Abstract
We demonstrate that the receptor binding moiety of Escherichia coli heat-labile enterotoxin (EtxB) can completely prevent autoimmune disease in a murine model of arthritis. Injection of male DBA/1 mice at the base of the tail with type II collagen in the presence of complete Freund’s adjuvant normally leads to arthritis, as evidenced by inflammatory infiltration and swelling of the joints. A separate injection of EtxB at the same time as collagen challenge prevented leukocyte infiltration, synovial hyperplasia, and degeneration of the articular cartilage and reduced clinical symptoms of disease by 82%. The principle biological property of EtxB is its ability to bind to the ubiquitous cell surface receptor GM1 ganglioside, and to other galactose-containing glycolipids and galactoproteins. The importance of receptor interaction in mediating protection from arthritis was demonstrated by the failure of a non-receptor-binding mutant of EtxB to elicit any protective effect. Analysis of T cell responses to collagen, in cultures of draining lymph node cells, revealed that protection was associated with a marked increase in interleukin 4 production concomitant with a reduction in interferon γ levels. Furthermore, in protected mice there was a significant reduction in anti-collagen antibody levels as well as an increase in the IgG1/IgG2a ratio. These observations show that protection is associated with a shift in the Th1/Th2 balance as well as a general reduction in the extent of the anti-type II collagen immune response. This suggests that EtxB-receptor-mediated modulation of lymphocyte responses provides a means of preventing autoimmune disease.
Autoimmune diseases remain a major health problem despite enormous efforts to understand the underlying causative mechanisms. The lack of clarity with regard to both the predisposing factors and the precise antigenic targets of the immune response have restricted the development of effective therapeutic approaches. However, recent evidence suggests that agents which modulate the nature of the immune response may be effective as a means of prophylaxis or treatment. We recently reported that the nontoxic B subunit of Escherichia coli heat-labile enterotoxin (EtxB) exerts profound modulatory effects on lymphocyte populations in vitro; notably it was shown to cause the polyclonal activation of B cells, to induce apoptosis in CD8+ T cells, and to have a negligible direct effect on CD4+ T cells (1–3). The failure of a non-receptor-binding mutant of EtxB, EtxB(G33D), to cause such differential activation and apoptosis of lymphocytes provided unequivocal evidence for a critical role of receptor interaction in mediating these events. The principle receptor to which EtxB binds is a ubiquitous cell surface glycolipid, GM1 ganglioside [ref. 4; Galβ3GalNAcβ4(NeuAcα3)-Galβ4Glcβ1Cer], which consists of a ceramide tail inserted in the outer leaflet of the plasma membrane linked to an exposed pentasaccharide moiety. In addition to binding GM1, EtxB binds weakly to other gangliosides, including non-galactose-containing GM2 and asialo-GM1 (5) as well as galactoproteins (6, 7). Although the precise function of these receptors remains unknown, our observations led us to suggest that their cross-linking by EtxB might be sufficient to modulate the environment in which an immune response to other antigens, including autoantigens, is initiated in vivo. To investigate this possibility, we have studied whether EtxB can prevent autoimmune disease in a well established model of arthritis.
Collagen-induced arthritis (CIA) is an autoimmune disease inducible in mice, rats, and primates by immunization with homologous or heterologous type II collagen (CII) in adjuvant (8–10). The resulting pathology resembles rheumatoid arthritis with profound leukocyte infiltration, hyperplasia of the synovium, and breakdown of cartilage and bone. Although the antibody response to CII appears to be important in the development of full pathology in CIA (11, 12), the disease is clearly T cell-dependent because (i) disease can be transferred into naive recipients using CD4+ T cells specific for CII from arthritic mice (13, 14), and (ii) it can be prevented by the in vivo administration of monoclonal antibodies to CD4, TCR, or class II major histocompatibility complex (MHC; refs. 15–17). Evidence suggests that the nature of the CD4+ T cell response with respect to the balance between Th1 cytokines [interferon γ (IFN-γ) and interleukin (IL)-2] and Th2 cytokines (IL-4, IL-10, and IL-13) plays an important role in CIA. As with other inflammatory autoimmune diseases, CIA appears to be promoted by a Th1-dominated response and is down-regulated if relative levels of Th2 cytokines are increased. Thus, early injection of IL-12 (a cytokine that enhances Th1 responses) or IFN-γ increases the severity of arthritis in collagen-challenged mice (18, 19), whereas treatment with IL-4, IL-13, or IL-10 prevents disease (20, 21). In addition, time course studies have shown that IFN-γ-producing T cells dominate the anti-CII response during disease onset but that later remission is accompanied by a decline in IFN-γ levels and increased IL-10 (22). Although the precise role of Th1 cytokines in the causation of CIA remains unclear, the ability of IFN-γ to promote the production of complement fixing IgG2a antibody and the ability to enhance macrophage activation and inflammatory mediator release are likely to be critical (23).
In this paper, we demonstrate that receptor binding by EtxB can prevent autoimmune disease in the DBA/1 mouse model of arthritis. Disease protection was evident both clinically and histologically when EtxB was given either at the same time or 21 days after collagen challenge. EtxB did not abrogate the induction of the anti-CII immune response; however, the profiles of T cell cytokines and antibody isotypes was substantially altered. These studies identify EtxB-receptor interaction as an important target for modulating adverse immune reactions and are suggestive of a role for GM1-mediated signaling events in controlling cells of the immune system.
MATERIALS AND METHODS
Mice.
Male DBA/1 mice were purchased from Harlan Olac (Bicester, U.K.) and maintained in the departmental animal facilities. Mice were 10 weeks of age at the time of immunization.
Antigens, Immunization, and Induction of Arthritis.
Recombinant preparations of EtxB and EtxB(G33D) were purified as reported (24). Both proteins are well characterized with respect to purity, physicochemical properties, and their ability to bind to GM1 (1). Either protein (100 μg) was injected s.c. into the dorsal flank in incomplete Freund’s adjuvant (IFA). CII collagen (from bovine nasal septum; Sigma) was dissolved in 0.05 M acetic acid. To induce arthritis, 100 μg of CII was injected s.c. at the base of the tail in complete Freund’s adjuvant (CFA) and in IFA 21 days later. For use in T cell assays, samples of CII were placed in boiling water for 40 min before extensive dialysis against Hanks’ balanced salt solution (Ca2+ and Mg2+ free; Flow Laboratories) and sterilization by filtration (0.2-μm filter; Millipore). Heating in this way aids the breakdown of the molecule, allowing more effective antigen presentation in in vitro assays.
Disease Assessment.
Mice were examined visually for the incidence of arthritis in the tarsal (ankle) joints at various time points; the arthritis was assessed by measuring the tarsal joints with a micrometer on day 45 after disease induction. On day 45, experiments were terminated and stifle (knee) joints were dissected out, fixed in neutral-buffered formalin, and decalcified. Longitudinal sections were stained with hemotoxylin and eosin and scored blind according to an established grading system (25): 0, normal; 1, synovial hyperplasia with pannus formation and mild inflammation or noninflammatory mild articular cartilage degeneration; 2, articular cartilage degeneration with synovial hyperplasia and pannus formation, with moderate to severe inflammation; 3, same as 2 but also with significant inflammation and evidence of inflammatory cells and debris in the joint space.
Assessment of T Cell Proliferation and Cytokine Production.
Mice were killed 16 days after immunization, and inguinal lymph node cell populations were derived and cultured at 3 × 106 viable cells per ml in either 2-ml volumes in 24-well plates or 8-ml volumes in 25-cm3 flasks (Nunc) as described (26). Cultures were established in the presence or absence of 50 μg/ml CII as indicated, and at desired time points, triplicate 100-μl samples were withdrawn for assessment of proliferation ([3H]thymidine incorporation; ref. 26) or cytokine production. For the assessment of IFN-γ and IL-4 levels, a modified ELISA technique was employed (1, 27) in which cell samples were cultured overnight at 37°C in a humidified atmosphere of 5% CO2 in capture antibody-coated ELISA plates before detection of cytokine. In this way, cytokine production by cells over a defined period of culture could be assessed in a way in which the effects of differential cytokine lability were minimized. Cytokine levels were calculated against standard curves produced with the appropriate recombinant cytokine by weighted probit analysis (28).
Measurement of Antibody Responses.
Serum samples were taken 15 days after immunization of mice and were analyzed for the presence of anti-bovine CII antibodies by ELISA (29). The subclass distribution of the anti-collagen antibody response was determined using ELISA with specific detecting antibodies for murine IgG1, IgG2a, IgG2b, and IgG3 (Serotec). Antibody titers were calculated by linear regression analysis on log-transformed data and are expressed as endpoints, where the endpoint is calculated as the mean optical density obtained from eight unimmunized mouse serum samples run on each ELISA plate.
Statistical Analysis.
Statistical significance was determined using a Student’s t test.
RESULTS
EtxB-Receptor Interaction Prevents CIA.
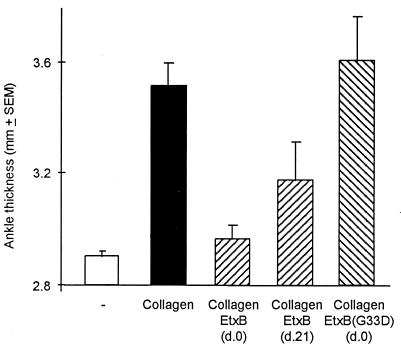
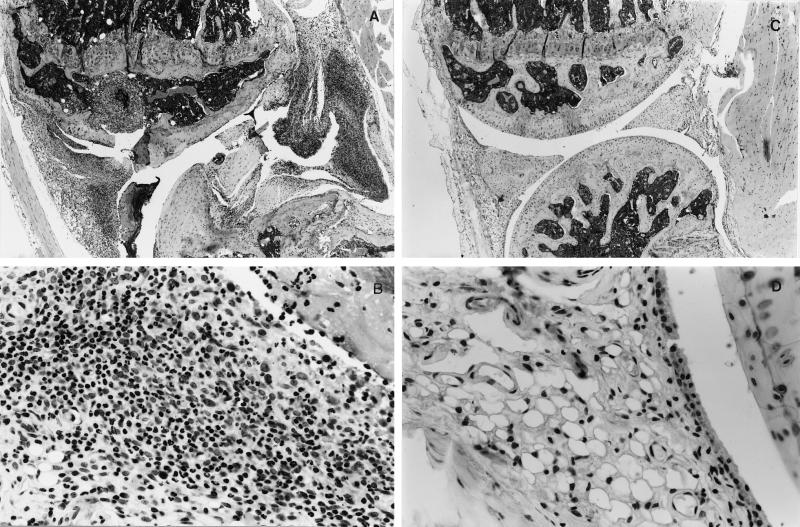
Immunization of male DBA/1 mice with bovine CII resulted in classical symptoms of arthritis evidenced by an increase in ankle thickness (Fig. 1). To determine if EtxB could ameliorate the induction of arthritis, mice were injected s.c. with 100 μg of EtxB into the dorsal flank and were challenged at the same time with CII at the base of the tail. The inclusion of EtxB resulted in a dramatic reduction in joint swelling to an extent that there was no significant increase in ankle thickness in comparison with unimmunized control mice (P > 0.85). In the representative experiment shown, EtxB injection on day 0 reduced joint swelling by 82% (P < 0.001). Such protection from arthritis was also evident from histological analysis of stifle joints. Challenge with collagen alone led to a marked disruption of joint structure in 71% of the animals (Table 1). This was characterized by a loss of articular surface and associated pannus formation (Fig. 2A), intense infiltration of the synovium with neutrophils and macrophages, and clear necrosis and loss of synoviocytes (Fig. 2B). In contrast, mice challenged with collagen and concomitantly injected with EtxB exhibited no joint destruction (Fig. 2C), and in 13 of 15 mice, there was no sign of any inflammation (Fig. 2D and Table 1). In the two animals in which histological abnormality was noted, the score of arthritis was very low, and the changes were restricted to slight synovial hyperplasia or mild diffuse neutrophil infiltration. In both cases, there were minimal changes to the articular surfaces.
Figure 1.
EtxB, but not EtxB(G33D), dramatically protects mice from the development of CIA. Groups of male DBA/1 mice (7–15 animals per group) were either unchallenged (□; negative control) or were each injected with 100 μg of bovine collagen in CFA on day 0 by s.c. injection at the base of tail. With collagen-injected mice, either the animals were left unprotected (▪; positive control) or attempts were made to prevent disease development by the separate s.c. administration into the flank of 100 μg of EtxB in IFA on either day 0 or day 21 (▧) or 100 μg of EtxB(G33D) in IFA on day 0 (▨). All animals, except the negative control, received a boosting dose of collagen in IFA s.c. at the base of tail on day 21, and disease severity was assessed on day 45 by measuring hind limb ankle thickness.
Table 1.
Histological signs of joint damage in collagen- induced arthritis
| Collagen* | Toxin† | Incidence‡ | Histological score§ |
|---|---|---|---|
| − | − | 0/7 (0%) | 0.00 ± 0.00 |
| + | − | 10/14 (71%) | 1.88 ± 0.35 |
| + | EtxB (day 0) | 2/15 (13%) | 0.62 ± 0.38 |
| + | EtxB (day 21) | 3/7 (43%) | 1.62 ± 1.59 |
| + | EtxB(G33D) (day 0) | 5/7 (71%) | 1.92 ± 0.49 |
Bovine CII given in CFA s.c. at the base of tail on day 0 and in IFA on day 21.
Either EtxB or EtxB(G33D) (100 μg) given in IFA s.c. on the dorsal flank on the day indicated.
The number of mice with arthritis assessed histologically 45 days after collagen injection over the total group size.
Sections of knee joints were scored blind for joint changes according to the following grading system: 0, normal; 1, synovial hyperplasia with pannus formation and mild inflammation or noninflammatory mild articular cartilage degeneration; 2, articular cartilage degeneration with synovial hyperplasia and pannus formation, and moderate to severe inflammation (polymorphonuclear leukocytes and macrophages); 3, Articular cartilage degeneration with synovial hyperplasia and pannus formation, severe inflammation (polymorphonuclear leukocytes and macrophages), and significant inflammation in joint space with polymorphonuclear leukocytes, macrophages, and debris. Data are shown as the mean ± SEM of the positive animals.
Figure 2.
EtxB protects mice from histopathological changes in the joint associated with arthritis. Stifle joints were removed on day 45 from mice challenged with either bovine collagen alone (unprotected, A and B) or bovine collagen and EtxB (protected, C and D), as described in the Fig. 1 legend. Low magnification reveals marked disruption of joint structure with loss of articular surface and associated pannus formation, with focal granulomatous inflammation and fibrosis of the epiphyseal cavities of the femur and tibia, and necrosis and intense inflammation of the synovium in unprotected mice (A). In contrast, there is no histological abnormality in joint microarchitecture of EtxB-protected mice (B). Microscopic examination of the synovium at higher magnification clearly shows necrosis and loss of synoviocytes with an extensive infiltration of neutrophils and macrophages in unprotected mice (C), whereas in protected animals (D), the synovial membrane comprises a regular layer of synoviocytes with underlying loose connective tissue.
To evaluate if EtxB could be used to modulate CIA if given to animals in which the pathological immune response to CII was established, injection of EtxB was delayed until day 21 after collagen challenge. By this time, antibody and T cell reactivity to CII is clearly demonstrable, and some animals exhibit initial signs of joint swelling and infiltration (data not shown). The results indicate that when mice were given EtxB at this late stage, the histological incidence of arthritis was reduced from 72% to 43% (Table 1), and there was a significant reduction in mean ankle thickness (Fig. 1; P = 0.05). Of the three mice that showed histological signs of disease, marked pathological alterations were present in only one (score = 3), with the other two showing only slight synovial hyperplasia in one stifle joint (score = 0.25).
To assess if EtxB-receptor interaction was necessary to elicit disease protection, mice were injected with CII plus a non-receptor-binding mutant of EtxB, EtxB(G33D). Such treatment failed to protect the animals from the development of arthritis, as evidenced by the lack of differences in the extent of ankle swelling (Fig. 1), the incidence of joint damage, or the histological score of disease (Table 1), compared with mice given CII alone. We conclude that EtxB can prevent both the induction and progression of CIA and that this is mediated by a mechanism dependent on receptor interaction.
Receptor Binding by EtxB Does Not Block the Establishment of an Anti-Collagen Immune Response.
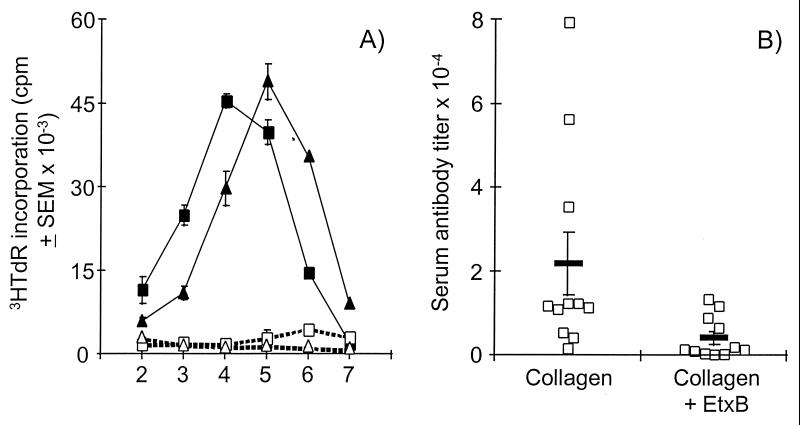
To elucidate how injection of EtxB abrogates the induction of CIA, we investigated whether receptor interaction prevents the establishment of either a T cell or antibody response to CII. To assess T cell reactivity to collagen, inguinal lymph node cells were isolated from groups of mice 16 days after injection with either EtxB plus CII (protected) or CII alone (unprotected), and their in vitro proliferative responses were measured in the presence or absence of CII. Strong proliferative responses to CII were detected in cultures of cells from both protected and unprotected animals (Fig. 3A). Although the magnitude of the responses was similar, there was a slight shift in the kinetics of the reaction with a delay of 1 day in attaining peak thymidine incorporation in cultures of cells from protected mice. Therefore, while there may be some change in the nature of the anti-CII T cell, response it is clear that protection is not due to EtxB inhibiting T cell reactivity to CII.
Figure 3.
Receptor interaction by EtxB does not prevent T cell reactivity to CII but does reduce the anti-CII antibody response. (A) Inguinal lymph node cells were isolated 16 days after injection with collagen and were cultured either in the presence (solid symbols) or absence (open symbols) or 50 μg/ml of heat denatured bovine CII. Identical cultures were established from either EtxB-protected (triangles) or unprotected (squares) mice. Proliferation in these cultures was measured on days 2 to 6 by assay of [3H]thymidine incorporation. (B) Serum samples from mice (n = 11) injected either with CII alone or with CII plus EtxB (as indicated) were collected 15 days after immunization and assayed for the presence of anti-bovine CII antibodies. End point titers of total anti-CII IgG were calculated by linear regression analysis for each animal and are shown (□) along with the mean (bar) for the group.
Analysis of serum samples taken from mice 15 days after challenge with either CII alone or CII in combination with EtxB revealed that both groups of animals had mounted an anti-CII antibody response (Fig. 3B). While there was considerable variation in the anti-CII Ig titer between mice, the mean antibody level in protected animals was approximately 5-fold lower than in the unprotected group (P < 0.03). Consequently, EtxB-mediated protection against arthritis is not due to a complete inhibition of anti-collagen antibody production but is associated with a reduction in the magnitude of that response.
Protection from Arthritis Is Associated with an Alteration in the Nature of the Anti-Collagen Immune Response.
While EtxB did not prevent the induction of either antibody or T cell reactivity to collagen, it is possible that protection was associated with a shift in the nature of these responses. To test this, the production of IFN-γ and IL-4 were assessed throughout the timecourse of lymphocyte proliferation to CII. Identical cultures of inguinal lymph node cells were established from protected and unprotected mice, and on days 2–6 triplicate samples were removed for cytokine measurement. The T cell response to CII in cultures from animals challenged with CII alone was associated with the production of high levels of IFN-γ peaking on day 3 of culture and remaining elevated throughout the test period (Fig. 4). In contrast, such cultures contained negligible quantities of IL-4, only slightly exceeding, on day 4, that produced by cells cultured in the absence of CII. Cells from protected mice produced IFN-γ in response to CII; however, the levels were lower and peaked 1 day later compared with those observed in cultures from unprotected mice. In addition, there was a sharp decline in the level of IFN-γ detected in cultures of cells from protected animals on days 5 and 6. Of greater importance was the observation that the anti-CII response in protected animals was associated with the release of high levels of IL-4. These data reveal that protection is associated with an 8-fold increase in the ratio of IL-4 to IFN-γ production when comparing the peak level of each cytokine detected. A similar shift in the cytokine profile did not occur after treatment with EtxB(G33D) (data not shown). We conclude that receptor interaction by EtxB in vivo causes a dramatic shift in the profile of cytokines produced in vitro in cultures of lymph node cells responding to CII.
Figure 4.
EtxB alters the nature of the T cell response to CII. Inguinal lymph node cells, taken from mice 15 days after challenge with either CII or CII plus EtxB, were cultured in the presence or absence of 50 μg/ml CII. On each day of lymph node cell culture, triplicate samples were removed and assayed for the production of IFN-γ or IL-4 over an 18-h period by standard ELISA. Cytokine levels were calculated against standard curves produced with the appropriate recombinant cytokine by weighted probit analysis. The quantities of each cytokine (as indicated) produced in lymph node cell cultures from unprotected mice in the presence (▪) or absence (□) of collagen and from protected mice in the presence (▨) or absence (▧) of collagen are shown.
To evaluate if there was also a shift in the nature of the immune response to CII in vivo in EtxB-protected mice, the IgG subclass distribution of anti-CII antibody was determined. Quantities of anti-CII IgG1, IgG2a, IgG2b, and IgG3 in serum samples, taken from mice 15 days after challenge with either EtxB plus CII or CII alone, were measured by ELISA. Fig. 5 shows the titer of each IgG subclass in the anti-CII response from protected and unprotected mice. The data show that all four IgG subclasses are represented in the anti-CII response from both unprotected and protected mice, but importantly, there is a clear increase in the IgG1/IgG2a ratio from 2.4 to 7.3 in those animals given CII plus EtxB. We conclude that injection of EtxB alters the nature of the T cell and antibody response to CII, such that protection against arthritis is associated with a reduction in the anti-CII antibody level and a shift toward a more Th2-dominated immune response.
Figure 5.
Administration of EtxB leads to a reduction in the levels of all IgG isotypes in the anti-CII response and an altered IgG1/IgG2a ratio. Serum samples from mice (n = 11) injected either with CII alone (A) or CII plus EtxB (B) were collected 15 days after immunization and assayed for the presence of anti-bovine CII antibodies of each IgG isotype (as indicated). End point titers of each isotype were calculated by linear regression analysis for each animal and the mean values (±SEM) for each group are shown.
DISCUSSION
This paper establishes that receptor-interaction by EtxB can prevent the induction of arthritis by modulating T cell and antibody responses to CII in vivo. The anti-CII T cell response in CIA is normally associated with the production of high levels of Th1 cytokines (22) and consequent help for the production of complement fixing IgG2a antibody (30, 31). Evidence suggests that these antibodies play an important role in initiating joint inflammation (11), which is accompanied by leukocyte infiltration and macrophage activation (32). These pathological changes facilitate the breakdown of cartilage and bone, which characterizes inflammatory arthritis. Our data clearly demonstrate that EtxB-mediated protection from CIA is associated with a change in the profile of cytokines produced by lymph node cells responding to CII. There was a reduction in levels of IFN-γ and a dramatic increase in IL-4 production, resulting in an 8-fold increase in the IL-4/IFN-γ ratio in protected versus unprotected mice. Since levels of IL-4 and not the absolute amount of IFN-γ determine the balance between Th2 and Th1 responses in vivo (33, 34), the functional outcome of an immune response is best predicted by the quantity of IL-4 and the ratio of IL-4 to IFN-γ (33–35). Thus the increased production of IL-4 and the shift in the IL-4/IFN-γ ratio suggest that EtxB alters the balance of anti-CII T cell reactivity from a Th1-dominated response to one favoring Th2 activation. In support of this contention, EtxB administration led to a dramatic reduction in the level of anti-CII IgG2a antibodies (P < 0.001) and an increase in the IgG1/IgG2a ratio. However, it should also be noted that the levels of all of the IgG isotypes to CII were markedly diminished in protected mice. We conclude that receptor interaction by EtxB causes a clear shift in the Th1/Th2 balance as well as a general reduction in the extent of the anti-CII immune response.
The alterations to the anti-CII response that result from EtxB administration are entirely consistent with the observed abrogation of CIA. Indeed, strategies that block Th1 cytokine release, or enhance the levels of Th2 cytokines, have been reported to protect animals from a variety of inflammatory diseases including CIA (20, 21, 36, 37). Our finding that protection could be achieved even once the pathological immune response to CII was established demonstrates that EtxB-receptor interaction can modulate an existing immune response. Alternative strategies for preventing inflammatory disorders have included the induction of mucosal tolerance after oral or intranasal delivery of autoantigens or peptides (38–40). Interestingly, a recent report demonstrated that direct chemical coupling of myelin basic protein (MBP) to cholera toxin B subunit (CtxB) can reduce the intragastric dose of MBP required to induce tolerance for delayed type hypersensitivity and consequent protection from experimental autoimmune encephalomyelitis (41). This effect is likely to depend on a mechanism involving enhanced receptor-mediated uptake of MBP from the gut lumen. Whether oral delivery in this way also involves modulation of the normal mucosal response in a similar way to that reported here remains to be established. However, the surprising observation that IFN-γ levels were enhanced after intragastric administration of CtxB–MBP is suggestive of another mechanism.
How might EtxB-receptor interaction modulate the nature of the immune response in CIA? It is clear that EtxB and its homologue, CtxB, have profound effects on T and B cells both in vitro and in vivo. In this regard, CD8+ T cells are depleted from the epithelial compartment of the small intestine after oral administration of CtxB (42) and rapidly undergo apoptosis in vitro if they are cultured with EtxB (2). By contrast, both EtxB and CtxB activate resting B cells causing an increase in the levels of class II MHC on the cell surface (3, 43). For EtxB, this increase in class II MHC is associated with enhanced expression of B7, ICAM-1, CD40, and CD25 and occurs in the absence of significant proliferation (3). None of the observed effects of EtxB are exhibited by EtxB(G33D), indicating a complete dependence on receptor interaction in their causation. While EtxB is known to bind to a variety of different gangliosides and galactoproteins, its principle receptor is likely to be GM1 ganglioside. The observation that CtxB exerts similar modulatory effects on lymphocytes and yet lacks the ability to bind GM2 or galactoproteins supports the contention that it is GM1–ganglioside interaction that profoundly alters lymphocyte responses.
The effect which receptor-binding has on the response to CII is consistent with its modulatory properties for lymphocyte populations. In particular, increased surface expression of class II MHC, B7, ICAM-1, and CD40 by B cells is likely to enhance their functional activity as antigen-presenting cells and therefore increase their involvement in stimulating the T cell response to CII. It is known that presentation of antigen by B cells favors the induction of Th2-dominated responses (44). Indeed, work by Day et al. has established that targeting autoantigen to B cells, by linking it to anti-IgD, can abrogate a pathological Th1 response to MBP and consequently prevent experimental autoimmune encephalomyelitis (45). If EtxB activates B cells and promotes Th2 responses, one might have expected an increase in the overall antibody response to CII or at least the elevated production of anti-CII IgG1 antibodies. However, while protection was associated with an increase in the relative proportion of IgG1, the titer of all IgG isotypes, including IgG1, was markedly reduced by EtxB administration. There are several possible explanations for this. First, the antibody response to CII during the normal development of CIA is likely to be stimulated by both the immunizing dose of CII and the subsequent presentation of autologous collagen released during pathological damage to the joints. Therefore, the prevention of joint damage that occurs after administration of EtxB would be expected to reduce antigenic load and result in lowered antibody titers. Support for this comes from our finding that the proportion of serum anti-collagen antibodies which recognize murine CII, rather than being specific for bovine CII, was markedly reduced in protected mice compared with that found in unprotected animals (unpublished observations). Second, a reduction in Th1 activity and associated IFN-γ production would limit the activation of macrophages and dendritic cells and hence decrease the overall magnitude of the in vivo T cell response. Because it is only the limited number of B cells that have Ig specific for CII which will be involved in its presentation to T cells, the polyclonal activation of the B cell population by EtxB is unlikely to compensate for the restricted activity of other antigen-presenting cells. Antigen-presenting cell activation by IFN-γ may also be decreased as a result of EtxB administration because GM1-binding is known to deplete CD8+ T cells (1, 2), which are a potent source of this cytokine (46). The removal of CD8+ T cell derived IFN-γ would also provide a mechanism for the observed Th1-to-Th2 switch in the anti-CII response. Although this population does not appear to be critical for disease induction, it has been found that CII-specific CD8+ T cell hybridomas can be derived from mice immunized with CII in CFA (47). Third, it remains possible that EtxB-receptor interaction may have other effects on the activity of antigen-presenting cells, for example, modulating uptake and processing or the expression of important costimulatory molecules. Such an effect could be mediated indirectly, for example, through enhanced secretion of IL-10, or be the direct result of EtxB binding to these cells.
The results of our studies clearly indicate that EtxB-receptor interaction can abrogate CIA, and suggest that agents which bind to GM1 may provide a means of preventing or treating human rheumatoid arthritis. The observation that EtxB induces a Th1-to-Th2 switch heralds the possibility that such agents will find wider therapeutic applications in controlling other inflammatory disorders, such as multiple sclerosis, type I diabetes, and autoimmune uveitis. All of these diseases are thought to be initiated by autoreactive Th1 cells (48), and evidence from relevant experimental models suggests that protection can be afforded by the induction of a Th2 response (36, 37, 48, 49). Indeed, a significant reduction in the severity of experimental autoimmune encephalomyelitis can be achieved after administration of EtxB at the time of challenge with MBP (unpublished data), highlighting the wider applicability of our findings in ameliorating inflammatory autoimmune diseases.
Acknowledgments
We thank C. J. Elson and S. J. Thompson for advice and help in the preparation of this manuscript, and A. J. Coad for assistance with the preparation of histological sections. This work was supported by grants from the Medical Research Council, The Wellcome Trust, and the Arthritis and Rheumatism Council.
ABBREVIATIONS
- CIA
collagen-induced arthritis
- CII
type II collagen
- Etx
E. coli heat-labile enterotoxin
- EtxB
B subunit of E. coli heat-labile enterotoxin
- IFN-γ
interferon γ
- IL
interleukin
- IFA
incomplete Freund’s adjuvant
- CFA
complete Freund’s adjuvant
- MBP
myelin basic protein
References
- 1.Nashar T O, Webb H M, Eaglestone S, Williams N A, Hirst T R. Proc Natl Acad Sci USA. 1996;93:226–230. doi: 10.1073/pnas.93.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nashar T O, Williams N A, Hirst T R. Int Immunol. 1996;8:731–736. doi: 10.1093/intimm/8.5.731. [DOI] [PubMed] [Google Scholar]
- 3.Nashar, T. O., Hirst, T. R. & Williams, N. A. (1997) Immunology, in press.
- 4.Hol W, Sixma T K, Merritt E A. In: Structure and Function of Escherichia coli Heat-Labile Enterotoxin and Cholera Toxin B-Pentamer. Moss J, Iglewski B, Vaughan M, Tu T, editors. Vol. 9. New York: Dekker; 1995. pp. 185–224. [Google Scholar]
- 5.Fukuta S, Magnani J L, Twiddy E M, Holmes R K, Ginsburg V. Infect Immun. 1988;56:1748–1753. doi: 10.1128/iai.56.7.1748-1753.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holmgren J, Fredman P, Lindblad M, Svennerholm A M, Svennerholm L. Infect Immun. 1982;38:424–433. doi: 10.1128/iai.38.2.424-433.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orlandi P A, Critchley D R, Fishman P H. Biochemistry. 1994;33:12886–12895. doi: 10.1021/bi00209a021. [DOI] [PubMed] [Google Scholar]
- 8.Courtenay J S, Dallman M J, Dayan A D, Martin A, Mosedale B. Nature (London) 1980;283:666–668. doi: 10.1038/283666a0. [DOI] [PubMed] [Google Scholar]
- 9.Trentham D E, Townes A S, Kang A H. J Exp Med. 1977;146:857–868. doi: 10.1084/jem.146.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoo T J, Kim S Y, Stuart J M, Floyd R A, Olson G A, Cremer M A, Kang A H. J Exp Med. 1988;168:777–782. doi: 10.1084/jem.168.2.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seki N, Sudo Y, Yoshioka T, Sugihara S, Fujitsu T, Sakuma S, Ogawa T, Hamaoka T, Senoh H, Fujiwara H. J Immunol. 1988;140:1477–1484. [PubMed] [Google Scholar]
- 12.Taylor P C, PlaterZyberk C, Maini R N. Eur J Immunol. 1995;25:763–769. doi: 10.1002/eji.1830250321. [DOI] [PubMed] [Google Scholar]
- 13.Holmdahl R, Klareskog L, Rubin K, Larsson E, Wigzeu H. Scand J Immunol. 1985;22:295–306. doi: 10.1111/j.1365-3083.1985.tb01884.x. [DOI] [PubMed] [Google Scholar]
- 14.Kadowaki K M, Matsuno H, Tsuji H, Tunru I. Clin Exp Immunol. 1994;97:212–218. doi: 10.1111/j.1365-2249.1994.tb06070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ranges G E, Sriram S, Cooper S M. J Exp Med. 1985;162:1105–1110. doi: 10.1084/jem.162.3.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chiocchia G, Boissier M C, Fournier C. Eur J Immunol. 1991;21:2899–2905. doi: 10.1002/eji.1830211202. [DOI] [PubMed] [Google Scholar]
- 17.Wooley P H, Luthra H S, Stuart J M, David C S. J Exp Med. 1981;154:688–700. doi: 10.1084/jem.154.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Germann T, Szeliga J, Hess H, Storkel S, Podlaski F J, Gately M K, Schmitt E, Rude E. Proc Natl Acad Sci USA. 1995;92:4823–4827. doi: 10.1073/pnas.92.11.4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boissier M C, Chiocchia G, Bessis N, Hajnal J, Garotta G, Nicoletti F, Fournier C. Eur J Immunol. 1995;25:1184–1190. doi: 10.1002/eji.1830250508. [DOI] [PubMed] [Google Scholar]
- 20.Bessis N, Boissier M C, Ferrara P, Blankenstein T, Fradelizi D, Fournier C. Eur J Immunol. 1996;26:2399–2403. doi: 10.1002/eji.1830261020. [DOI] [PubMed] [Google Scholar]
- 21.Walmsley M, Katsikis P D, Abney E, Parry S, Williams R O, Maini R N, Feldmann M. Arthritis Rheum. 1996;39:495–503. doi: 10.1002/art.1780390318. [DOI] [PubMed] [Google Scholar]
- 22.Mauri C, Williams R O, Walmsley M, Feldmann M. Eur J Immunol. 1996;26:1511–1518. doi: 10.1002/eji.1830260716. [DOI] [PubMed] [Google Scholar]
- 23.Snapper C M, Paul W E. Science. 1987;236:944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- 24.Amin T, Hirst T R. Protein Expression Purif. 1994;5:198–204. doi: 10.1006/prep.1994.1031. [DOI] [PubMed] [Google Scholar]
- 25.Thompson S J, Thompson H S G, Harper N, Day M J, Coad A J, Elson C J, Staines N A. Immunology. 1993;79:152–157. [PMC free article] [PubMed] [Google Scholar]
- 26.Williams N A, Hill T J, Hooper D C. Immunology. 1991;72:34–39. [PMC free article] [PubMed] [Google Scholar]
- 27.Harper H M, Cochrane L, Williams N A. Immunology. 1996;89:449–456. doi: 10.1046/j.1365-2567.1996.d01-760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bailey M, Williams N A, Wilson A D, Stokes C R. J Immunol Methods. 1992;153:261–262. doi: 10.1016/0022-1759(92)90329-r. [DOI] [PubMed] [Google Scholar]
- 29.Staines N A, Ekong T A N, Thompson H S G, Isaacs A B, Loryman B, Major P J, Hobbs S M, Devey M E. J Autoimmun. 1990;3:643–657. doi: 10.1016/s0896-8411(05)80032-0. [DOI] [PubMed] [Google Scholar]
- 30.Watson W C, Townes A S. J Exp Med. 1985;162:1878–1891. doi: 10.1084/jem.162.6.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stasiuk L M, Abehsira-Amar O, Fournier C. Cell Immunol. 1996;173:269–275. doi: 10.1006/cimm.1996.0277. [DOI] [PubMed] [Google Scholar]
- 32.Kasama T, Strieter R M, Lukacs N W, Lincoln P M, Burdick M D, Kunkel S L. J Clin Invest. 1995;95:2868–2876. doi: 10.1172/JCI117993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sadick M D, Heinzel F P, Holaday B J, Pu R T, Dawkins R S, Locksley R M. J Exp Med. 1990;171:115–127. doi: 10.1084/jem.171.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morris L, Troutt A B, McLeod K S, Kelso A, Handman E, Aebischer T. Infect Immun. 1993;61:3459–3465. doi: 10.1128/iai.61.8.3459-3465.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelso A. Immunol Today. 1995;16:374–379. doi: 10.1016/0167-5699(95)80004-2. [DOI] [PubMed] [Google Scholar]
- 36.Baron J L, Madri J A, Ruddle N H, Hashim G, Janeway C A., Jr J Exp Med. 1993;177:57–68. doi: 10.1084/jem.177.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Racke M K, Bonomo A, Scott D E, Cannella B, Levine A, Raine C S, Shevach E M, Rocken M. J Exp Med. 1994;180:1961–1966. doi: 10.1084/jem.180.5.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.NaglerAnderson C, Bober L A, Robinson M E, Siskind G W, Thorbecke G J. Proc Natl Acad Sci USA. 1986;83:7443–7446. doi: 10.1073/pnas.83.19.7443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thompson H S G, Harper N, Bevan D J, Staines N A. Autoimmunity. 1994;16:189–199. doi: 10.3109/08916939308993327. [DOI] [PubMed] [Google Scholar]
- 40.Metzler B, Wraith D C. Int Immunol. 1993;5:1159–1165. doi: 10.1093/intimm/5.9.1159. [DOI] [PubMed] [Google Scholar]
- 41.Sun J B, Rask C, Olsson T, Holmgren J, Czerkinsky C. Proc Natl Acad Sci USA. 1996;93:7196–7201. doi: 10.1073/pnas.93.14.7196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elson C O, Holland S P, Dertzbaugh M T, Cuff C F, Anderson A O. J Immunol. 1995;154:1032–1040. [PubMed] [Google Scholar]
- 43.Francis M L, Ryan J, Jobling M G, Holmes R K, Moss J, Mond J J. J Immunol. 1992;148:1999–2005. [PubMed] [Google Scholar]
- 44.Gajewski T F, Pinnas M, Wong T, Fitch F W. J Immunol. 1991;146:1750–1758. [PubMed] [Google Scholar]
- 45.Day M J, Tse A G D, Puklavec M, Simmonds S J, Mason D W. J Exp Med. 1992;175:655–659. doi: 10.1084/jem.175.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramshaw I, Ruby J, Ramsay A, Adaa G, Karupiah G. Immunol Rev. 1992;127:157–182. doi: 10.1111/j.1600-065x.1992.tb01413.x. [DOI] [PubMed] [Google Scholar]
- 47.Chiocchia G, Boissier M C, Ronziere M C, Herbage D, Fournier C. J Immunol. 1990;145:519–525. [PubMed] [Google Scholar]
- 48.Rocken M, Racke M, Shevach E M. Immunol Today. 1996;17:225–231. doi: 10.1016/0167-5699(96)80556-1. [DOI] [PubMed] [Google Scholar]
- 49.Saoudi A, Kulun J, Huygen K, De Kozak Y, Velu T, Goldman M, Druet P, Bellon B. Eur J Immunol. 1993;23:3096–3103. doi: 10.1002/eji.1830231208. [DOI] [PubMed] [Google Scholar]