Abstract
The cell wall of Sulfolobus acidocaldarius has been isolated. Cells were mechanically disrupted with a French press, and the cytoplasmic membrane was removed by extracting cell-envelope fragments with Triton X-100. The Triton-insoluble cell wall material retained the characteristic subunit structure when examined in the electron microscope. Isolated cell wall fragments formed in open sheets that were easily separated from cytoplasmic contamination. Chemical studies showed that the Triton-insoluble cell wall fragments consisted of lipoprotein with small amounts of carbohydrate and hexosamine. The amino acid composition indicated a highly charged hydrophobic cell surface. The presence of diaminopimelic acid with only traces of muramic acid indicates that the cell envelope does not have a rigid peptidoglycan layer. The results of chemical analyses and electron microscopy suggest a wall-membrane interaction stabilizing the cell envelope. The chemical and physical properties of this type of cell envelope would appear to form the basis for a new major division of bacteria with the definitive characteristics of a morphologically distinct subunit cell wall devoid of peptidoglycan.
Full text
PDF

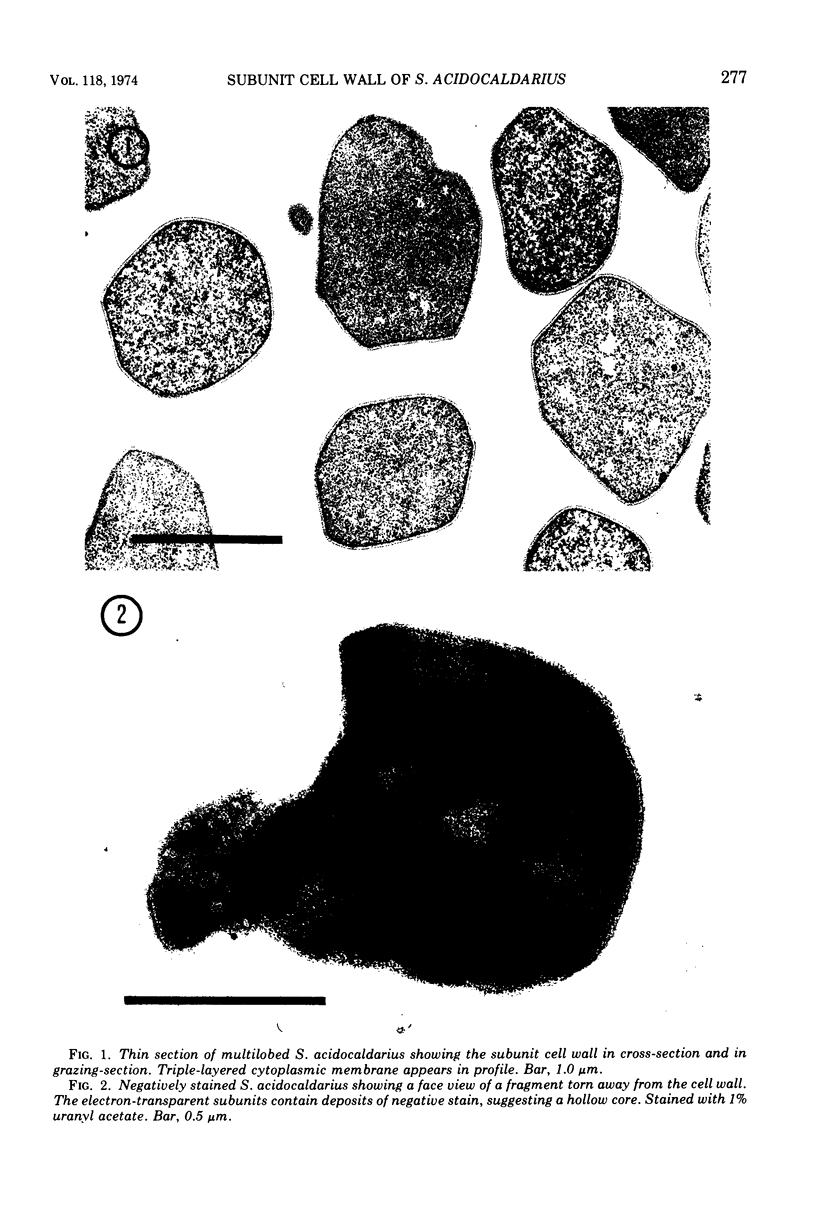







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams G. A. Extraction of lipopolysaccharides from gram-negative bacteria with dimethyl sulfoxide. Can J Biochem. 1967 Mar;45(3):422–426. doi: 10.1139/o67-050. [DOI] [PubMed] [Google Scholar]
- BROWN A. D. ASPECTS OF BACTERIAL RESPONSE TO THE IONIC ENVIRONMENT. Bacteriol Rev. 1964 Sep;28:296–329. doi: 10.1128/br.28.3.296-329.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdsell D. C., Cota-Robles E. H. Lysis of spheroplasts of Escherichia coli by a non-ionic detergent. Biochem Biophys Res Commun. 1968 May 10;31(3):438–446. doi: 10.1016/0006-291x(68)90496-8. [DOI] [PubMed] [Google Scholar]
- Braun V., Rehn K. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of the E. coli cell wall. The specific effect of trypsin on the membrane structure. Eur J Biochem. 1969 Oct;10(3):426–438. doi: 10.1111/j.1432-1033.1969.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Brock T. D., Brock K. M., Belly R. T., Weiss R. L. Sulfolobus: a new genus of sulfur-oxidizing bacteria living at low pH and high temperature. Arch Mikrobiol. 1972;84(1):54–68. doi: 10.1007/BF00408082. [DOI] [PubMed] [Google Scholar]
- Brock T. D., Brock M. L., Bott T. L., Edwards M. R. Microbial life at 90 C: the sulfur bacteria of Boulder Spring. J Bacteriol. 1971 Jul;107(1):303–314. doi: 10.1128/jb.107.1.303-314.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrick L., Jr, Berk R. S. Membranous inclusions of Pseudomonas aeruginosa. J Bacteriol. 1971 Apr;106(1):250–256. doi: 10.1128/jb.106.1.250-256.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K. Y., Doy C. H., Mercer E. H. Ultrastructure of the obligate halophilic bacterium Halobacterium halobium. J Bacteriol. 1967 Jul;94(1):196–201. doi: 10.1128/jb.94.1.196-201.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum E. H., Siehr D. J. Thiobacillus thiooxidans cell wall amino acids and monosaccharides. J Bacteriol. 1967 Dec;94(6):2069–2070. doi: 10.1128/jb.94.6.2069-2070.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe J. M., Featherston W. R., Stadelman W. J., Banwart G. J. Amino acid composition of certain bacterial cell-wall proteins. Appl Microbiol. 1965 Sep;13(5):650–652. doi: 10.1128/am.13.5.650-652.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Peterson D. E., Bernlohr R. W. Determinaion of muraic acid, ornithine, and diaminopimelic acid during automatic amino aci analysis. Anal Biochem. 1970 Feb;33(2):238–243. doi: 10.1016/0003-2697(70)90292-7. [DOI] [PubMed] [Google Scholar]
- REPASKE R. Lysis of gram-negative bacteria by lysozyme. Biochim Biophys Acta. 1956 Oct;22(1):189–191. doi: 10.1016/0006-3002(56)90240-2. [DOI] [PubMed] [Google Scholar]
- Steensland H., Larsen H. A study of the cell envelope of the halobacteria. J Gen Microbiol. 1969 Mar;55(3):325–336. doi: 10.1099/00221287-55-3-325. [DOI] [PubMed] [Google Scholar]







