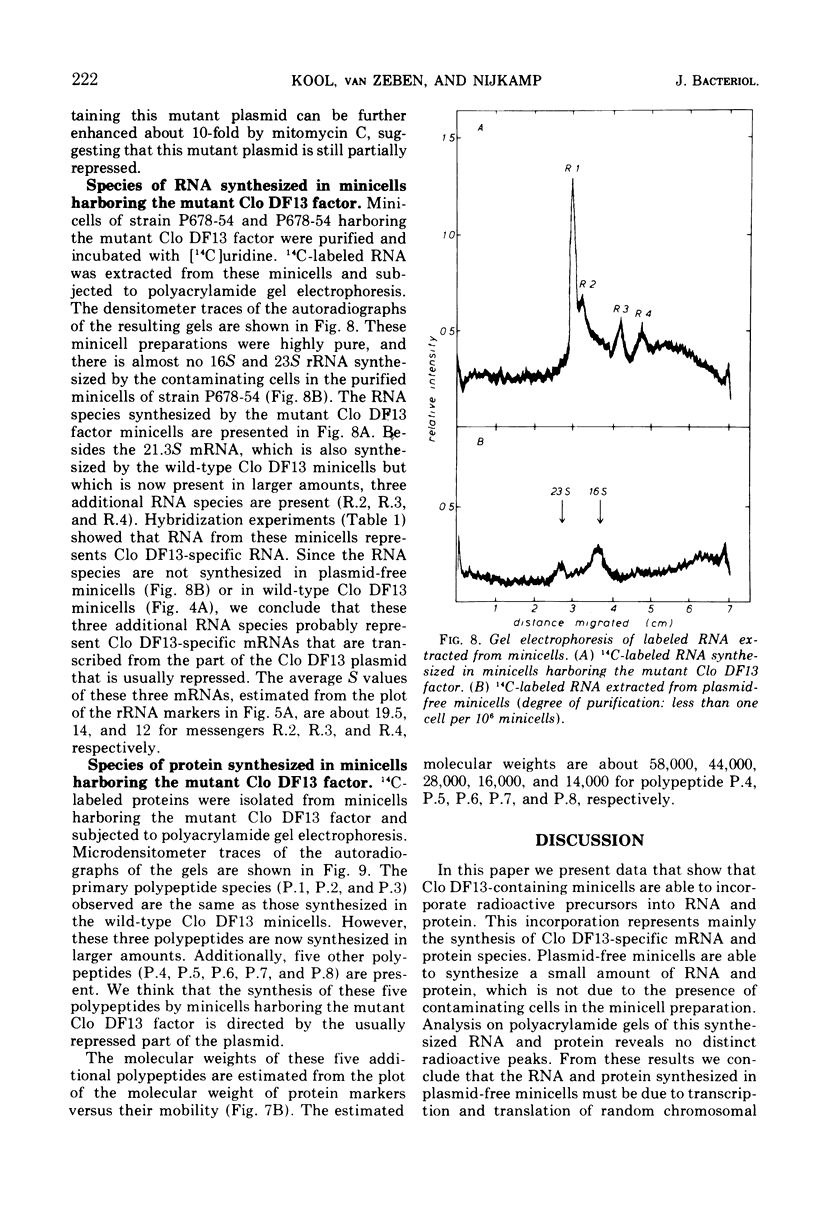
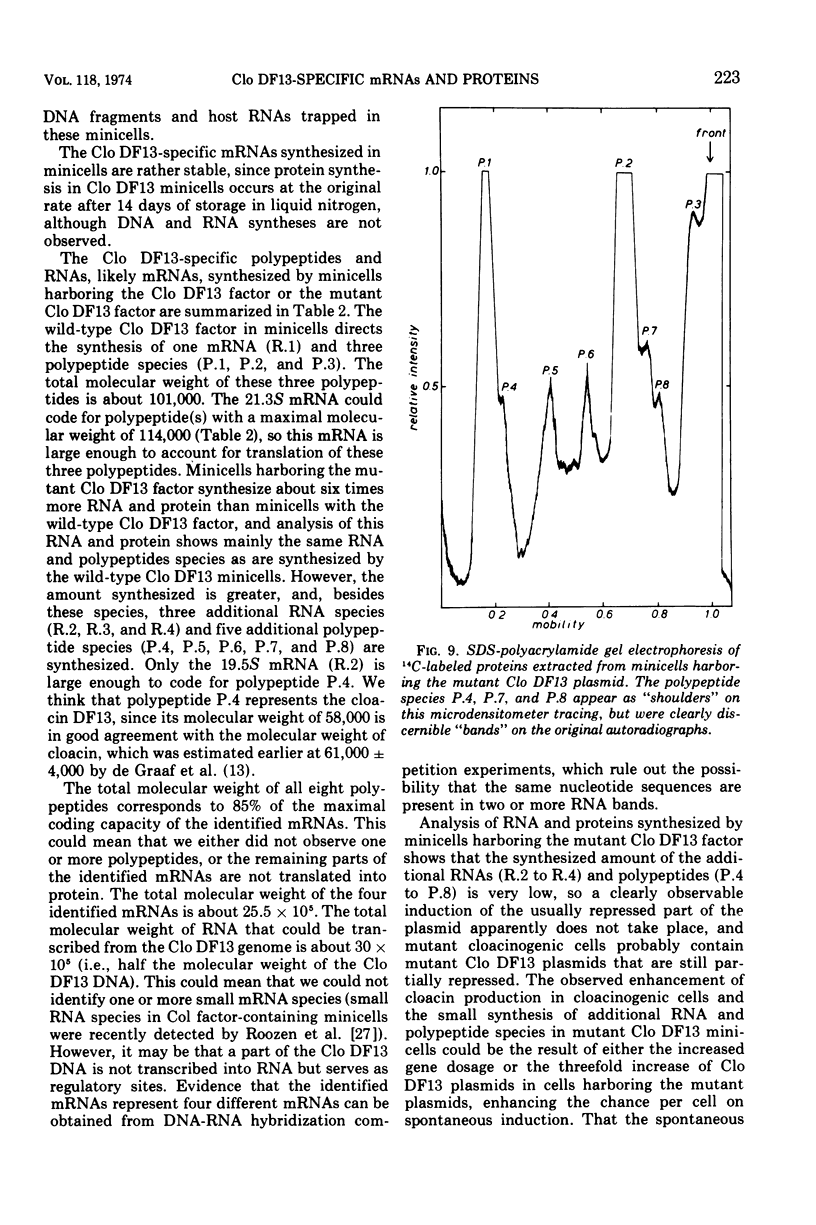
It has previously been shown that the cloacinogenic factor Clo DF13 (Clo DF13) segregates into minicells of strain Escherichia coli P678-54 that harbors Clo DF13 and that this Clo DF13 factor is the only deoxyribonucleic acid (DNA) present in these otherwise chromosomeless minicells. The study reported here shows that minicells prepared from P678-54(Clo DF13) are able to incorporate radioactive precursors into ribonucleic acid (RNA) and protein. The RNA synthesized in these purified minicells is Clo DF13 specific, as shown by RNA-DNA hybridization experiments. The results indicate that all the de novo synthesized gene products in Clo DF13 minicells are Clo DF13 specific. Polyacrylamide gel electrophoretic patterns show that in these minicells at least three polypeptides (molecular weight about 70,000, 20,000, and 11,000) and one major species of messenger RNA (mRNA) (S value about 21.3) are synthesized. To investigate the factor in its induced state, we isolated a Clo DF13 mutant with an enhanced level of cloacin production. Minicells harboring this Clo DF13 mutant produce five additional polypeptides (molecular weight about 58,000, 44,000, 28,000, 16,000, and 14,000). Three additional mRNA species (S value about 19.5, 14, and 12) could be distinguished. The total molecular weight of the eight polypeptides corresponds to 85% of the total coding capacity of the mRNAs (303,000). The total molecular weight of the four mRNAs is 2.55 × 106, which covers 85% of the Clo DF13 DNA (molecular weight 6 × 106).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler H. I., Fisher W. D., Cohen A., Hardigree A. A. MINIATURE escherichia coli CELLS DEFICIENT IN DNA. Proc Natl Acad Sci U S A. 1967 Feb;57(2):321–326. doi: 10.1073/pnas.57.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer H., Yuan D. RNA chain growth-rate in Escherichia coli. J Mol Biol. 1968 Dec 14;38(2):163–180. doi: 10.1016/0022-2836(68)90404-x. [DOI] [PubMed] [Google Scholar]
- CURTIS S. R., 3rd CHROMOSOMAL ABERRATIONS ASSOCIATED WITH MUTATIONS TO BACTERIOPHAGE RESISTANCE IN ESCHERICHIA COLI. J Bacteriol. 1965 Jan;89:28–40. doi: 10.1128/jb.89.1.28-40.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Click R. E., Tint B. L. Comparative sedimentation rates of plant, bacterial and animal ribosomal RNA. J Mol Biol. 1967 Apr 14;25(1):111–122. doi: 10.1016/0022-2836(67)90282-3. [DOI] [PubMed] [Google Scholar]
- Clowes R. C. Molecular structure of bacterial plasmids. Bacteriol Rev. 1972 Sep;36(3):361–405. doi: 10.1128/br.36.3.361-405.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Graaf F. K., Planta R. J., Stouthamer A. H. Effect of a bacteriocin produced by Enterobacter cloacae on protein biosynthesis. Biochim Biophys Acta. 1971 Jun 17;240(1):123–136. [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREDERICQ P. Colicins and colicinogenic factors. Symp Soc Exp Biol. 1958;12:104–122. [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Goebel W., Schrempf H. Isolation of minicircular deoxyribonucleic acids from wild strains of Escherichia coli and their relationship to other bacterial plasmids. J Bacteriol. 1972 Sep;111(3):696–704. doi: 10.1128/jb.111.3.696-704.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy K. G., Meynell G. G. A model relating the replication and expression of colicin factor E2-P9. Genet Res. 1972 Dec;20(3):331–334. doi: 10.1017/s0016672300013847. [DOI] [PubMed] [Google Scholar]
- Inselburg J. Segregation into and replication of plasmid deoxyribonucleic acid in chromosomeless segregants of Escherichia coli. J Bacteriol. 1970 Jun;102(3):642–647. doi: 10.1128/jb.102.3.642-647.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRBY K. S. ISOLATION AND CHARACTERIZATION OF RIBOSOMAL RIBONUCLEIC ACID. Biochem J. 1965 Jul;96:266–269. doi: 10.1042/bj0960266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass L. R., Yarmolinsky M. B. Segregation of functional sex factor into minicells. Proc Natl Acad Sci U S A. 1970 Jul;66(3):815–822. doi: 10.1073/pnas.66.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kool A. J., Nijkamp H. J. Isolation and characterization of immunity temperature sensitive mutants of Enterobacter cloacae harbouring the cloacinogenic factor DF13. Mol Gen Genet. 1972;114(4):312–324. doi: 10.1007/BF00267500. [DOI] [PubMed] [Google Scholar]
- Kool A. J., Pranger M., Nijkamp H. J. Proteins synthesized by a non-induced bacteriocinogenic factor in minicells of Escherichia coli. Mol Gen Genet. 1972;115(4):314–323. doi: 10.1007/BF00333170. [DOI] [PubMed] [Google Scholar]
- Levy S. B., Norman P. Segregation of transferable R factors into Escherichia coli minicells. Nature. 1970 Aug 8;227(5258):606–607. doi: 10.1038/227606a0. [DOI] [PubMed] [Google Scholar]
- Levy S. B. Physical and functional characteristics of R-factor deoxyribonucleic acid segregated into Escherichia coli minicells. J Bacteriol. 1971 Oct;108(1):300–308. doi: 10.1128/jb.108.1.300-308.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewicki P. P., Sinskey A. J. Precision of RNA separation by polyacrylamide gel electrophoresis. Anal Biochem. 1970 Feb;33(2):273–278. doi: 10.1016/0003-2697(70)90297-6. [DOI] [PubMed] [Google Scholar]
- Novick R. P. Extrachromosomal inheritance in bacteria. Bacteriol Rev. 1969 Jun;33(2):210–263. doi: 10.1128/br.33.2.210-263.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Roozen K. J., Fenwick R. G., Jr, Curtiss R., 3rd Synthesis of ribonucleic acid and protein in plasmid-containing minicells of Escherichia coli K-12. J Bacteriol. 1971 Jul;107(1):21–33. doi: 10.1128/jb.107.1.21-33.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirbasku D. A., Buchanan J. M. Patterns of ribonucleic acid synthesis in T5-infected Escherichia coli. II. Separation of high molecular weight ribonucleic acid species by disc electrophoresis on acrylamide gel columns. J Biol Chem. 1970 May 25;245(10):2679–2692. [PubMed] [Google Scholar]
- Stouthamer A. H., Tieze G. A. Bacteriocin production by members of the genus Klebsiella. Antonie Van Leeuwenhoek. 1966;32(2):171–182. doi: 10.1007/BF02097457. [DOI] [PubMed] [Google Scholar]
- Summers W. C. The process of infection with coliphage T7. I. Characterization of T7 RNA by polyacrylamide gel electrophoretic analysis. Virology. 1969 Oct;39(2):175–181. doi: 10.1016/0042-6822(69)90037-3. [DOI] [PubMed] [Google Scholar]
- Tieze G. A., Stouthamer A. H., Jansz H. S., Zandberg J., van Bruggen E. F. A bacteriocinogenic factor of Enterobacter cloacae. Mol Gen Genet. 1969;106(1):48–65. [PubMed] [Google Scholar]
- Veltkamp E., Barendsen W., Nijkamp H. J. Influence of protein and ribonucleic acid synthesis on the replication of the bacteriocinogenic factor Clo DF13 in Escherichia coli cells and minicells. J Bacteriol. 1974 Apr;118(1):165–174. doi: 10.1128/jb.118.1.165-174.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veltkamp E., Nijkamp H. J. The role of DNA polymerase I, II and 3 in the replication of the bacteriocinogenic factor Clo DF 13. Mol Gen Genet. 1973 Sep 27;125(4):329–340. doi: 10.1007/BF00276588. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wiegers U., Hilz H. Rapid isolation of undegraded polysomal RNA without phenol. FEBS Lett. 1972 Jun 1;23(1):77–82. doi: 10.1016/0014-5793(72)80289-8. [DOI] [PubMed] [Google Scholar]
- de Graaf F. K., Niekus H. G., Klootwijk J. Inactivation of bacterial ribosomes in vivo and in vitro by cloacin DF13. FEBS Lett. 1973 Sep 1;35(1):161–165. doi: 10.1016/0014-5793(73)80601-5. [DOI] [PubMed] [Google Scholar]
- de Graaf F. K., Tieze G. A., Wendelaar Bonga S., Stouthamer A. H. Purification and genetic determination of bacteriocin production in Enterobacter cloacae. J Bacteriol. 1968 Feb;95(2):631–640. doi: 10.1128/jb.95.2.631-640.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]


