Synopsis
Epidemiological studies have shown that migraine headaches are a common finding in the general population, often associated with a high degree of disability. Additionally, migraine has a reported co-morbidity with other medical conditions, most notably with chronic pains such as temporomandibular disorders (TMD). The pathophysiological mechanisms involved with migraine are suggestive of an increased and prolonged hyperexcitability to stimuli, especially within the trigeminal distribution. Since migraine is mediated via branches of the trigeminal nerve it has the potential to mimic other types of pains, such as toothache or sinusitis. Therefore, it is recommended that oral and maxillofacial surgeons be familiar with the diagnostic criteria for migraine headaches in order to identify and appropriately treat such individuals who present to their clinics.
Keywords: Migraine Headache, Diagnosis, Epidemiology, Pathophysiology, Oral Surgery, Pain
Introduction
Neurovascular pains, otherwise known as headaches, are a group of pain disorders that are felt as cephalalgias, or pains in the head. Likely they are a heterogeneous group of disorders that share a common anatomic region of presentation, the head, but have somewhat separate pathophysiological mechanisms. For the most part, neurovascular pains are currently viewed as arising from neuronal firing of nociceptors associated with intracranial blood vessels and dura. For this reason these pain disorders were historically referred to as ‘vascular pains’ [1], but further research has identified both peripheral and central aspects of the nervous system are playing crucial roles in the initiation and perpetuation of these pains [2,3]. This brought about a change in nomenclature regarding headache, which are now referred to as ‘neurovascular pains disorders’, stressing the neuropathic aspect of migraine. (Table 1)
Table 1.
List of Primary Headache Disorders
|
Research has continued to more closely associate headache disorders with neuropathic mechanisms, which is starting to result in a shift in how headaches are viewed – from both clinical and research perspectives. In the midst of this paradigm shift this article seeks to provide an overview of the epidemiologic data and pathophysiologic mechanisms of one type of neurovascular pain, that being migraine headaches. The reason for focusing on migraine headache is because they are known to be common in the population [4,5] and have been reported to have the most disability associated with them [6,7], making migraine headache the prototypic neurovascular pain disorder. Migraine headache in the United States has resulted in $13 billion in lost productivity and $1 billion in direct healthcare costs [8]. For these reasons migraine headache likely has a significant impact on the practice of oral and maxillofacial surgery, as well as dentistry as a whole, despite this relationship not being systematically investigated. Therefore, this article seeks to clarify what migraine headache is and is not, outline who it presents in the population, and superficially review key underlying pathophysiological mechanisms.
Even though this review focuses specifically on migraine headache, clinicians should be aware of the various other headache disorders that have been defined and which can impact care in a similar fashion as migraine headache. For readers interested in the specific diagnostic criteria of these other headache disorders, please refer to The International Classification of Headache Disorders, 2nd Edition [9]. For information beyond the diagnostic criteria, the reader may wish to consult with any number of available textbooks, such as Wolff’s Headache and Other Head Pain, 7th edition [10] and The Headaches, 3rd edition [11].
What is a migraine headache?
The case definition of migraine headache has been established by a panel of experts and presented within The International Classification of Headache Disorders, 2nd Edition [9]. This disorder is characterized by the repeated occurrence of these paroxysmal head pains and not an isolated event, much in the same way epilepsy is defined. An additional similarity is the presentation of aura in about 10% of people who experience migraines. The aura experienced with migraines is defined as a focal neurological symptom that usually precedes or accompanies the onset of headache. The most typical type of aura experienced is visual aura, such as scintillations and/or scotoma, but other type auras may occur, like aphasia, hemiplesia, and hypoesthesia. These auras are thought to be related to a wave of spreading hyperexcitability and then depression over areas of cortical neurons. Even though these varied presentations have been recognized, the disorder of having migraine headaches seems to be consistent with one syndrome and the presentation of aura symptomotology being related to the neuroanatomic region involved in susceptible individuals. (Table 2)
Table 2.
Diagnostic Criteria for Migraine Headaches
Diagnostic Criteria for Episodic Migraine
|
Therefore, a single occurrence of intense, unilateral, throbbing head pain made worse with physical activity, with or without aura, is not considered strictly a migraine headache. Such an occasional migraine-like headache can be experienced by anyone, but the condition of having migraine headaches is the repeated occurrence of headache attacks that fit the specific diagnostic criteria. This prevents the misclassification of potentially life-threatening events, such as a subarachnoid bleed (see below), from being inappropriately diagnosed as a migraine headache.
Primary verse secondary headache diagnoses
Overall, all headache disorders are classified into two groups based on whether there is an identifiable underlying pathologic etiology. Any headache disorder identified to have an underlying causative factor, such as associated with an aneurysm or hyponatremia, is referred to as a secondary headache. This is an important distinction. All headache types can arise from mechanical impingement of some component of the trigeminal nociceptive system, central or peripheral, or an imbalance in homeostasis, infectious or otherwise. Furthermore, when these secondary causes of headache mimic migraine headache [12], they also can respond to typical migraine headache treatments [13]. These migraine-like head pains likely involve some of the same pathophysiological mechanisms as primary migraine headache, such as triggering the same primary afferent nociceptors (see Pathophysiology section below), but may be initiated by a life threatening event – such as a subarachnoid hemorrhage or the impingement by a space-occupying lesion. To guard against misdiagnosis, clinicians should follow the defined IHS diagnostic criteria and be aware of the typical ‘red-flags’ that may signal the presence of an ominous reason for head pain (see Table 3) [14]. If the individual’s clinical presentation is consistent with a secondary cause of headache, appropriate and timely referral for further diagnostic work-up is mandated. For these reasons, clinicians need to assess their patients for the possibility of such ‘secondary’ causes when considering a headache diagnosis.
Table 3.
List of Signs & Symptoms of Secondary Headache
|
Episodic verse chronic migraine diagnoses
The most recent version of the diagnostic criteria of headache further separates migraine by duration, termed episodic migraine for those that are intermittent as discrete headaches and chronic migraine for those that seem to be daily and continuous in nature [9]. This was instituted because chronic migraine and other continuous headache disorders were being referred to as chronic daily headache in the literature, and difficulty investigating the pathophysiological aspects of this heterogeneous disorder were being encountered [15,16].
This is a relatively new concept with the underlying premise that individuals with chronic migraine first experienced episodic migraine and then transformed into the chronic version [17], also sometimes referred to as analgesic rebound or medication over-use headache [18]. A proposed transforming factor is frequent use of short-acting analgesics and vasoconstricting agents at a rate of daily or near daily for 2 years or more [19,20], which has clinical implications for clinicians who regularly prescribe such medications. Other transforming factors have been suggested [21], but at present only anecdotal evidence have been reported from basic science research.
Important differences between episodic and chronic migraine are that individuals with chronic migraine have less pronounced features classically defined as migraine, such as nausea, photophobia, aura and significant pain reduction with analgesic intake that was effective in the past [17]. The significant aspect of this, as can be imagined, is that transformation to chronic migraine is thought to be associated with greater disability and worse prognosis [22–24], but this remains to be thoroughly investigated. Therefore the classification of episodic and chronic migraine was revised to specifically draw the clinician’s attention to the greater understanding that these two headache presentations are thought to both be migraine headaches, with the assumption that similar underlying pathophysiological mechanisms are present [17].
Where are “headaches” located?
The term ‘headache’ has been defined by Dorland’s Illustrated Medical Dictionary as, “pain in the head; cephalalgia” [25]. It is our understanding from interactions with patients and colleagues that headache, as a construct, is most consistently thought of as a pain in the head felt above the orbito-meatal line and posterior to include the entire back of the head; thereby excluding the orofacial regions of the nose, sinuses, jaws, temporomandibular joints and ears. This unstated assumption can be misleading, especially since such an anatomical distinction has not been made by the International Headache Society’s classification subcommittee, as recently revised in their published diagnostic criteria for headache disorders [9]. Within this review the definition of headache encompasses the entire head, including the orofacial region, and is therefore consistent with the recognized diagnostic criteria.
The presentation of headaches within the orofacial region has been documented by several case series [26–32]. This has obvious implications to clinical practice, necessitating the clinician consider the various headache disorders as part of the differential diagnosis when patients present with a complaint of orofacial pain. Understanding which individuals are most likely to present with a headache disorder (i.e. epidemiology), what characteristics of these headache disorders are, (i.e. diagnostic criteria) and some knowledge of the underlying pain mechanisms (i.e. pathophysiology) will be helpful in arriving at a definitive diagnosis and appropriate treatment plan. From clinical experience the most common odontogenic neurovascular presentation of “toothache” is migraine headache, which is consistent with existing epidemiological research.
There is a concept that such neurovascular pains can arise in extracranial tissues, such as via the interaction of blood vessels and nerve within tooth dental pulps. This is opposed to the accepted belief that pain arises from intracranial tissues and is then referred and perceived within the extracranial tissues. This concept of extracranial mechanism of neurovascular pains has been termed “vascular toothache” or “vascular-type craniofacial pain” [33–35]. Currently this is not a concept that is firmly accepted by the majority of pain clinicians since treatment targeting this peripheral blood vessel/nerve interface, such as dental extractions, has not been observed to palliate these pains. This clinical evidence thereby suggests the likely source of pain is intracranial and referred to be perceived in the periphery by the individual who is experiencing such pain. Therefore, the practicing oral and maxillofacial surgeon needs to be familiar with these concepts to accurately diagnose and treat patients with such a presentation.
Epidemiology Aspects of Migraine Headache
Prevalence of migraine
The 1-year-period prevalence of migraine in adults has been noted to range between 10 and 14%, with females experiencing migraines three times more often than males [7,36–38] (Figure 1). The prevalence increases from infancy until around 40 years of age, as does the occurrence of new people developing migraine, and then decreases with aging [39–43].
Figure 1. Prevalence of Migraine Headache Separated by Gender.
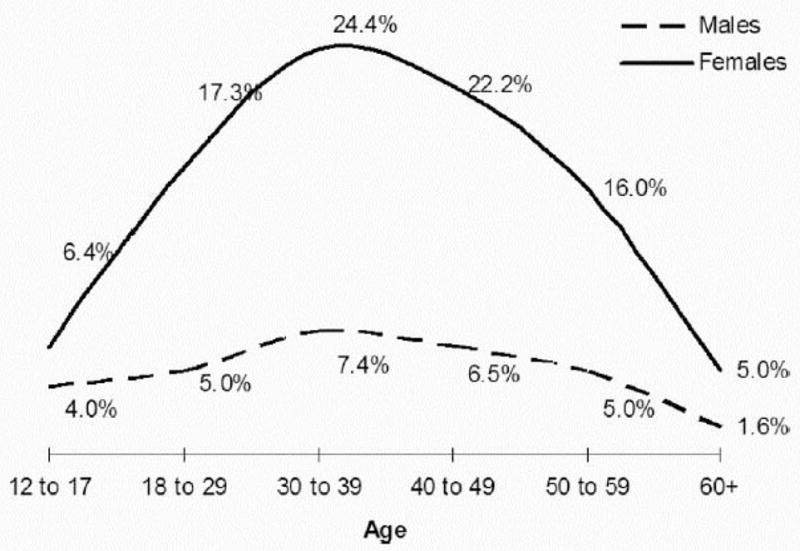
The presence of migraine is considerably higher in females, especially during the ages of 20 to 50 years old. From Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF. Migraine prevalence, disease burden, and the need for preventative therapy. Neurology. 2007;68:343–349, with permission.
These prevalence estimates are similar across studies in the Americas and Europe [39,40,42,44–46], but some racial differences have been observed. Within the United States, migraine has been shown to be more prevalent among Caucasians (≈15%), followed by African Americans (≈12%) and then Asian Americans (≈7%) [47]. Similar differences were observed between these racial populations by continent, whereby the Americas and Europe having the higher prevalence (≈13%), while Asia and Africa had lower prevalence (≈5%) [4].
Cross-sectional and longitudinal studies have noted an association between low education and low income with migraine [7,36,43,44,48,49]. This association has been explained to be mediated through lack of access to health care, stress, and poor living conditions [48]. Lower educational attainment, however, could be a consequence of a migraine disposition’s influencing the ability to obtain vocational education even before the onset of migraine attacks [43].
Risk factors in migraine headache
There are two components that accompany the idea of risk factors related to migraine headache, which are; 1) the risk for developing the condition of experiencing migraine headaches, and 2) the risk of triggering a migraine headache attack. Even though the literature at times does not consistently and clearly distinguish between these two types of risk factors, they are presented separately based on our understanding of the literature.
Risk factors for having the condition of migraine
An increased risk of having migraine headache has been noted among people who have a first degree relative with migraine [43,48,50] and between twins, with a pair-wise concordance rate being significantly higher in monozygotic twins (43%) compared to dizygotic twins (12%) [51]. This suggests that there is some underlying genetic factor(s) that predispose people to developing the condition of migraine headaches. As can be imagined, this is becoming an explosive area of research within the post-genomic era. With that being said, the evidence of genetic mutations resulting in pathophysiological mechanisms have been scant. What is known and accepted will be briefly outlined below.
A rare subtype known as familial hemiplegic migraine, named from its clinical presentation of transient hemiplegic aura, is thought to have a prominent hereditability [52]. Research has shown it to be an autosomal dominant disorder involving multiple different mutations of P/Q-type voltage gated calcium channels (for specific information about mechanisms, see Pathophysiology of Migraine section below) [53,54]. Preliminary reports suggest other genetic mutations are somehow involved with increased risk of having the condition of migraine. Recently an endothelial nitric oxide synthase polymorphism, homozygous Asp298, was found to have a 3-fold increased risk for migraine [55], while MTHFR C677T genotype, which is known to be associated with an increase risk for stroke, had a 2-fold increase for migraine [56].
Even with all these positive relationships between migraine and genetic factors, the current opinion is that genetics alone cannot explain the presence of the condition, and therefore, migraine is thought of as a multifactorial disorder [57]. Many different non-genetic factors such as stress, psychic tension, depression, and sleep problems have been implicated in increasing the risk of having the condition of migraine headaches [58–62]. However, we need to understand that research has not determined the exact nature of this association since it has two directions: depression increases the risk of developing migraine, as well as the risk of having depression increases with having migraine [48]. Therefore the description of such risk factors continues to be ambiguous and the diagnosis of having migraine headaches remains as a description of clinical features and not genotyping.
Risk factors for triggering migraines
Changes in hormone levels (e.g. menstruation, ovulation, oral contraceptives), sleep disturbance, weather changes, psychological factors, alcohol and nutrition (e.g. chocolate) are suggested to be risk factors for triggering migraine headaches [58–67]. More specifically, the risk of occurrence and persistence of headache despite treatment increases with menstruation [61], while hormone replacement therapy appears to contribute to prolongation of migraine headaches into older age [68]. Finally, few studies have investigated factors associated with a reduction in risk of migraines being triggered, such as daily sunshine duration of three hours or more [61]. (Table 4)
Table 4.
Risk, expressed as an Odds Ratio, of Migraine Headache
| Variable | % with risk factor | Migraine (n=453)
|
|
|---|---|---|---|
| OR† | 95% CI† | ||
| Gender (female vs. male) | 42.0 | 6.6* | 3.0, 14.8 |
| Mean age (per decennial increase) | 42.7 | 0.6* | 0.4, 0.8 |
| Familial disposition (yes vs. no) | 32.9 | 3.1* | 1.6, 6.2 |
| Vocational education (none vs. any) | 26.9 | 2.9* | 1.5,5.7 |
| Marital status (single vs. married) | 17.0 | 1.2 | 0.5, 2.8 |
| Employment (not working vs. working) | 12.8 | 0.6 | 0.2, 1.8 |
| Self-rated health (bad vs. good) | 15.9 | 1.8 | 0.8, 3.8 |
| Problems at home or at work (yes vs. no) | 43.3 | 0.7 | 0.4, 1.4 |
| Having a close friend (no vs. yes) | 39.3 | 1.9 | 0.9, 3.9 |
| Regular physical exeicise (no vs. yes) | 62.5 | 1.1 | 0.5, 2.3 |
| Snoring (yes vs. no) | 39.7 | 2.0 | 0.9, 4.2 |
| Feelings of fatigue (yes vs. no) | 38.6 | 0.9 | 0.5, 1.9 |
| Sleeping problems (yes vs. no) | 21.0 | 1.3 | 0.6, 2.8 |
| Feeling refreshed when awakening (no vs. yes) | 49.7 | 1.1 | 0.5, 2.1 |
| Mean hours of sleep per night (per 1-hour decrease) | 7.0 | 1.1 | 0.8, 1.6 |
| Having a high work load (yes vs. no) | 34.9 | 2.2* | 1.1, 4.3 |
| Having too little time to do work (yes vs. no) | 20 .5 | 0.9 | 0.4, 2.1 |
| Being able to relax after work (no vs. yes) | 9.3 | 0.6 | 0.2, 1.9 |
| Exposure to noise at work (yes vs. no) | 22.9 | 1.8 | 0.9, 3.9 |
| Exposure to vapors at work (yes vs. no) | 16.3 | 1.6 | 0.6, 4.0 |
| Use of oral contraceptives (yes vs. no) | 10.9 | 1.5 | 0.5, 4.5 |
| Frequent tension -type headache (yes vs. no) | 25.6 | 2.5* | 13, 5.0 |
p ≤ 0.05.
OR odds ratio: CI, confidence interval.
Subjects with migraine were not Included in the at-risk group for tension-type headache
Odds ratios, adjusted for age and gender, for incidence of migraine headache between 1989 and 2001 in relation to factors reported in a Danish population-based follow-up study. Adapted from Lyngberg AC, Rasmussen BK, Jorgensen T, Jensen R. Incidence of primary headache: A Danish epidemiologic follow-up study. American Journal of Epidemiology. 2005;161:1066–1073, with permission.
Co-morbidity disorders with migraine headache
Co-morbidity of migraine with other pain conditions
Migraine has been noted to be associated to other chronic pain conditions, including temporomandibular disorders (TMD). In a cross-sectional study, based on reviewing the records of one medical insurance population, almost 28% (n=115) patients of those 408 subjects with a TMD diagnosis had a diagnosis of migraine headaches [69]. In a population-based study, subjects with TMD pain were 1.8 times more likely to report headache than subjects without TMD pain (95% CI, 1.1–3.2) [70]. Furthermore, the presence of headaches was shown to increase the risk of developing TMD over a 3-years time period within a population study of 1,996 11-year-olds (Odds Ratio (OR)=2.65: 95% CI, 1.6–4.4) [71].
Migraine has been associated with chronic pain conditions outside the head and neck region. In an adult population-based study it was found that migraine was strongly related to chronic spinal pain, such as chronic back or neck problems (OR=5.2; 95%CI=4.1–6.4) [72]. In another study a relationship was noted between headache frequency and co-morbid musculoskeletal symptoms, defined as pain or stiffness in muscles and joints [73]. The magnitude of this association increased with the frequency of headache, with individuals who experience headaches on 15 or more days per month (women: OR=5.3; 95%CI: 4.4–6.5 while men OR=3.6; 95%CI: 2.9–4.5). Furthermore, a high prevalence of fibromyalgia has been found among subjects with migraine [74,75].
This increased odds of having co-morbid musculoskeletal pain when diagnosed with migraine was also observed among children and young adults. In a cross-sectional population-based study, including over 9000 adolescents and young adults revealed a strong association between headache felt more than 30 days per year and frequent low back pain (OR=3.4; 95%CI: 2.3–5.0) [76]. In a prospective study, including 1756 third and fifth grade school children followed over four years, children with headache were more likely to report persistent musculoskeletal pain at follow-up than children without headache [77]. Such data supports the emerging understanding that headaches, and likely migraines in specific, are a risk factor for the development of musculoskeletal pains including painful TMD [71,78]. (Table 5)
Table 5.
Conditions Co-morbid with Migraine Headache
| Category | Condition |
|---|---|
| Psychiatric | Depression |
| Anxiety | |
| Panic disorder | |
| Bipolar | |
| Neurological | Epilepsy |
| Tourette’sa | |
| Vascular | Raynaud’s phenomenon |
| Blood pressure (inconsistent) | |
| Ischemic stroke, sub-clinical stroke, white matter abnormalities | |
| Heart | Patent foramen ovalea |
| Mitral valve prolapsea | |
| Atrial septal aneurysma | |
| Other | Snoring/sleep apneaa |
| Asthma/allergy | |
| Systemic lupus erythematosusa | |
| Non-headache pain |
Data from clinical samples only.
From Scher AI, Bigal ME, Lipton RB. Comorbidity of migraine. Curr Opin Neurol. 2005;18:305–310, with permission.
Co-morbidity of migraine and other diseases
The role of co-morbidity with other diseases in the occurrence and persistence of chronic headache has to be considered. Several co-morbid diseases such as clinical and sub-clinical cardiovascular disorders, epilepsy and psychiatric disorders such as depression and anxiety disorders, are frequently noted among people experiencing migraine headaches [79].
The association between migraine headache and coronary heart disease has been assessed by several studies. Hospital and population-based epidemiological studies have demonstrated that people with migraines have an increased likelihood of several classic risk factors for cardiovascular disease such as high blood pressure, negative cholesterol profile, and parental history of early myocardial infarction [79]. Furthermore, ischemic stroke occurs more frequently in migraineurs, with the association made worse being female, older, smoking cigarettes, and the use oral contraceptives [80,81]. Also, a relationship was found between family history of vascular disorders (i.e. stroke, arterial hypertension, myocardial infarction) and migraine, in a study including children (OR=1.8; 95%CI:1.0–3.5) [82].
A relationship between migraine and epilepsy has long been postulated, with recent clinical and epidemiological studies having demonstrated that both are highly co-morbid [83–85]. Although migraine prevalence in patients with epilepsy is high (14% to 20%), only a few (1.7% to 3%) experience seizures in close temporal proximity to migraine [84,85].
Psychiatric disorders have been also investigated as co-morbidities related to migraine. Cross-sectional, cohorts and bidirectional associations between migraine and a variety of psychiatric and somatic conditions have been reported in the literature. In a cross-sectional study, magnitude of the associations of an odds ratio between 2.4 and 3.1 was found for three psychiatric disorders: depression (OR=2.4, 95% CI 1.8–3.1), anxiety (OR=3.1, 95% CI 2.0–4.9) and panic attacks (OR=3.1, 95% CI 2.2–4.3) [86]. A 2-year population-based cohort evaluated the bidirectional relations between migraine, severe non-migraine headache, and depression. Results showed that depression increased only the risk of developing migraine (Relative Risk (RR)=3.4; 95%CI:1.4–8.7), over a 2-year period. In addition, migraine at baseline increased the risk of developing depression (RR= 5.8; 95% CI 2.7–12.3) [66]. In another cross-sectional study, including over 50 000 adults age 20 and older, migraine headache was positively associated with depression (OR= 2.7; 95% CI 2.3–3.2) and anxiety disorders (OR=3.2; 95% CI 2.8–3.6) [62]. These associations are not specific to migraine, where non-migraine headache was related with depression (OR=2.2; 95%CI 2.0–2.5) and anxiety disorders (OR=2.7; 95% CI 2.4–3.0) as well. It is interesting to observe a linear trend associated with headache frequency: migraine headache occurring on fewer than 7 days per month, 7–14 days per month or 15 or more days per month, the associations with depression in terms of odds ratios were 2.0 (95% CI 1.6–2.5), 4.2 (95% CI 3.2–5.6), and 6.4 (95% CI 4.4–9.3), respectively [62]. This ‘dose-response curve’ strongly implicates the involvement of migraine headache with mood disorders.
Pathophysiological Mechanisms of Migraine Headache
The pathogenesis of migraine is incompletely understood [87]. Currently, migraine is thought of as a disorder of trigeminal sensory processing, generated centrally, probably at the level of the brainstem. Recent discoveries demonstrated that the neuronal events mediating migraine originate within the trigeminovascular system and its central projections [88]. (Figure 2)
Figure 2. Tissues Innervated by the Three Branches of the Trigeminal Nerve.

The vast majority of the brain and associated structures are innervated by branches of the ophthalmic branch nerve of the trigeminal nerve (yellow). A small portion are innervated by the maxillary branch (orange) and mandibular branch (red). Stimulation of intracranial nociceptive fibers in the colored areas is thought to result in pain being perceived in the corresponding trigeminal branches of the orofacial region, including the teeth and alveolar bone. From Alonso AA & Nixdorf DR. Case series of four different headache types presenting as tooth pain. J Endod 2006;32:1110–1113, with permission.
The headache pain felt during migraine attacks are thought to be caused by the very same nerves that are sensing pain, primary afferent nociceptive neurons. Antidromic transmission of nerve impulses result in a release of vasoactive neuropeptides, such as substance P, calcitonin gene-related peptide (CGRP) and neurokinin, which promote vasodilation and plasma protein leakage [89]. This physiological process has been termed ‘neurogenic inflammation’, since all the cardinal signs of inflammation are resultant from neuronal impulses as opposed to infectious agents. Current opinion suggests that the release of such vasoactive peptides leads to the subsequent release of serotonin, histamine, bradykinin, and prostaglandins. These algogenic substances, which are both brain-generated and blood-born, further sensitize the primary afferent nociceptor [90–92].
This understanding of the pathophysiology has led to the development of a class of medications, known as triptans. This class of medications are 5-HT1B and 5-HT1D receptor agonists, meaning they bind to specific subtypes of serontonin receptors located on both blood vessels and nociceptive neurons within the brain respectively. The resulting action is the inhibition of the ‘neurogenic inflammation’ and vasoconstriction of the cerebral blood vessels, as well as raising the depolarization threshold of the primary afferent neuron [93]. For these reasons it has proven to be more efficacious than analgesics and vasoconstrictors alone or in combination as an abortive medication [93]. As a greater understanding of migraine pathophysiology occurs, novel treatment approaches will undoubtedly be developed that exploit can this knowledge. (Figure 3)
Figure 3. Neurogenic Inflammation and Action of 5-TH1B/D Agonists.
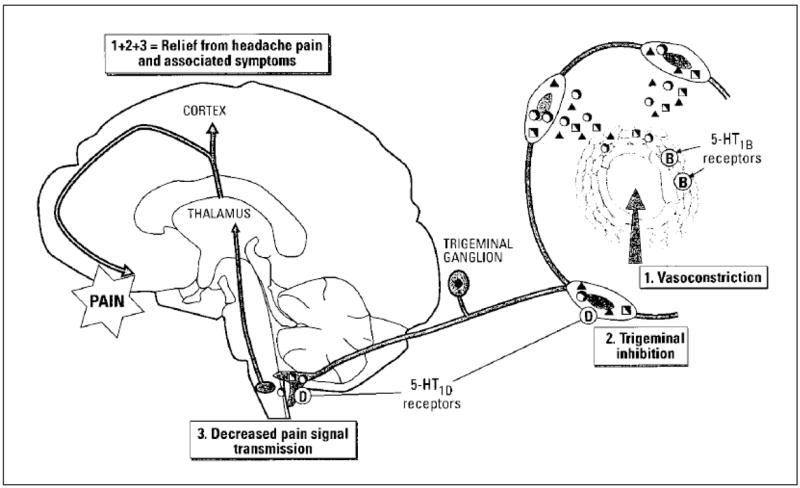
This figure depicts the three actions of 5-HT1B/D receptor agonist activity: 1) Reducing vasodilation caused by the release of release of inflammatory mediators, 2) Inhibiting the further neuronal release of these inflammatory mediators, and 3) Decreasing the transmission of noxious stimuli at the level of the first synapse in the brainstem. From Hargreaves RJ, Shepheard SL. Pathophysiology of migraine--new insights. Can J Neurol Sci. 1999;26(suppl 3):S12–S19, with permission.
Animal studies exploring the implications of ‘neurogenic inflammation’ revealed both intracranial and extracranial hypersensitivity is present [94,95]. This same type of hypersensitivity has been observed in humans experiencing migraine headache [96,97] and has been termed central sensitization. The clinical presentation of central sensitization is known as allodynia and hyperesthesia. Both of these presentations have been shown to occur within and outside trigeminally innervated structures during migraine headache attacks [96,97]. Interestingly, such hypersensitivity has been shown to occur in between migraine headaches as well [98,99]. This is thought to be due to a temporary increase at least in the sensitivity of the second-order neurons that receive converging inputs from the skin in various body sites, such as the dura mater and the periorbital skin [100]. Allodynia has been demonstrated to be continuously present when episodic migraines have transformed into chronic migraines [22], likely representing synaptic strengthening of this pathway resulting in less chance for reversal of this hypersensitive state. (Figure 4)
Figure 4. Trigeminovascular Pain Pathway of Migraine.
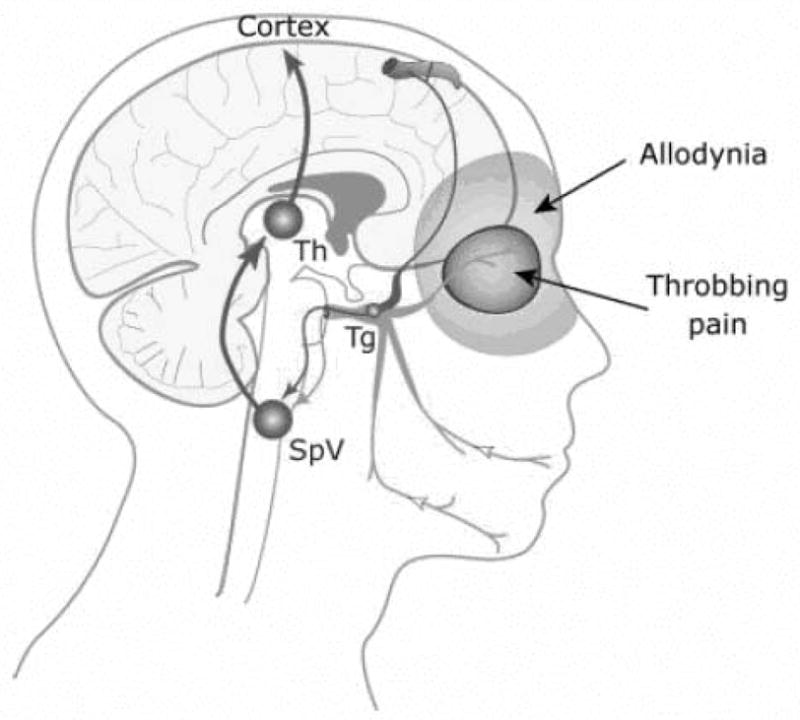
This figure exhibits the innervation of a cerebral blood vessel from a branch of the ophthalmic nerve (in red) with pain referral and allodynia being present in the somatic distribution of the same trigeminal nerve, Ipsilateral periorbital tissues. Tg = Trigeminal Ganglion; SpV = Spinal Trigeminal Nucleus; Th = Thalamic Nueclei From Burstein R, Jakubowski M. Unitary hypothesis for multiple triggers of pain and strain of migraine. J Comp Neurol. 2005;493:9–14, with permission.
The periaqueductal gray matter (PAG) is the center of a powerful descending anti-nociceptive pathway [101]. The PAG regulates nociceptive, autonomic and behavioral responses to threat [102]. The potential for PAG dysfunction to be involved in migraine was observed when patients had electrodes implantation into the PAG provoked headaches that resembled migraines [103]. Functional imaging studies performed during migraine headache attacks suggest that allodynia commonly seen in migraineurs involve the brainstem descending pain modulating system (e.g. PAG dysfunction) [104,105]. Recent research has revealed that abnormal modulation of the brain nociceptive systems, specifically via dysfunction of the PAG, is further involved in the chronification of migraine headaches. This is thought to occur with each migraine headache event, resulting in a shift from episodic migraines to chronic daily headache with whole body hypersensitivity in individuals who experience frequent migraine for a protracted length of time [88]. (Figure 5)
Figure 5. Evidence of Periaqueductal Gray Matter (PAG) and related Nuclei Involved in Migraine.
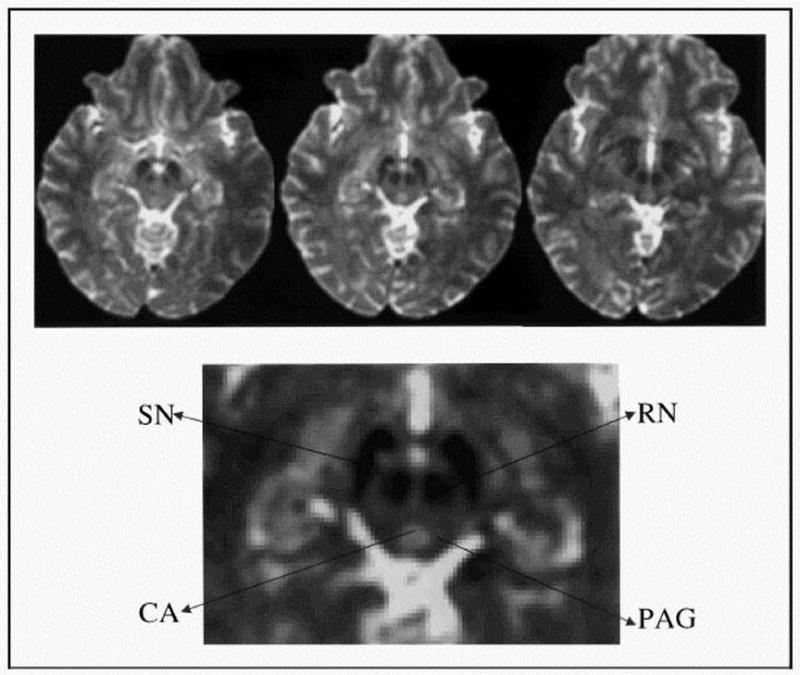
Three contiguous transverse slices, using functional magnetic resonance imaging, reveal changes in blood flow within the brainstem. Arrows pointing away from areas of hypointensity correspond to the substantia nigra (SN) and red nucleus (RN). The cerebral aqueduct (CA), which is hyperintense, is surrounded by the periaqueductal gray matter (PAG), which also is hypointense. From Welch KMA, Nagesh V, Aurora SK, Gelman N. Periaqueductal gray matter dysfunction in migraine: Cause of the burden of illness? Headache. 2001;41:629–637, with permission.
The aforementioned knowledge regarding pathophysiological mechanisms lacks information as to how or why the inciting event of antidromic transmission occurs along the afferent trigeminal fiber innervating the blood vessels and dura of the brain. Research on this issue has largely been at the cellular and genetic levels. Most researchers agree that susceptibility is inherited and that its clinical presentation is strongly modulated by both internal and external factors. Pioneering research revealed polymorphisms in genes regulating ion translocation have been implicated in two subtypes of familial hemiplegic migraine, which is a rare migraine disorder, and have been mapped to chromosome 19p13 [106]. Point mutations have been described to affect calcium channels and ATP-utilizing Na+/K+ ion pumps with these mutations being found on neurons and astrocytes [107,108]. This suggests multiple different pathophysiological mechanisms result in a specific, and rare, clinical presentation of familial hemiplegic migraine. Translating this information into more the common migraine disorders, which are undoubtedly more heterogeneous in nature, explains why specific genetic abnormalities have not yet been identified.
The new cloned receptor for serotonin, 5-HT7, attracts attention [109]. Recently research suggests that the receptor may play a role in migraine and other central nervous system disorders including anxiety and cognitive disturbances. Furthermore, it may also be involved in other pain conditions, such as epilepsy, depression, memory, and sleep [110]. Therefore, development of knowledge along this line of research may yield a better understanding of these co-morbid disorders and specific treatments.
In summary, migraine is regarded as a neurovascular disorder with alterations in trigeminal sensory processing. This disorder, which likely is associated with genetic alterations such as calcium channel abnormalities [53,54] and serotonin receptor activity [109,110], has a centrally generated trigger [111] that results in vasodilatation and primary afferent nociceptor hypersensitivity [91]. This results in lower sensory thresholds during the migraine headache attack [96,97]. Furthermore, individuals who have experienced frequent migraine headaches show dysfunction of their descending antinociceptive pathway [88], which is thought to result in persistent whole-body hypersensitivity [22] and exaggerated pain response to stimulation.
Discussion
Clinical relevance of migraine headaches
Epidemiological data demonstrates that migraine headaches are common [6,36] and because of this migraineurs will routinely present within the oral and maxillofacial clinical setting. The individuals with migraine headache have specific issues unique to them that may alter the ultimate course of treatment. The two most compelling arguments for knowing about migraine and other headache disorders is because they may present as non-odontogenic reasons for toothache [29] and sinus pain [111], as well as the possibility that life-threatening pathological processes may be present. Such pains are known to motivate individuals to be evaluated by their dentists, suggesting that dental specialists should be familiar with these concepts and the diagnostic process. Furthermore, since migraine headache and secondary reasons causing migraine-like headache can be episodic or chronic in nature, both should be included in the differential diagnosis of intermittent and daily continuous orofacial pains when an odontogenic source is not readily identifiable.
Migraine has consistently been shown to be associated with other chronic pains including TMD and other musculoskeletal pain disorders [70,74,75]. Emerging evidence is suggestive that migraine headaches may be a risk factor for the development of TMD [71,78], which implies that the phenotypical expression of migraine results in an alteration in central processing of stimuli including nociception. Since the disorder of migraine headache actively uses the trigeminal nociceptive pathway, it is not unexpected to suggest that these individuals can be more sensitive to stimulation, such as may occur with oral surgical procedures. Therefore, procedures that will cause acute pain within trigeminally-innervated structures will likely be perceived as being more severe, longer lasting and be expressed as having a greater degree of interference in individuals who have migraine headaches. This will especially be true of people with chronic migraine, since continuous hypersensitivity is known to be present [22]. For this reason it is recommended that such patients be identified prior to the initiation of surgical treatment so that appropriate measures can be taken to improve their post-procedural outcome. One such measure may be to delay surgical treatment until better headache control is obtained, which is thought to be a clinical measure suggesting reversal of some of the hypersensitivity.
Migraine headache have been strongly associated with depression and anxiety [43,44], which is a similar finding in other chronic pain conditions. This association noted by clinical research is supported by neuroscience research that suggests common receptors are involved [109,110]. Together, this data suggests a close relationship between migraine headaches and mood disorders. Since a co-morbid presentation of depression and anxiety is common, people with migraine headaches should be screened for such mood disorders because such disorders are known to decrease compliance with prescribed pre- and post-operative directions.
Along similar lines, migraine headache has been associated with an increased risk of cardiovascular events, most notably stroke. This risk is increased in females who smoke and are taking exogenous estrogen supplements, such as for contraceptive reasons. Migraine headaches are also known to be co-morbid with seizures disorders. Therefore, the presence of these co-morbid disorders should be taken into account when prescribing medications, providing anesthesia services, and performing surgical treatments on these individuals.
Conclusions
Clinicians should be familiar with the concept that various neurovascular pain disorders, specifically migraine and secondary headaches, may present as pain anywhere within the trigeminally-innervated tissues. For these reasons clinicians should consider including such diagnoses within the differential diagnosis when patients have a pain complaint that seems non-odontogenic in nature. Surgical procedures performed on individuals with migraine headaches, especially the chronic migraine, may experience increased post-operative pain intensity, duration and unpleasantness. Furthermore, individuals with migraine are known to have an increased probability of co-morbid conditions, most notably other chronic pains, depression and anxiety, seizures and stroke.
Acknowledgments
This work was supported in part by Grant No. RR023247 from the National Institutes of Health
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Graham JR, Wolff HG. Mechanisms of migraine headache and action of ergotamine tartate. Arch Neurol Psychiatry. 1938;39:737–763. [Google Scholar]
- 2.Moskowitz MA. The neurobiology of vascular head pain. Ann Neurol. 1984;16(2):157–168. doi: 10.1002/ana.410160202. [DOI] [PubMed] [Google Scholar]
- 3.Woods RP, Iacoboni M, Mazziotta JC. Bilateral spreading cerebral hypoperfusion during spontaneous migraine headache. N Engl J Med. 1994;331(25):1689–1692. doi: 10.1056/NEJM199412223312505. [DOI] [PubMed] [Google Scholar]
- 4.Scher AI, Stewart WF, Lipton RB. Migraine and headache: A meta-analytic approach. In: Crombie IK, Croft PR, Linton SJ, LeResche L, Von Korff M, editors. Epidemiology of Pain. Seattle: IASP Press; 1999. pp. 159–170. [Google Scholar]
- 5.Rasmussen BK. Epidemiology of migraine. In: Olesen J, Goadsby PJ, Ramadan NM, Tfelt-Hansen P, Welch KMA, editors. The Headaches. 3. Philadelphia: Lippincott Williams & Wilkins; 2006. pp. 235–242. [Google Scholar]
- 6.Lipton RB, Hamelsky SW, Stewart WF. Epidemiology and impact of headache. In: Silberstein SD, Lipton RB, Dalessio D, editors. Wolff’s Headache and Other Head Pain. 7. Oxford, UK: Oxford Press; 2001. pp. 85–107. [Google Scholar]
- 7.Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in the united states: Data from the American Migraine Study II. Headache. 2001;41(7):646–857. doi: 10.1046/j.1526-4610.2001.041007646.x. [DOI] [PubMed] [Google Scholar]
- 8.Hu XH, Markson LE, Lipton RB, Stewart WF, Berger ML. Burden of migraine in the United States. Arch Intern Med. 1999;159(8):813–818. doi: 10.1001/archinte.159.8.813. [DOI] [PubMed] [Google Scholar]
- 9.Headache Classification Subcommittee of the International Headache Society. The international classification of headache disorders, 2nd edition (ICHD-II) Cephalalgia. 2004;24(Suppl 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 10.Silberstein SD, Lipton RB, Dalessio DJ. Wolff’s Headache and Other Head Pain. 7. New York: Oxford Press; 2001. [Google Scholar]
- 11.Olesen J, Goadsby PJ, Ramadan NM, Tfelt-Hansen P, Welch KMA. The Headaches. 3. Philadelphia: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 12.Day JW, Raskin NH. Thunderclap headache: Symptoms of unruptured cerebral aneurysm. Lancet. 1986;29(2):1247–1248. [PubMed] [Google Scholar]
- 13.Rosenberg JH, Silberstein SD. The headache of SAH responds to Sumatriptan. Headache. 2005;45(5):597–598. doi: 10.1111/j.1526-4610.2005.05114.x. [DOI] [PubMed] [Google Scholar]
- 14.Saper JR, Silbertstein SD, Gordon CD, Hamel RL, Swidan S. Handbook of headache management: A practical guide to diagnosis and treatment of head, neck, and facial pain. 2. Philadelphia: Lippincott Williams & Wilkins; 1999. pp. 15–31. [Google Scholar]
- 15.Kaniecki RG. Diagnostic challenges in headache: Migraine as the wolf in sheep’s clothing. Neurology. 2002;58(Suppl 6):S1–S2. doi: 10.1212/wnl.58.9_suppl_6.s1. [DOI] [PubMed] [Google Scholar]
- 16.Welch KMA, Goadsby PJ. Chronic daily headache: Nosology and pathophysiology. Curr Opin Neurol. 2002;15(3):287–295. doi: 10.1097/00019052-200206000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Silbertstein SD, Olesen J. Chronic migraines. In: Olesen J, Goadsby PJ, Ramadan NM, Tfelt-Hansen P, Welch KMA, editors. The Headaches. 3. Philadelphia: Lippincott Williams & Wilkins; 2006. pp. 613–617. [Google Scholar]
- 18.Mathew NT, Reuveni U, Perez F. Transformed or evolutive migraine. Headache. 1987;27(2):102–106. doi: 10.1111/j.1526-4610.1987.hed2702102.x. [DOI] [PubMed] [Google Scholar]
- 19.Limmroth V, Katsarava Z, Fritsche G, Przywara S, Diener HC. Features of medication overuse headache following overuse of different acute headache drugs. Neurology. 2002;59(7):1011–1014. doi: 10.1212/wnl.59.7.1011. [DOI] [PubMed] [Google Scholar]
- 20.Bahra A, Walsh M, Menon S, Goadsby PJ. Does chronic daily headache arise de novo in association with regular use of analgesics? Headache. 2003;43(3):179–190. doi: 10.1046/j.1526-4610.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- 21.Bigal ME, Sheftell FD, Rapoport AM, Tepper SJ, Lipton RB. Chronic daily headache: Identification of factors associated with induction and transformation. Headache. 2002;42(7):575–581. doi: 10.1046/j.1526-4610.2002.02143.x. [DOI] [PubMed] [Google Scholar]
- 22.Young WB, Richardson ES, Shukla P. Brush allodynia in hospitalized headache patients. Headache. 2005;45(8):999–1003. doi: 10.1111/j.1526-4610.2005.05180.x. [DOI] [PubMed] [Google Scholar]
- 23.Katsarava Z, Limmroth V, Finke M, Diener HC, Fritsche G. Rates and predictors for relapse in medication overuse headache: A 1-year prospective study. Neurology. 2003;60(10):1682–1683. doi: 10.1212/01.wnl.0000063322.14078.90. [DOI] [PubMed] [Google Scholar]
- 24.Wang SJ, Fuh JL, Lu SR, Juang KD. Outcomes and predictors of chronic daily headache in adolescents: A 2-year longitudinal study. Neurology. 2007;68(8):591–596. doi: 10.1212/01.wnl.0000252800.82704.62. [DOI] [PubMed] [Google Scholar]
- 25.Dorland’s Illustrated Medical Dictionary. 27. Philadelphia: Saunders; 1988. [Google Scholar]
- 26.Delcanho RE, Graff-Radford SB. Chronic paroxysmal hemicrania presenting as toothache. J Orofac Pain. 1993;7(3):300–306. [PubMed] [Google Scholar]
- 27.Benoliel R, Sharav Y. SUNCT syndrome: Case report and literature review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85(2):158–161. doi: 10.1016/s1079-2104(98)90419-x. [DOI] [PubMed] [Google Scholar]
- 28.Benoliel R, Sharav Y. Paroxysmal hemicrania. case studies and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85(3):285–292. doi: 10.1016/s1079-2104(98)90010-5. [DOI] [PubMed] [Google Scholar]
- 29.Alonso AA, Nixdorf DR. Case series of four different headache types presenting as tooth pain. J Endod. 2006;32(11):1110–1113. doi: 10.1016/j.joen.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 30.Moncada E, Graff-Radford SB. Benign indomethacin-responsive headaches presenting in the orofacial region: Eight case reports. J Orofac Pain. 1995;9(3):276–284. [PubMed] [Google Scholar]
- 31.Namazi MR. Presentation of migraine as odontalgia. Headache. 2001;41(4):420–421. doi: 10.1046/j.1526-4610.2001.111006420.x. [DOI] [PubMed] [Google Scholar]
- 32.Sarlani E, Schwartz AH, Greenspan JD, Grace EG. Chronic paroxysmal hemicrania: A case report and review of the literature. J Orofac Pain. 2003;17(1):74–78. [PubMed] [Google Scholar]
- 33.Benoliel R, Elishoov H, Sharav Y. Orofacial pain with vascular-type features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84(5):506–512. doi: 10.1016/s1079-2104(97)90267-5. [DOI] [PubMed] [Google Scholar]
- 34.Czernisky R, Benoliel R, Sharav Y. Odontalgia in vascular orofacial pain. J Orofac Pain. 1999;13(3):196–200. [PubMed] [Google Scholar]
- 35.Sharav Y, Benoliel R, Elishoov H. 8th World Congress on Pain. Seattle: IASP Press; 1996. Vascular orofacial pain: Diagnostic features; p. 155. [Google Scholar]
- 36.Stewart WF, Lipton RB, Celentano DD, Reed ML. Prevalence of migraine headache in the United States. relation to age, income, race, and other sociodemographic factors. JAMA. 1992;267(1):64–69. [PubMed] [Google Scholar]
- 37.Lipton RB, Diamond S, Reed M, Diamond ML, Stewart WF. Migraine diagnosis and treatment: Results from the American Migraine Study II. Headache. 2001;41(7):638–645. doi: 10.1046/j.1526-4610.2001.041007638.x. [DOI] [PubMed] [Google Scholar]
- 38.Pryse-Phillips W, Findlay H, Tugwell P, Edmeads J, Murray TJ, Nelson RF. A Canadian population survey on the clinical, epidemiologic and societal impact of migraine and tension-type headache. Can J Neurol Sci. 1992;19(3):333–339. [PubMed] [Google Scholar]
- 39.Rasmussen BK, Jensen R, Schroll M, Olesen J. Epidemiology of headache in a general population--a prevalence study. J Clin Epidemiol. 1991;44(11):1147–1157. doi: 10.1016/0895-4356(91)90147-2. [DOI] [PubMed] [Google Scholar]
- 40.Launer LJ, Terwindt GM, Ferrari MD. The prevalence and characteristics of migraine in a population-based cohort: The GEM study. Neurology. 1999;53(3):537–542. doi: 10.1212/wnl.53.3.537. [DOI] [PubMed] [Google Scholar]
- 41.Henry P, Michel P, Brochet B, Dartigues JF, Tison S, Salamon R. A nationwide survey of migraine in France: Prevalence and clinical features in adults. GRIM. Cephalalgia. 1992;12(4):229–237. doi: 10.1046/j.1468-2982.1992.1204229.x. [DOI] [PubMed] [Google Scholar]
- 42.Hagen K, Zwart JA, Vatten L, Stovner L, Bovim G. Prevalence of migraine and non-migrainous headache--head-HUNT, a large population-based study. Cephalalgia. 2000;20(10):900–906. doi: 10.1046/j.1468-2982.2000.00145.x. [DOI] [PubMed] [Google Scholar]
- 43.Lyngberg AC, Rasmussen BK, Jorgensen T, Jensen R. Incidence of primary headache: A Danish epidemiologic follow-up study. Am J Epidemiol. 2005;161(11):1066–1073. doi: 10.1093/aje/kwi139. [DOI] [PubMed] [Google Scholar]
- 44.Breslau N, Davis GC, Andreski P. Migraine, psychiatric disorders, and suicide attempts: An epidemiologic study of young adults. Psychiatry Res. 1991;37(1):11–23. doi: 10.1016/0165-1781(91)90102-u. [DOI] [PubMed] [Google Scholar]
- 45.Edmeads J, Findlay H, Tugwell P, Pryse-Phillips W, Nelson RF, Murray TJ. Impact of migraine and tension-type headache on life-style, consulting behaviour, and medication use: A Canadian population survey. Can J Neurol Sci. 1993;20(2):131–137. doi: 10.1017/s0317167100047697. [DOI] [PubMed] [Google Scholar]
- 46.O’Brien B, Goeree R, Streiner D. Prevalence of migraine headache in Canada: A population-based survey. Int J Epidemiol. 1994;23(5):1020–1026. doi: 10.1093/ije/23.5.1020. [DOI] [PubMed] [Google Scholar]
- 47.Stewart WF, Lipton RB, Liberman J. Variation of migraine prevalence by race. Neurology. 1996;47(1):52–59. doi: 10.1212/wnl.47.1.52. [DOI] [PubMed] [Google Scholar]
- 48.Breslau N, Rasmussen BK. The impact of migraine: Epidemiology, risk factors, and co-morbidities. Neurology. 2001;56(Suppl 1):S4–S12. doi: 10.1212/wnl.56.suppl_1.s4. [DOI] [PubMed] [Google Scholar]
- 49.Schwartz BS, Stewart WF, Simon D, Lipton RB. Epidemiology of tension-type headache. JAMA. 1998;279(5):381–383. doi: 10.1001/jama.279.5.381. [DOI] [PubMed] [Google Scholar]
- 50.Stewart WF, Staffa J, Lipton RB, Ottman R. Familial risk of migraine: Population-based study. Ann Neurol. 1997;41(2):166–172. doi: 10.1002/ana.410410207. [DOI] [PubMed] [Google Scholar]
- 51.Ulrich V, Gervil M, Kyvik KO, Losen J, Russell MB. Evidence of a genetic factor in migraine with aura: A population-based Danish twin study. Ann Neurol. 1999;45(2):242–246. doi: 10.1002/1531-8249(199902)45:2<242::aid-ana15>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 52.Clarke JM. On recurrent motor paralysis in migraine. BMJ. 1910;1:1534–1538. doi: 10.1136/bmj.1.2582.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ophoff RA, Terwindt GM, Vergouwe MN, et al. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell. 1996;87(3):543–552. doi: 10.1016/s0092-8674(00)81373-2. [DOI] [PubMed] [Google Scholar]
- 54.Ducros A, Denier C, Joutel A, et al. The clinical spectrum of familial hemiplegic migraine associated with mutations in a neuronal calcium channel. N Engl J Med. 2001;345(1):17–24. doi: 10.1056/NEJM200107053450103. [DOI] [PubMed] [Google Scholar]
- 55.Borroni G, Rao R, Liberini P, et al. Endothelial nitric oxide synthase (Glu298ASP) polymorphism is an independent risk factor for migraine with aura. Headache. 2006;46(10):1575–1579. doi: 10.1111/j.1526-4610.2006.00614.x. [DOI] [PubMed] [Google Scholar]
- 56.Scher AI, Terwindt GM, Verschuren WM, et al. Migraine and MTHFR C677T genotype in a population-based sample. Ann Neurol. 2006;59(2):372–375. doi: 10.1002/ana.20755. [DOI] [PubMed] [Google Scholar]
- 57.Ferrari MD, Nann J, Palotie A. Genetics of migraine. In: Olesen J, Goadsby PJ, Ramadan NM, Tfelt-Hansen P, Welch KMA, editors. The Headaches. 3. Philadelphia: Lippincott Williams & Wilkins; 2006. pp. 251–267. [Google Scholar]
- 58.Göder R, Fritzer G, Kapsokalyvas A, et al. Polysomnographic findings in nights preceding a migraine attack. Cephalalgia. 2001;21(1):31–37. doi: 10.1046/j.1468-2982.2001.00141.x. [DOI] [PubMed] [Google Scholar]
- 59.Kelman L, Rains JC. Headache and sleep: Examination of sleep patterns and complaints in a large clinical sample of migraneurs. Headache. 2001;45(7):904–910. doi: 10.1111/j.1526-4610.2005.05159.x. [DOI] [PubMed] [Google Scholar]
- 60.Spierings EL, Ranke AH, Honkoop PC. Precipitating and aggravating factors of migraine versus tension-type headache. Headache. 2001;41(6):554–558. doi: 10.1046/j.1526-4610.2001.041006554.x. [DOI] [PubMed] [Google Scholar]
- 61.Wober C, Holzhammer J, Zeitlhofer J, Wessely P, Wober-Bingol C. Trigger factors of migraine and tension-type headache: Experience and knowledge of the patients. J Headache Pain. 2006;7(4):188–195. doi: 10.1007/s10194-006-0305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zwart JA, Dyb G, Hagen K, et al. Depression and anxiety disorders associated with headache frequency. The Nord-Trondelag Health Study. Eur J Neurol. 2003;10(2):147–152. doi: 10.1046/j.1468-1331.2003.00551.x. [DOI] [PubMed] [Google Scholar]
- 63.MacGregor EA, Hackshaw A. Prevalence of migraine on each day of the natural menstrual cycle. Neurology. 2004;63(2):351–353. doi: 10.1212/01.wnl.0000133134.68143.2e. [DOI] [PubMed] [Google Scholar]
- 64.MacGregor EA, Frith A, Ellis J, Aspinall L, Hackshaw A. Incidence of migraine relative to menstrual cycle phases of rising and falling estrogen. Neurology. 2006;67(12):2154–2158. doi: 10.1212/01.wnl.0000233888.18228.19. [DOI] [PubMed] [Google Scholar]
- 65.Misakian AL, Langer RD, Bensenor IM, et al. Postmenopausal hormone therapy and migraine headache. J Womens Health. 2003;12(10):1027–1036. doi: 10.1089/154099903322643956. [DOI] [PubMed] [Google Scholar]
- 66.Breslau N, Lipton RB, Stewart WF, Schultz LR, Welch KM. Cormorbidity of migraine and depression: Investigating potential etiology and prognosis. Neurology. 2003;60(8):1308–1312. doi: 10.1212/01.wnl.0000058907.41080.54. [DOI] [PubMed] [Google Scholar]
- 67.Loder E. Menstrual migraine: Clinical considerations in light of revised diagnostic criteria. Neurol Sci. 2005;26(Suppl 2):S121–S124. doi: 10.1007/s10072-005-0423-8. [DOI] [PubMed] [Google Scholar]
- 68.MacGregor EA. Oestrogen and attacks of migraine with and without aura. Lancet Neurol. 2004;3(6):354–361. doi: 10.1016/S1474-4422(04)00768-9. [DOI] [PubMed] [Google Scholar]
- 69.Shimshak DG, Kent RL, DeFuria M. Medical claims profiles of subjects with temporomandibular joint disorders. Cranio. 1997;15(2):150–158. doi: 10.1080/08869634.1997.11752121. [DOI] [PubMed] [Google Scholar]
- 70.Ciancaglini R, Radaelli G. The relationship between headache and symptoms of temporomandibular disorder in the general population. J Dent. 2001;29(2):93–98. doi: 10.1016/s0300-5712(00)00042-7. [DOI] [PubMed] [Google Scholar]
- 71.LeResche L, Mancl LA, Drangsholt M, Huang G, Von Korff M. Predictors of onset of facial pain and temporomandibular disorders in early adolescence. Pain. 2007;129(3):269–278. doi: 10.1016/j.pain.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Von Korff M, Crane P, Lane M, et al. Chronic spinal pain and physical mental morbidity in the United States: Results from the national comorbidity survey replication. Pain. 2005;113(3):331–339. doi: 10.1016/j.pain.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 73.Hagen K, Einarsen C, Zwart JA, Svebak S, Bovim G. The co-occurrence of headache and musculoskeletal symptoms amongst 51,050 adults in Norway. Eur J Neurol. 2002;9(5):527–533. doi: 10.1046/j.1468-1331.2002.00451.x. [DOI] [PubMed] [Google Scholar]
- 74.Ifergane G, Buskila D, Simiseshvely N, Zeev K, Cohen H. Prevalence of fibromyalgia syndrome in migraine patients. Cephalalgia. 2006;26(4):451–456. doi: 10.1111/j.1468-2982.2005.01060.x. [DOI] [PubMed] [Google Scholar]
- 75.Marcus DA, Bernstein C, Rudy TE. Fibromyalgia and headache: An epidemiological study supporting migraine as part of the fibromyalgia syndrome. Clin Rheumatol. 2005;24(6):595–601. doi: 10.1007/s10067-005-1121-x. [DOI] [PubMed] [Google Scholar]
- 76.Hestbaek L, Leboeuf-Yde C, Kyvik KO, et al. Comorbidity with low back pain: A cross-sectional population-based survey of 12- to 22-year-olds. Spine. 2004;29(13):1483–1491. doi: 10.1097/01.brs.0000129230.52977.86. [DOI] [PubMed] [Google Scholar]
- 77.El-Metwally A, Salminen JJ, Auvinen A, Kautiainen H, Mikkelsson M. Prognosis of non-specific musculoskeletal pain in preadolescents: A prospective 4-year follow-up study. Pain. 2004;110(3):550–559. doi: 10.1016/j.pain.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 78.LeResche L, Mancl L, Drangsholt M, Lipton RB. Association of severe headache and migraine with TMD in adolescents. J Dent Res. 2006;85(A):114. [Google Scholar]
- 79.Scher AI, Bigal ME, Lipton RB. Comorbidity of migraine. Curr Opin Neurol. 2005;18(3):305–310. doi: 10.1097/01.wco.0000169750.52406.a2. [DOI] [PubMed] [Google Scholar]
- 80.Rubinstein SM, Peerdeman SM, vanTulder MW, Riphagen I, Haldeman S. Hospital and population-based epidemiological studies have demonstrated the relationship between migraine and ischemic stroke. Stroke. 2005;36(7):1575–1580. doi: 10.1161/01.STR.0000169919.73219.30. [DOI] [PubMed] [Google Scholar]
- 81.Kurth T, Gaziano JM, Cook NR, Logorscino G, Diener HC, Buring JE. Migraine and risk of cardiovascular disease in women. JAMA. 2006;296(3):283–291. doi: 10.1001/jama.296.3.283. [DOI] [PubMed] [Google Scholar]
- 82.Lanzi G, Termine C, Rossi M, et al. Are vascular disorders more prevalent in the relatives of children and adolescents with migraine? Cephalalgia. 2003;23(9):886–891. doi: 10.1046/j.1468-2982.2003.00600.x. [DOI] [PubMed] [Google Scholar]
- 83.Lipton RB, Ottman R, Ehrenberg BL, Hauser WA. Comorbidity of migraine: The connection between migraine and epilepsy. Neurology. 1994;44(Suppl 7):S28–S32. [PubMed] [Google Scholar]
- 84.Marks DA, Ehrenberg BL. Migraine-related seizures in adults with epilepsy, with EEG correlation. Neurology. 1993;43(12):2476–2483. doi: 10.1212/wnl.43.12.2476. [DOI] [PubMed] [Google Scholar]
- 85.Velioglu SK, Ozmenoglu M. Migraine-related seizures in an epileptic population. Cephalalgia. 1999;19(9):797–801. doi: 10.1046/j.1468-2982.1999.1909797.x. [DOI] [PubMed] [Google Scholar]
- 86.McWilliams LA, Goodwin RD, Cox BJ. Depression and anxiety associated with three pain conditions: Results from a nationally representative sample. Pain. 2004;111(1–2):77–83. doi: 10.1016/j.pain.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 87.Cutrer FM. Pathophysiology of migraine. Semin Neurol. 2006;26(2):171–180. doi: 10.1055/s-2006-939917. [DOI] [PubMed] [Google Scholar]
- 88.Welch KM. Contemporary concepts of migraine pathogenesis. Neurology. 2003;61(suppl 4):S2–S8. doi: 10.1212/wnl.61.8_suppl_4.s2. [DOI] [PubMed] [Google Scholar]
- 89.Hargreaves RJ, Shepheard SL. Pathophysiology of migraine--new insights. Can J Neurol Sci. 1999;26(suppl 3):S12–S19. doi: 10.1017/s0317167100000147. [DOI] [PubMed] [Google Scholar]
- 90.Moskowitz MA. Trigeminovascular system. Cephalalgia. 1992;12(3):133–136. doi: 10.1046/j.1468-2982.1992.1203127.x. [DOI] [PubMed] [Google Scholar]
- 91.Fusco M, D’Andrea G, Micciche F, Stecca A, Bernardini D, Cananzi AL. Neurogenic inflammation in primary headaches. Neurol Sci. 2003;24(Suppl 2):S61–S64. doi: 10.1007/s100720300043. [DOI] [PubMed] [Google Scholar]
- 92.Davidoff RA. Migraine, Manifestations, Pathogenesis, and Management. 2. Oxford: Oxford University Press; 2002. pp. 228–273. [Google Scholar]
- 93.Saxena PR, Tfelt-Hansen P. Triptans, 5-HT1B/D receptor agonists in acute treatment of migraines. In: Olesen J, Goadsby PJ, Ramadan NM, Tfelt-Hansen P, Welch KMA, editors. The Headaches. 3. Philadelphia: Lippincott Williams & Wilkins; 2006. pp. 469–503. [Google Scholar]
- 94.Strassman AM, Raymond SA, Burstein R. Sensitization of meningeal sensory neurons and the origin of headaches. Nature. 1996;384(6609):560–564. doi: 10.1038/384560a0. [DOI] [PubMed] [Google Scholar]
- 95.Burstein R, Yamamura H, Malick A, Strassman AM. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J Neurophysiol. 1998;79(2):964–982. doi: 10.1152/jn.1998.79.2.964. [DOI] [PubMed] [Google Scholar]
- 96.Burstein R, Yarnitsky D, Goor-Aryeh I, Ransil BJ, Bajwa ZH. An association between migraine and cutaneous allodynia. Ann Neurol. 2000;47(5):614–624. [PubMed] [Google Scholar]
- 97.Burstein R, Cutrer MF, Yarnitsky D. The development of cutaneous allodynia during a migraine attack: Clinical evidence for the sequential recruitment of spinal and supraspinal nociceptive neurons in migraine. Brain. 2000;123(Pt 8):1703–1709. doi: 10.1093/brain/123.8.1703. [DOI] [PubMed] [Google Scholar]
- 98.Drummond PD. Scalp tenderness and sensitivity to pain in migraine and tension headache. Headache. 1987;27(1):45–50. doi: 10.1111/j.1526-4610.1987.hed2701045.x. [DOI] [PubMed] [Google Scholar]
- 99.Smith R, Hasse LA, Vonder Meulen MB. Scalp pain-threshold and early migraine treatment. Headache. 2004;44(5):482. [Google Scholar]
- 100.Moskowitz MA, Waeber C. Migraine enters the molecular era. Neuroscientist. 1996;2(3):191–200. [Google Scholar]
- 101.Fields HL, Basbaum AI. Central nervous system mechanisms of pain modulation. In: Melzack R, Wall PD, editors. Textbook of Pain. 3. Edinburg, UK: Churchill Livingstone; 1994. pp. 243–257. [Google Scholar]
- 102.Bandler R, Carrive P. Integrated defense reaction elicited by excitatory amino acid microinjection in the midbrain periaqueductal grey region of the unrestrained cat. Brain Res. 1988;439(1–2):95–106. doi: 10.1016/0006-8993(88)91465-5. [DOI] [PubMed] [Google Scholar]
- 103.Raskin NH, Hosobuchi Y, Lamb S. Headache may arise from perturbation of brain. Headache. 1987;27(8):416–420. doi: 10.1111/j.1526-4610.1987.hed2708416.x. [DOI] [PubMed] [Google Scholar]
- 104.Weiller C, May A, Limmroth V, et al. Brain stem activation in spontaneous human migraine attacks. Nature Medicine. 1995;1(7):658–660. doi: 10.1038/nm0795-658. [DOI] [PubMed] [Google Scholar]
- 105.Welch KMA, Nagesh V, Aurora SK, Gelman N. Periaqueductal gray matter dysfunction in migraine: Cause of the burden of illness? Headache. 2001;41(7):629–637. doi: 10.1046/j.1526-4610.2001.041007629.x. [DOI] [PubMed] [Google Scholar]
- 106.Qian J, Noebels JL. Presynaptic Ca2+ channels and neurotransmitter release at the terminal of a mouse cortical neuron. J Neurosci. 2001;21(11):3721–3728. doi: 10.1523/JNEUROSCI.21-11-03721.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McGrail KM, Phillips JM, Sweadner KJ. Immunofluorescent localization of three Na,K-ATPase isozymes in the rat central nervous system: Both neurons and glia can express more than one Na,K-ATPase. J Neurosci. 1991;11(2):381–391. doi: 10.1523/JNEUROSCI.11-02-00381.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Agosti RM. 5HT1F- and 5HT7-receptor agonists for the treatment of migraines. CNS Neurol Disord Drug Targets. 2007;6(4):235–237. doi: 10.2174/187152707781387242. [DOI] [PubMed] [Google Scholar]
- 109.Witkin JM, Baez M, Yu J, Barton ME, Shannon HE. Constitutive deletion of the serotonin-7 (5-HT(7)) receptor decreases electrical and chemical seizure thresholds. Epilepsy Res. 2007;75(1):39–45. doi: 10.1016/j.eplepsyres.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 110.Giffin NJ, Kaube H. The electrophysiology of migraine. Curr Opin Neurol. 2002;15(3):303–309. doi: 10.1097/00019052-200206000-00013. [DOI] [PubMed] [Google Scholar]
- 111.Cady RK, Dodick DW, Levine HL, et al. Sinus headache: A neurology, otolaryngology, allergy, and primary care consensus on diagnosis and treatment. Mayo Clin Proc. 2005;80(7):908–916. doi: 10.4065/80.7.908. [DOI] [PubMed] [Google Scholar]


