Abstract
In management of metabolic syndrome, the traditional Chinese medicine (TCM) is an excellent representative in alternative and complementary medicines with a complete theory system and substantial herb remedies. In this article, basic principle of TCM is introduced and 22 traditional Chinese herbs are reviewed for their potential activities in the treatment of metabolic syndrome. Three herbs, ginseng, rhizoma coptidis (berberine, the major active compound) and bitter melon, were discussed in detail on their therapeutic potentials. Ginseng extracts made from root, rootlet, berry and leaf of Panax quinquefolium (American ginseng) and Panax ginseng (Asian ginseng), are proved for anti-hyperglycemia, insulin sensitization, islet protection, anti-obesity and anti-oxidation in many model systems. Energy expenditure is enhanced by ginseng through thermogenesis. Ginseng-specific saponins (ginsenosides) are considered as the major bioactive compounds for the metabolic activities of ginseng. Berberine from rhizoma coptidis is an oral hypoglycemic agent. It also has anti-obesity and anti-dyslipidemia activities. The action mechanism is related to inhibition of mitochondrial function, stimulation of glycolysis, activation of AMPK pathway, suppression of adipogenesis and induction of low-density lipoprotein (LDL) receptor expression. Bitter melon or bitter gourd (Momordica charantia) is able to reduce blood glucose and lipids in both normal and diabetic animals. It may also protect β cells, enhance insulin sensitivity and reduce oxidative stress. Although evidence from animals and humans consistently supports the therapeutic activities of ginseng, berberine and bitter melon, multi-center large-scale clinical trials have not been conducted to evaluate the efficacy and safety of these herbal medicines.
Metabolic syndrome characterized by insulin resistance has become a health thread worldwide in the past 20 years (1). In survey of 8,814 people in USA, prevalence of metabolic syndrome was over 40% in people of 60–69 year old (2). In the other geographic areas in the world including Europe and Asia, the prevalence of metabolic syndrome has been increasing dramatically (1). Metabolic syndrome and associated diseases have been one of major burdens in health care system in many industrialized countries.
The concept of metabolic syndrome was generated by Kylin in 1923, who described a cluster of medical conditions, such as hypertension, hyperglycemia and gout (3). The concept did not attract much attention until Dr. Gerald Reaven introduced the syndrome X in 1988 (4), which is similar to the metabolic syndrome. In 1999, WHO (World Health Organization) and EGIR (European Group for the Study of Insulin Resistance) released their diagnostic criteria for the metabolic syndrome, respectively (5; 6). Now, there are at least 6 sets of diagnostic criteria for the syndrome from different organizations over the world (1; 7). The primary contents of diagnostic criteria are similar among these organizations. They are hyperglycemia, insulin resistance, central obesity, hypertension, elevated triglycerides and decreased high-density lipoprotein-cholesterol (HDL-C). In the treatment of metabolic syndrome, the traditional Chinese medicine (TCM) is an excellent example in alternative and complementary medicine with a long history, unique theory system and variety of herb remedies.
TCM ON METABOLIC SYNDROME
TCM is a medical system developed on the basis of Taoist philosophy. The theory of TCM was first documented in an ancient Chinese book, Huangdi Neijing (Yellow Thearch’s Inner classic), which was composed two thousand years ago in China (8). The book provides the foundation for diagnostic methods and therapeutic strategies including acupuncture in TCM. It proposes that the human body contains Yin, Yang and Five Elements (agents). The Five Elements are metal, wood, water, fire and earth. A perfect balance among these contents is required for the maintenance of health in the body. A disease is a consequence of disbalance of Yin and Yang or the Five Elements from cold, heat, emotions or other influences. Qi (air) and blood serve as mediators in communication between Yin and Yang, and among the Five Elements. The primary aim in the treatment of illness is to restore the balance, and replenish Qi or blood. Herbal medicines, acupuncture, and massage are often used to restore the balance in the clinical practice in TCM (9).
Obesity and type 2 diabetes are two major diseases in the metabolic syndrome. According to the book Huangdi Neijing, obesity is a result of over eating, and diabetes is referred as Xiaoke disease which is a consequence of obesity. The complications of Xiaoke disease include stroke, carbuncle and foot gangrene (10; 11). In Chinese, Xiao means losing body weight and Ke means thirsty, which are similar to the symptoms of diabetes, losing body weight in the presence of increased drinking, eating and urination. In the TCM theory, Xiaoke is considered a result of Yin deficiency with dryness-heat. The treatment of diabetes should be focused on replenishing Yin (fluid) and evacuating fire (heat) from the body (12).
TCM prefers herbology, a Chinese art of combining different medical herbs into one therapy through prescription (13). According to TCM, a disease may have a set of identical symptoms among different patients, but the background (Qi, blood, Yin, Yang, and Five Elements) for development of the same disease is quite different in patients. Each patient should receive personalized herbology because of the difference in the background. In the personalized treatment, multiple herbs are often prescribed to form a special formula and single herb is not often used individually.
The bioactivities of many Chinese herbs have been identified with modern technologies in chemistry and pharmacology. With pharmacological concepts in medicine, many scientists made an effort in isolation and purification of bioactive components in herbs with a hope to enrich the therapeutic activities. This strategy is used widely to study Chinese herbs in China and many other countries. Up to now, hundreds of traditional Chinese herbs and active components have been tested for treatment of diabetes, dyslipidemia and obesity. In this review, twenty two of the herbs were selected from the literature to represent the current understanding of Chinese herbs in the treatment of metabolic syndrome (Table 1). Among these, the well-recognized herbs are ginseng, coptis, bitter melon and tea. Since the effect of tea on metabolic syndrome is reviewed in a separate article in this special issue, we focus on ginseng, berberine from coptis, and bitter melon in this article.
Tab 1.
Effects of traditional Chinese herbs on metabolic syndrome
| Name | Plant | Extract | Effect | Mechanism | Model | Ref. |
|---|---|---|---|---|---|---|
| Aloe vera leaves | AloeveraL. Var.Chinens is (Haw.) Berger | Pulp extracts, ethanolic extract | FBG, TC, TG, FFA, hepatic transaminases, phospholipids ↓
Insulin ↑ |
Hydroperoxides, GSH ↓ | T1DM (STZ) rats, T2DM rats | (118)
(119) (120) |
| Cortex Cinnamoni | Cinnamomum zeylanicum | Bark extract, aqueous extract | FBG, insulin, protein-bound sugars, TC, TG ↓
HDL ↑ |
α-glycosidase ↓
Glucose uptake, insulin-signaling pathway in skeletal muscle ↑ |
Fructose-fed rats, T2DM patients, db/db mice | (121)
(122) (123) (124) (125) |
| Crataegus | Crataegus cuneats Sieb. Et Zucc. | Alcoholic extract of fruit, aqueous extract of leaf | TC, TG, LDL, VLDL, lipid deposits in liver and aorta, FBG ↓ | HFD rats, T1DM(STZ) rats | (126)
(127) |
|
| Fructus Corni | Cornus officinalis Sieb. et Zucc. | Anthocyanin and ursolic acid, alcohol extract, oleanolic acid | Lipid accumulation in liver, TG, body weight, FBG ↓
Insulin, C-peptide ↑ |
Proliferation of islet and postprandial insulin, GLUT4 ↑ | HFD mice, T2DM rats | (128)
(129) (130) |
| Fructus Ligustri Lucidi | Ligustrum lucidum Ait. | Decoction | FBG ↓ | T1DM (alloxan) mice | (131) | |
| Fructus Lycii | Lycium barbarum L. | Decoction, polysaccharide fractions | Anti-oxidative, FBG, TC, TG, MDA ↓
HDL, SOD activity ↑ |
T1DM (STZ or alloxan) rats, T1DM (alloxan) rabbits | (132)
(133) (134) |
|
| Ganoder ma Lucidum | Ganoderma Iucidum (Leyss.ex Fr.) Karst. | Polysaccharides, ganoderan B | TC, TG, LDL, LPO, fecal cholate, FBG ↓
Insulin, HDL, fecal TC, coprostanol ↑ |
Ca2+ inflow to the pancreaticβ cells, insulin-releasing, hepatic glucokinase, phosphofructokinase and glucose-6-phosphate dehydrogenase ↑
HMG-CoA reductase, hepatic glucose-6-phosphate, glycogen synthetase ↓ Antioxidation |
HFD rats, hamsters and minipigs, T1DM (STZ or alloxan) mice | (135)
(136) (137) (138) (139) (140) |
| Rhizoma Zingiberis | Zingiber officinale Roscoe | Aqueous extract, juice, ethanol extract, methanolic extract | FBG, TC, TG, blood pressure, body weight, LDL oxidation, atherosclerosis, LPO, lipoproteins, phospholipids ↓
Insulin, HDL, GSH-Px ↑ |
T1DM (STZ) rats, fructose fed rats, APOE-deficient mice, hyperlipidemic rats, cholesterol fed rabbits | (141)
(142) (143) (144) (145) (146) (147) (148) |
|
| Radix Glycyrrhizae | Glycyrriza Uralensis Fisch.G.Glabra L. | Ethanolic extract, flavonoid oil | FBG, visceral adipose tissues, body weight ↓ | Genes for β-oxidation, genes for fatty acid synthesis ↓
PPARγ ↑ |
KK-Ay mice, HFD mice | (149)
(150) (151) |
| Radix Notoginseng | Panax notoginseng(Burk.)F.H. Chen | n-Butanol extract, pulverized root, ginsenoside Rg1 | TC, TG, LDL, FBG ↓ | Accumulation of abnormal lipid ↓
FXR/LXR α agonist |
HFD or high-cholesterol fed rats, T1DM (alloxan) mice, APOE-deficient mice | (152)
(153) (154) (155) |
| Radix Platycodi | Platycodon grandiflorum | Crude saponins, aqueous extract, platycodin saponin | TG, LDL, TG, liver surface fat pads, calorie intake, body weight ↓ | Intestinal absorption of dietary fat ↓ | Lipid emulsion fed rats, HFD mice, HFD rats | (156)
(157) (158) |
| Pumpkin | Cucurbita moschata(Duch. ex Lam.) Duch. ex Poiret | Water extract, protein-bound polysaccharide | Fatty liver, TC, TG, FBG ↓
Glucose tolerance, insulin ↑ |
Lipid synthesis ↓
Fatty acid breakdown, hepatic β-oxidation ↑ |
HFD mice, T1DM(alloxan) rats | (159)
(160) |
| Radix Astragaliseu Hedysari | Astragalus membranaceus (Fisch.) Bunge var. mongholicus (Bunge) Hsiao and Astragalus membranaceus (Fisch.) Bunge | Astragalus polysaccharide | FBG ↓
Insulin sensitivity, glucose-insulin tolerance, ACTH, glucagon, insulin ↑ |
PTP1B↓, insulin antagonistic hormones, Th1/Th2 cytokine ratio, apoptotic β cell percentage ↓
Glycogen synthesis, PPARγ, islet mass ↑ |
T1DM (STZ) mice and rats, TNF-α injected rats | (161)
(162) (163) |
| Radix et Rhizom Rhei | Rheum palmatum L. | Aqueous extract and alcohol extract, wine steamed Radix et Rhizoma Rhei | FBG, LPO ↓
Oral glucose tolerance ↑ |
Insulin-stimulated glucose uptake ↑
α-glucoamylase ↓ |
APOE-deficient mice, T1DM (STZ) mice | (164)
(155) (165) |
| Radix Puerariae | Pueraria lobata (Willd.) Ohwi. | Ethanol extract, puerarin | FBG, glycation products, insulin, abdominal fat, TC, TG, body mass ↓ | Antioxidant enzymes, GLUT4 and intracellular insulin signaling, α-adrenoceptors on the adrenal gland, the secretion of β-endorphin, glucose utilization, β-endorphin-like imunoreactivity ↑
Aldose reductase ↓ |
D-galactose fed rats, T1DM (STZ) rats, rats with islet damage induced by hydrogen peroxide, HFD rats, ovariectomized rats | (166)
(167) (168) (169) (170) (171) (172) |
| Radix Rehmanniae | Rehmannia glutinosa Libosch. | FBG, urea nitrogen, 5-hydroxymethylfurfural, thiobarbituric acid reactive substance ↓ | T1DM (STZ) rats after nephrectomy | (173) | ||
| Radix Salviae Miltiorrhizae | Salvia miltiorrhiza Bge. | TG ↓ | Bcl-2 protein, cerebral SOD↑
Cerebral MDA, LPO, Bax ↓ |
Four-vessel occlusion and reperfusion rats, APOE-deficient mice, high cholesterol diet fed rabbits | (174)
(155) (175) |
|
| Rhizoma Polygonati | Polygonatum sibiricum Red. | Methanol extract | FBG ↓ | Hepatic glucose output, GLUT2 ↓ | KK-Ay mice, Wistar obese rats, T1DM (STZ) mice | (176)
(177) (178) (179) |
| Rhizoma Polygonati Odorati | Polygonatum odoratum (Mill.) Druce | Water extract, asteroidal glycoside | FBG, TG, glycosylated hemoglobin ↓ | α-glucosidase in digestive canal ↓
Whole body glucose disposal rates, glycogen, glycogen synthase ↑ |
Diabetic mice and rats, 90% pancreatectomized rats | (180)
(181) |
| Salix matsudana leaves | Salix matsudana Koidz. | Polyphenol fractions, apigenin-7-O-d-glucoside, luteolin-7-O-d-glucoside and chrysoeriol-7-O-d-glucoside | Body weight, parametrial adipose tissue weights, TC ↓ | α-amylase and palmitic acid uptake into small intestinal ↓
Norepinephrine-induced lipolysis ↑ |
HFD mice | (182)
(183) |
| Semen Cassiae | Cassia obtusifolia L. | Water extract | TC, TG, LDL, apoB, body weight, Lee’s index, insulin, MDA↓ | Hyperlipidemic mice, HFD rat | (184)
(185) |
|
| Tea | Camellia sinensis | Black tea, green tea, water extracts, crude tea polysaccharides, tea polysaccharide fraction, oolong tea | FBG, phosphate, body weight, GSP, TC, TG, FFA, LDL, systolic blood pressure, LPO ↓
GSH, vitamin, glucose tolerance, adiponectin, LDL particle size, insulin, HDL↑ |
Insulin response, enzyme activities of carbohydrate metabolism and antioxidant defenses, PPARα and PPARγ ↑
Weight of the intestine ↓ |
Healthy humans,
HFD rats, T1DM (alloxan) mice, T1DM (STZ) rats, KK-A(y)/TaJcl mice, fructose-fed hamster, patients with coronary artery disease |
(186)
(187) (188) (189) (190) (191) (192) (193) (194) |
ACTH, Adrenocorticotropic hormone; APOE, apolipoprotein E; FBG, fasting blood glucose; FFA, free fatty acids; HDL, high-density lipoprotein; HFD, high-fat diet; HMG-CoA reductase, 3-hydroxy-3-methyl-glutaryl-CoA reductase; GSH, Glutathione; GSP, glucosylated serum protein; LDL, low-density lipoprotein; LPO, lipid peroxidation; MDA, malondialdehyde; PPARγ, peroxisome proliferator-activated receptor γ; Ref., reference; SOD, superoxidase dismutase; TC, total cholesterol; TG, triglycerides; VLDL, very low-density lipoprotein.
In TCM, the major function of Asian ginseng is to replenish Qi, and American ginseng is used to restore Yin. Coptis and bitter melon are both bitter herbs and may serve to evacuate fire from the human body. In the theory of TCM, these herbs are beneficial to patients with obesity and diabetes.
GINSENG
Ginseng is one of the most popular Chinese herbal medicines. In TCM, ginseng is only referred as the root of Panax ginseng C. A. Mey.. However, the root of Panax quinquefolium L. (American ginseng) is also called ginseng sometimes. Since the major effects of ginseng include adaptogen, aphrodisiac and nourishing stimulant, which are required for treatment of aging, ginseng has been historically used in the treatment of most ageing-associated diseases (14). Metabolic syndrome has a high prevalence in aging population. Ginseng has been widely studied for treatment of diabetes, dyslipidemia and obesity. Interestingly, in addition to ginseng root, ginseng berry and leaf were also shown to reduce blood glucose in diabetic models (15; 16).
1. Ginsenosides
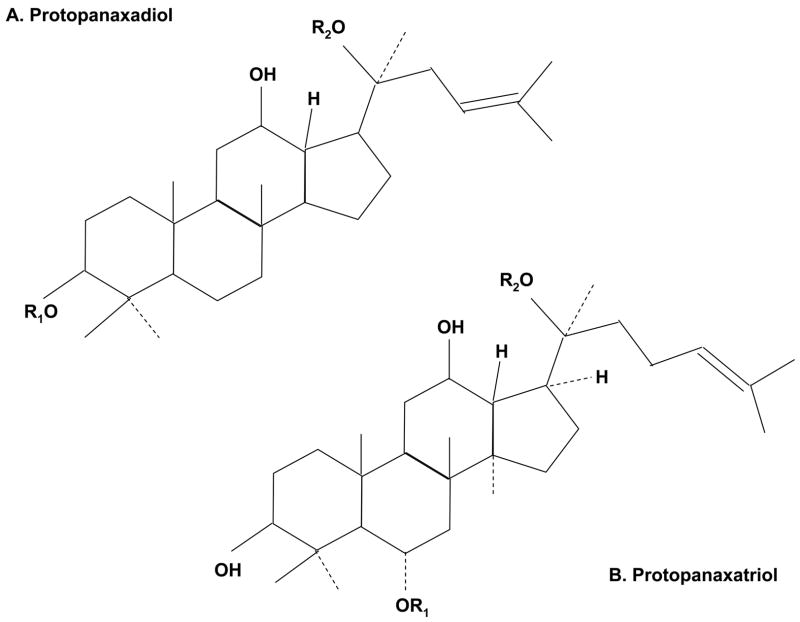
Ginseng contains many bioactive compounds. The representative compounds are ginseng-specific saponins (ginsenosides, Fig. 1), which have clear bioactivities in the regulation of blood glucose and blood pressure. The total contents of ginsenosides are 2.6% – 6.6% of dry weight of ginseng roots in Asian ginseng, red ginseng (heated and marinated Asian ginseng) and American ginseng (17). The ginsenosides are divided into several different compounds, such as Rb1, Rb2, Rc, Rd, Re and Rg1. In addition to ginsenosides, some glycans and peptides isolated from ginseng root may also have hypoglycemic effect in mouse models of diabetes (18). In terms of stability, ginsenosides are better than the glycans and peptides. Ginsenosides can be administrated orally or through injection. However, the bioactivities in glycans and peptides are inactivated in the stomach or intestine if taken orally. When ginseng is taken orally, ginsenosides should be the major active compounds in the blood. Ginsenosides are expensive since the price of ginseng root is much higher than many other herbs. As a result, the crude extract of ginseng root is often used instead of ginsenosides in many laboratory studies.
Fig. 1.
Structure of ginsenosides (ginseng-specific saponins). A. The ginseng saponins of protopanaxadiol include Ra1, Ra2, Ra3, Rb1, Rb2, Rb3, Rc, Rd, Rg2, Rg3, Rs1, Rs2, et al. B. The ginseng saponins of protopanaxatriol include Re, Rf, Rg1, Rg2, Rh1, et al.
2. Berry and leaf of American ginseng
A recent study reported that ginseng leaf and berry have a higher level of ginsenosides than the ginseng root, and they are also effective in the treatment of diabetes (16). In ob/ob mice, ginseng berry extract was tested in the regulation of blood glucose. After 12-day injection of the berry extract, hyperglycemia was reduced significantly and glucose tolerance was improved markedly. Fasting and postprandial insulin levels were decreased significantly. In the hyperinsulinemic-euglycemic clamp assay, insulin-stimulated glucose disposal was increased by more than twofold in the treated mice. The ginseng berry extract was found to reduce food intake, decrease body weight, and enhance energy expenditure as indicated by increased body temperature. Analysis of compounds in the berry extract indicated that ginsenoside Re was the major component in the ginseng berry extract. Injection of ginsenoside Re for 12 days produced a similar efficacy on glucose metabolism in ob/ob mice. However, the body weight and energy expenditure were not changed by ginsenoside Re, suggesting that the effect on energy metabolism might be induced by other components in the berry extract (19). The berry extract was also tested in db/db mice, and similar results were obtained for glucose and energy metabolism (20). The berry extract was also able to reduce body weight in the lean littermates (20), suggesting that the ginseng bioactivities are observed in both lean and genetic obese mice.
The effects of ginseng root and ginseng berry on metabolic syndrome were compared in a study in ob/ob mice (21). The results showed that the extract of ginseng berry had much stronger effects than ginseng root extract in reduction of blood glucose, improving glucose tolerance and lowering body weight. The root extract had modest effects on glucose metabolism and exhibited no activity in the regulation of body weight (21). The difference might be due to the higher content of ginsenosides in the ginseng berry (19).
In addition to ginseng berry, ginseng leaf also has a high content of ginsenosides, and therapeutic effects of leaf extract were observed in diabetic (ob/ob) mice (22). In the leaf extract, all of the major ginsenosides were detected by high performance liquid chromatography (HPLC), and their contents were even higher than those in the ginseng berry and root. In this study, the leaf extract was shown to reduce blood glucose, increase body temperature and decrease body weight after 12-day injection in ob/ob mice (22).
3. Ginseng on glucose metabolism
In dietary obese mice, an ethanol extract of wild ginseng significantly inhibited body weight gain, decreased blood glucose, triglycerides and free fatty acids (FFA) levels, and improved insulin sensitivity (23). The sizes of white and brown adipocytes were decreased by 50% in the high dose group. Extracts generated using different methods were compared for the metabolic activities of ginseng (24). Vinegar-processed ginseng radix has a better action in the correction of metabolic disorders (24).
Efficacy and safety of ginseng on glucose metabolism have been confirmed in patients with type 2 diabetes in double-blinded placebo-controlled studies (25; 26). One report showed that administration of ginseng elevated mood, improved psychophysical and physical performance, reduced fasting blood glucose (FBG), hemoglobin A1c (HbA1c) and body weight in patients (25). Another report indicated that 12 weeks of supplementation with Korean red ginseng decreased plasma glucose and insulin levels, and improved insulin sensitivity significantly in patients (26). The efficacy of American ginseng was tested in lowering postprandial blood glucose (PBG) in human subjects (15; 27–30). The PBG was generated by 25 g glucose administration in oral glucose tolerance test (OGTT). The results showed that doses of 1 g to 9 g American ginseng had the same effect on reducing PBG. In the diabetic subjects, administrating at 0 to 2 h before the glucose challenge, ginseng generated equal effects. In the healthy subjects, American ginseng was administrated at 40 min prior to the OGTT and was able to reduce the PBG (15; 27–30). Korean red ginseng exhibited similar activities on lowering PBG (31).
4. Ginseng on lipid metabolism
In addition to glucose metabolism, ginseng is also shown to regulate lipid metabolism. The lipid metabolism by ginseng was reported 20 years ago. In one study, ginseng was administrated through diet supplement (32). American ginseng and Chinese red ginseng led to reduction of cholesterol and triglycerides levels in liver and serum of avian. The decrease of cholesterol and low-density lipoprotein-cholesterol (LDL-C) was related to the suppression of β-hydroxy-β-methylglutaryl-CoA (HMG-CoA) reductase and cholesterol 7 α-hydroxylase activities (32). In rats and patients fed on high-cholesterol diet to generate hyperlipidemia, administration of red ginseng powder reduced plasma total cholesterol, triglycerides, FFA, platelet adhesiveness, and increased HDL-C significantly (33). In a clinical trial, administration of ginseng extract led to reduction of total cholesterol, triglycerides and LDL-C, and induction of HDL (34). These effects were attributed to the potent antioxidant effect of ginseng (34).
Ginsenosides are considered as the active components in the regulation of lipid metabolism and body weight. In mice with diet-induced hyperlipidemia, ginsenosides had an additive effect to aerobic exercise in the regulation of lipid metabolism, and antioxidant capacity (35). In obese animals fed on a high-fat diet, ginsenosides isolated from ginseng are able to reduce food intake, fat composition and serum leptin level, prevent weight gain and increase in serum triglycerides (36; 37). However, opposite results were obtained by another group using mice. Injection of ginsenosides prepared from Korean red ginseng impaired lipid metabolism through inhibition of PPARα function (38). Thus, more experiments are required to evaluate the efficacy of ginseng on lipid metabolism.
5. Ginseng on β cells
Some studies suggest that ginseng is able to increase insulin secretion. In alloxan diabetic mice, the hypoglycemic effect of ginseng radix extracts was associated with elevation of blood insulin (39). The ginseng activity was abolished by injection of antisera against insulin. Impaired insulin response to glucose in the mice was restored, and insulin release, especially glucose-induced insulin release from isolated rat pancreases, was stimulated by the ginseng extracts (39). The active components of ginseng include ginsenosides and DPG-3-2, a polysaccharide fraction, in the stimulation of insulin secretion (40; 41). In cultured islets, ginsenosides are able to augment glucose-stimulated insulin secretion directly (42).
Stimulation of insulin secretion by ginseng extracts may be related to biosynthesis of insulin, release of neurotransmitter acetylcholine, and protection of β-cells from apoptosis. Firstly, biosynthesis of insulin was increased significantly in the pancreatic islets by ginseng extract DPG-3-2 in vivo and in vitro (41). Secondly, release of insulin is increased by ginsenoside Rh2. This mechanism is related to release of neurotransmitter acetylcholine from nerve terminals in pancreases and stimulation of muscarinic M (3) receptors in β-cells (40; 43). Finally, ginseng may prevent β-cell apoptosis (44). This mechanism is related to inhibition of uncoupling protein 2 (UCP2) expression in mitochondria, and an increase in ATP production. Ginseng was shown to induce expression of anti-apoptotic factor Bcl-2 while suppress expression of pro-apoptotic factor caspase-9 (44). In β-cell line MIN6N8 cells, ginseng extract and ginsenosides were able to inhibit cytokine-induced apoptosis (45). The antioxidant activity of ginsenosides was involved in the anti-apoptosis activity as production of free radicals, such as nitric oxide (NO), and reactive oxygen species (ROS) was reduced by ginsenosides (45).
6. Mechanism of ginsenosides action
Although the metabolic activities of ginseng have been well established, the molecular mechanism underlying the biological activities remains largely unknown for ginseng. There are very limited reports about the molecular mechanism of ginseng activities. In the regulation of insulin sensitivity, ginsenoside Re was found to enhance glucose uptake in 3T3-L1 adipocytes by enhancing insulin-induced glucose transporter 4 (GLUT4) translocation (46). Inhibition of intracellular inflammatory molecules including JNK and NF-κB may be involved in this mechanism (46). Serine phosphorylation of insulin receptor substrate (IRS) is a major mechanism of insulin resistance. The phosphorylation leads to interruption of signal transduction from insulin receptor to downstream molecules such as Phosphoinositide 3-kinase (PI3K). The phosphorylation is catalyzed by many serine kinases, such as JNK, IKK, S6K and PKC, in the inflammatory signaling pathways (47). Inhibition of JNK and IKK will be able to reduce the serine phosphorylation in the IRS proteins and prevent inhibition of IRS function. It is likely that ginseng extracts increase insulin signaling activity by suppressing inflammatory response.
In the regulation of food intake and nutrient absorption, ginsenosides were found to inhibit Neuropeptide Y (NPY) expression in the hypothalamus (37) and inhibit pancreatic lipase (36). NPY is a neurotransmitter with well-known function in the stimulation of food intake in the brain (48). Pancreatic lipase is an enzyme secreted from the pancreatic exocrine cells to break apart fat molecules through hydrolysis. The effect of ginseng on lipid metabolism may be a result of reduced food intake, decreased lipid absorption and enhanced insulin sensitivity.
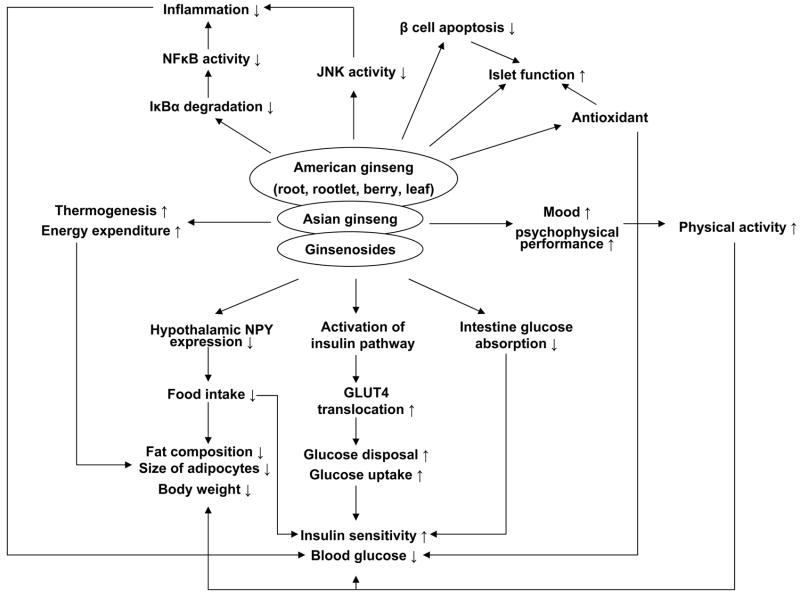
In summary, ginseng has anti-hyperglycemia, and anti-obesity effects (Fig. 2). The mechanism of action is related to insulin sensitization, insulin secretion, β-cell protection, thermogenesis and antioxidantion. It’s a promising herbal remedy in the treatment of metabolic syndrome. However, the cellular and molecular mechanisms of the metabolic activities of ginseng remain largely unknown. More research is required to establish the signaling pathway for ginseng.
Fig. 2.
Ginseng is able to improve glucose metabolism through multiple pathways: 1) Glucose disposal is increased partially due to activation of insulin signaling pathway and GLUT4 translocation by ginseng; 2) Ginseng is able to suppress food intake through inhibition of NPY expression in hypothalamus; 3) Physical activity is increased with ginseng through improvement of mood and psychophysical performance; 4) Fat composition and body weight are reduced partially related to up-regulation of thermogenesis and energy expenditure; 5) Antioxidant and anti-inflammation of ginseng may be involved in the mechanisms of insulin sensitization; 6) Ginseng protects islet function through antioxidant and inhibition of β cell apoptosis.
BERBERINE
Berberine, a botanical alkaloid in the root and bark of several plants, is the major active component of rhizoma coptidis, a popular traditional Chinese medication in the treatment of diabetes and infections. Rhizoma coptidis is prepared from rhizoma of several herbs including Coptis chinensis French, Coptis deltoidea C. Y. Cheng et Hsiao and Coptis teetoides C. Y. Cheng. Rhizoma Coptidis was recorded as a medication as early as A.D. 200 in The Herbal Classic of the Divine Plowman (Shen Nong Ben Cao Jing). In about A.D. 500, the anti-diabetes activity of Rhizoma Coptidis was recorded for the first time in a book “Note of Elite Physicians” by Hongjing Tao. However, in most records or books, the major therapeutic activity of Rhizoma Coptidis is claimed for the treatment of infection and inflammation because infectious diseases were much more popular than diabetes in ancient China (49; 50).
Berberine (Fig. 3) is the bioactive compound in Rhizoma Coptidis for anti-diabetes and anti-infection. The content of berberine in Rhizoma Coptidis is 5.2%–7.7%. Since Rhizoma Coptidis is expensive, most berberine used in clinical application is prepared from herbs other than Rhizoma Coptidis, such as Berberis amurense Rupr. and Phellodendron amurense Rupr.. Berberine has many chemical forms, i.e., berberine hydrochloride, berberine sulfate, berberine citrate or phosphate (50). Among then, berberine hydrochloride is the most common form. Berberine has a well-established antimicrobial activity in the control of infection by bacteria, viruses, fungi, protozoans and helminthes (8, 14). In China, berberine is an over-the-counter drug for the treatment of gastrointestinal infections, such as bacteria diarrhea. In 1988, the hypoglycemic effect of berberine was found when berberine was used to treat diarrhea in diabetic patients in China (13). Since then, berberine has been used as an anti-hyperglycemic agent by many physicians in China. There is substantial numbers of clinical reports about the hypoglycemic action of berberine in Chinese literatures.
Fig. 3.
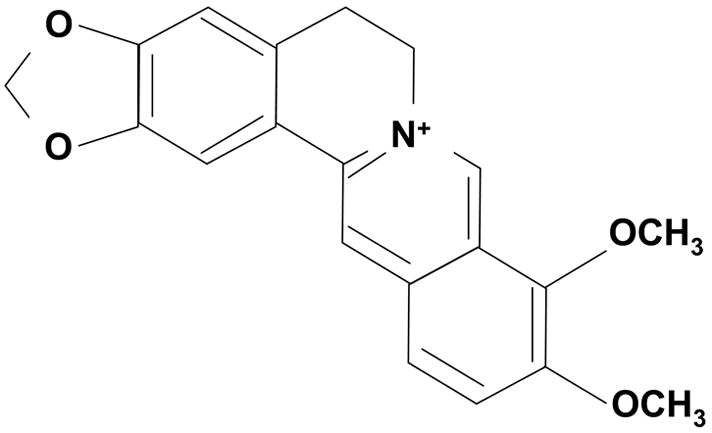
Structure of berberine
1. Efficacy of berberine on diabetic patients
Berberine was reported to have a comparable activity to sulphonureas or metformin in reducing blood glucose in diabetic patients in the Chinese literatures. Our study confirmed that administration of berberine (0.5 g t.i.d.) at the beginning of each major meals was able to reduce FBG as well as PBG in adult patients with newly-diagnosed type 2 diabetes (51). HbA1c of the patients was decreased by 2.0% with berberine treatment, which is comparable to that of metformin. In the poorly-controlled diabetic patients, berberine reduced HbA1c by 0.8%. Besides the hypoglycemic action, a beneficial effect of berberine on lipid metabolism was also observed (51; 52). However, up to now, there is no multicenter, well controlled, long-term clinical trial to evaluate the efficacy of berberine in the treatment of diabetes.
2. Berberine on glucose metabolism in animals
Berberine was shown to reduce weight gain, enhance insulin sensitivity and decrease blood glucose in both dietary and genetic animal models of type 2 diabetes. In obese rats fed on a high-fat diet, berberine was reported to decreases body weight, FBG, PBG, fasting insulin and HOMA-IR (53–55). In rats with type 2 diabetes induced by high-fat diet and low dose of streptozotocin (STZ), FBG was decreased and insulin tolerance was improved significantly by berberine (55; 56). In db/db mice, body weight was reduced and glucose tolerance was improved by berberine (54). Berberine also restored the pancreas damaged by alloxan in Wistar rats (57).
3. Berberine on lipid metabolism
Effects of berberine on lipid metabolism were evaluated in animals and human subjects. In rats fed on high-fat diet, berberine was shown to decrease serum triglycerides (55–57). Triglycerides deposition in liver and muscle was reduced significantly, and liver steatosis was prevented by berberine administration (55; 58). In addition to the activity in obese and diabetic rats, berberine reduces serum FFA in normal lean rats (55; 56). Recently, berberine was demonstrated to reduce cholesterol. Two clinical trials demonstrated that berberine was able to decrease triglycerides by 35% and 22%, serum cholesterol by 29% and 16%, and LDL-C by 25% and 20% in subjects with dyslipidemia (52; 59). In animal, berberine was shown to decrease triglycerides, serum cholesterol and LDL-C markedly in hamster or diabetic rats fed with high-cholesterol diet (52; 57; 60). However, berberine seems to have little or no effects on HDL level (52; 59).
4. Insulin-independent effects of berberine in vitro
Regarding mechanism of berberine action, the first study was conducted using hepatocytes (HepG2 cell line) and published in 2002 (61). In this study, berberine was shown to have insulin-independent activity in the stimulation of glucose consumption in cultured cells, and this activity is similar to that of metformin. Berberine did not induce insulin secretion in β cells (β-TC3 cell line) (61). In later studies conducted by several labs, the insulin-independent activity of berberine was confirmed in other cell models including muscle cells (L6 and C2C12 cell lines) and adipocytes (3T3-L1 cell line) (53; 54; 62–66). In the presence of insulin, berberine was able to enhance the insulin-induced glucose consumption and glucose uptake. Since berberine is a strong stimulus for glucose uptake, several groups investigated the effect of berberine on glucose transporters. The conclusions were controversial. Two groups reported that berberine was able to stimulate GLUT4 translocation (54; 66), but this activity was not observed by other groups (63; 64). One study indicated that berberine was able to enhance GLUT1 expression (63). However, this effect was not observed in other studies (53; 64). Our study suggests that berberine may have a weak direct effect on the translocation or expression of glucose transporters, and this is only detectable with high concentration of berberine (≥10 μM). Regulation of GLUTs is not the major mechanism by which berberine stimulate glucose metabolism (53).
5. Berberine activates AMPK
AMPK (AMP-activated protein kinase) is a target for berberine in the regulation of glucose metabolism. AMPK is an important energy-sensing/signaling system in mammalian cells. It is a member of a metabolite-sensing protein kinase family that acts as a fuel gauge by monitoring cellular energy levels, such as AMP/ATP ratio (67). Activation of AMPK is well known to increase insulin sensitivity and mitochondrial function (68). Phosphorylation of Thr-172 within the activation loop of the catalytic domain of the alpha subunit (AMPKα) is necessary for AMPK activity. Five published studies consistently demonstrate that berberine is a strong inducer for Thr-172 phosphorylation in AMPK (53; 54; 60; 62; 63). In cells, the phosphorylation was increased at 0.5 h after exposure to berberine and the increase was maintained for at least 16 h (53).
6. Berberine inhibits mitochondrial function
Berberine may activate AMPK through increasing AMP/ATP ratio (53; 62). Berberine inhibits ATP biosynthesis in mitochondria. It was reported 20 years ago that berberine inhibited NAD-linked respiration in isolated mitochondria of rat liver in vitro (69; 70). This activity was confirmed in a recent study published in 2003 (71). In the mitochondria, berberine was shown to inhibit monoamine oxidase directly (72–74). The mitochondrial inhibition was demonstrated in living cells in our recent study (53). In search for mechanism of increased AMP/ATP ratio, we investigated berberine effect on oxygen consumption by living cells. We observed that berberine inhibited oxygen consumption and enhanced glycolysis as indicated by increased lactate production. As the efficiency of ATP biosynthesis by glycolysis is much lower compared to that in mitochondria, this change is responsible for the increased AMP/ATP ratio. Based on these observations, we hypothesized that AMPK activation may be a consequence of mitochondrial inhibition by berberine (53). The study also suggests that modest inhibition of mitochondrial function may contribute to improvement of insulin sensitivity. This possibility is supported by a recent report in which inhibition of mitochondrial oxidative phosphorylation through gene knockout provided protection of mice from diet-induced insulin resistance, diabetes and obesity (75). This mechanism may be used by berberine in the up-regulation of glucose and lipid metabolism. However, this possibility remains to be tested in vivo.
7. Berberine as an α-glucosidase inhibitor
Berberine may reduce glucose absorption in intestine. It is interesting that berberine also acts as an α-glucosidase inhibitor. α-Glucosidase is an intestinal enzyme for digestion of carbohydrates, such as starch and table sugar, into monosaccharides. Inhibition of this enzyme will lead to suppression of absorption of dietary carbohydrates. α-Glucosidase inhibitor is a class of oral hypoglycemic agent for type 2 diabetes that works by reducing glucose absorption. α-Glucosidase activity was inhibited by berberine in Caco-2 cells (76). Glucose transportation cross the intestinal epithelium was also decreased after berberine treatment (77). These two events may be involved in control of blood glucose by berberine.
8. Berberine inhibits adipogenesis
Inhibition of adipogenesis may contribute to anti-obesity activity of berberine. Berberine was shown to suppress adipocytes differentiation and reduce lipid accumulation in 3T3-L1 adipocytes (54; 65; 78–80). In the cells treated by berberine, expression of several lipogenic genes including PPARγ, C/EBPα, SREBP-1c, fatty acid synthase, acetyl-CoA carboxylase, acyl-CoA synthase, lipoprotein lipase, aP2 and CD36 was suppressed. Since PPARγ and C/EBPα are master transcription regulators of adipogenesis and most of these genes (fatty acid synthase, acetyl-CoA carboxylase, acyl-CoA synthase, lipoprotein lipase, aP2 and CD36) are targets of the two transcription factors (81). Inhibition of PPARγ and C/EBPα is likely the key mechanism for inhibition of adipogenesis by berberine.
9. Berberine increases LDLR mRNA level
The mechanism by which berberine reduces cholesterol is related to the increased expression of LDL receptor (LDLR) in both mRNA and protein in liver (52; 82). The increase in mRNA of LDLR is a result of extended half-life of mRNA in HepG2 cells (52; 82). Activation of extracellular signal-regulated kinases (ERK) by berberine may contribute to the increased mRNA stability, and the ERK activation by berberine is specific to liver as no ERK activation was observed in other type of cells (53; 83; 84). Activation of JNK by berberine was reported and might be involved in the elevation of LDLR (85). The effect of berberine on LDLR was blocked by a JNK inhibitor.
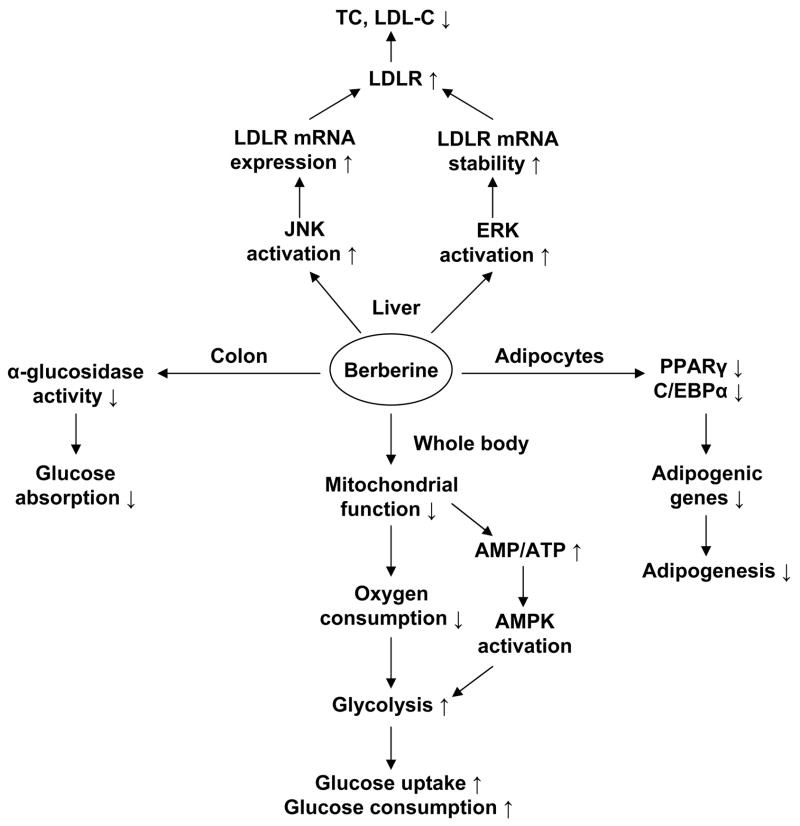
In summary, berberine, a single compound identified from a Chinese herb, has a promising activity in the control of blood glucose and lipid in patients (Fig. 4). The metabolic activity has been confirmed in various animal models, and the action mechanism has been suggested by recent studies. However, the mitochondrial inhibition and AMPK signaling pathway remain to be understood in detail, and tested in vivo for the metabolic activities of berberine. Additionally, the role of berberine in the regulation of gut flora is not clear, and required to be investigated given the anti-bacteria activity of berberine in the intestine. The gut flora are the microorganisms that normally live in the digestive tract and can regulate energy absorption in their hosts through fermentation of dietary fibers (86).
Fig. 4.
Mechanism of berberine in regulation of metabolism: 1) Berberine enhances glucose uptake through induction of glycolysis, which is due to inhibition of aerobic respiratory; 2) AMPK activation stimulated by berberine is a consequence of inhibition of mitochondrial function; 3) Berberine suppresses adipogenesis through inhibition of PPARγ and C/EBPα function; 4) Berberine is able to decrease intestinal glucose absorption by inhibition of α-glucosidase; 5) Berberine up-regulates LDL receptor (LDLR) expression through increasing LDLR mRNA, which is related to inactivation of ERK and activation of JNK pathway.
BITTER MELON
Bitter melon (bitter gourd, karolla or cerasee) is a popular vegetable as well as an herb in China. The species name of bitter melon is Momordica charantia. Bitter melon has been used as an herb for at least 600 years in South China (87).
1. “Plant insulin” of bitter melon
Bitter melon is known for its “plant insulin”, a polypeptide with 166 residues, which exerts a potent hypoglycemic effect after subcutaneous injection (88). Oral administration of “plant insulin” is not effective in the regulation of glucose metabolism as the peptide is inactivated in the gut. Requirement for injection limited its clinical application. In addition to the “plant insulin”, bitter melon may also contain unknown bioactive components since a substantial number of reports indicate that bitter melon is able to exert a hypoglycemic effect in a variety of animal models through oral administration.
2. Hypoglycemic effect of bitter melon on animal models
In normal mice, oral administration of bitter melon extracts decreased blood glucose (89; 90). This was observed in the absence of change in intestinal absorption of glucose, insulin secretion or liver and kidney function. In STZ-induced diabetic mice, bitter melon was able to decrease blood glucose. Several possibilities were proposed for the effect of bitter melon. These include suppression of STZ-induced peroxidation and apoptosis in β-cells, and increasing glycogen content in liver and muscle, and activation of hepatic glucokinase, hexokinase, and phosphofructokinase (91). In KK-Ay mice, a genetic type 2 diabetic model, insulin sensitivity was enhanced significantly by a water extract of bitter melon (92). The effect was observed with enhanced GLUT4 translocation (92). Bitter melon was shown to enhance exercise effect on reducing blood glucose and insulin in KK-Ay mice (93).
In alloxan-induced diabetic rats, blood glucose was decreased and cataract formation, a side effect of hyperglycemia, was delayed with oral administration of bitter melon (94). Acetone extract of bitter melon was reported to reduce blood glucose and cholesterol to normal range after 15–30 days administration. The blood glucose kept in the low level even after 15 days of discontinuation of the treatment (95). Ethanol extract of bitter melon decreased FBG in normal and STZ diabetic rats. Gluconeogenesis was inhibited through down-regulation of hepatic glucose-6-phosphatase and fructose-1,6-bisphosphatase activities, and glucose oxidation was enhanced by up-regulation of glucose-6-phosphate dehydrogenase (G6PDH) activity in red cells and hepatocytes (96).
Efficacy of bitter melon was compared with other drugs in the animals. One study suggested that bitter melon had comparable hypoglycemic effect to glibenclamide in alloxan diabetic rats (97). However, another study indicated that the hypoglycemic effects of bitter melon were weaker than tolbutamide in the normal rats or metformin in STZ diabetic rats (98). The difference may be due to bitter melon had stronger effect in alloxan diabetic rats than in STZ diabetic rats. Some reports indicated that bitter melon had no hypoglycemic effects on healthy or type 1 diabetic animals (99–101). The discrepancy may be a result of difference in origin or preparation of bitter melon.
3. Hypolipidemic effect of bitter melon
Hypolipidemic effect of bitter melon was reported with the hypoglycemic effect in diabetic rats. Administration of bitter melon extracts decreased serum cholesterol, triglycerides, LDL-C, urea, creatinine, alanine transaminase (ALT), aspartate transaminase (AST), and increased serum HDL-C, suggesting that bitter melon may correct hyperlipidemia and protect hepatic-renal functions (90; 102; 103). Ten weeks administration of bitter melon fruit extract nearly restored the increased triglycerides, TC, phospholipids, LPO and malonedialdehyde (MDA), together with decreased HDL-C (104). The disturbed activities of lipogenic enzymes in liver and in kidney were also restored by bitter melon (105). Once the bitter melon extract was withdrawn, the hyperglycemia and dyslipidemia appeared again (106).
4. Mechanism of bitter melon
Antioxidant and protection of β-cells are considered the major mechanisms of bitter melon in the treatment of diabetes. In the STZ-induced diabetic rats, expression of glutathione S-transferase (GST) isoenzymes was altered in the liver, kidney and testis. This disturbance was reverted by administration of bitter melon extract (107). In addition to meat, seeds of bitter melon also exerted rapid protective effects against lipid peroxidation by scavenging of free radicals (108). In vitro and in vivo, bitter melon markedly reduced the STZ-induced lipid peroxidation and apoptosis in islets of mice and RIN cells (109). The number of β-cells was significantly increased after bitter melon treatment in STZ rats (110).
Additionally, bitter melon may also act through inhibition of glucose absorption, enhancement of glucose disposal and recover of impaired peripheral nerves. Firstly, Na+- and K+-dependent glucose absorption was reduced in the brush border membrane vesicles of the jejunum by administration of bitter melon juice (111; 112). Another study indicated that bitter melon supplementation was able to inhibit digestion of carbohydrate by reducing maltase activity in intestine (113). Secondly, the lyophilized juice of bitter melon was able to stimulate the glucose uptake in L6 myotubes, which may be via the insulin pathway since it can be completely blocked by wortmannin, an inhibitor of PI3 kinase. Finally, the structural abnormalities of peripheral nerves were normalized with bitter melon in STZ rats (111; 112).
5. Clinical trials on bitter melon
Hypoglycemic effect of bitter melon extract was also tested in more than one hundred patients with type 2 diabetes. Administration of the fruit juice or homogenized suspension of bitter melon led to significant reduction of both FBG and PBG (114; 115). Nearly 75% of the patients had good response to bitter melon. Bitter melon administrated together with 50% dose of metformin or glibenclamide led to a greater reduction in blood glucose than that by full doses of the drugs, which indicated that bitter melon had synergistic effect with other oral hypoglycemic agents (116). However, these results are not supported by a recent clinical trial with forty patients that is randomized, double-blind, placebo-controlled (117). In that study, HbA1c was decreased by 0.22% in favor of bitter melon, and the difference is not significant. No significant effects were observed in FBG, total cholesterol and body weight under treatment with bitter melon. Thus, the clinical application of bitter melon needs more evidence from clinical trials.
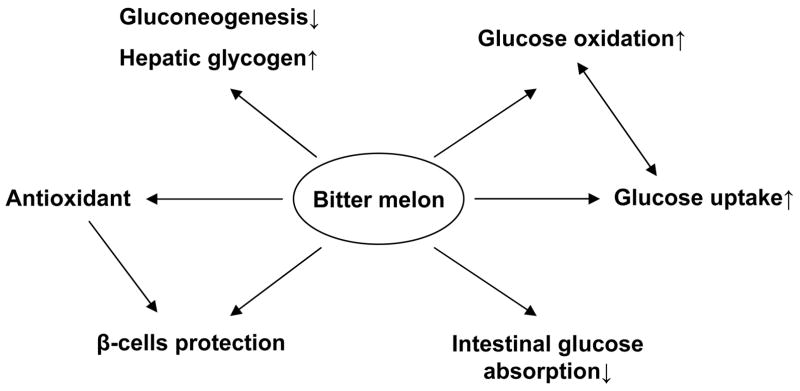
In summary, bitter melon has some activities in the regulation of glucose and lipid metabolism (Fig. 5), which were tested in animals and patients. The results are controversial. The action mechanism remains to be established. The therapeutic efficacy of bitter melon needs to be evaluated in the clinical trials with large sample size.
Fig. 5.
Mechanism of bitter melon in reduction of blood glucose. Antioxidant and β-cells protection are considered the major mechanisms of bitter melon action in the treatment of diabetes. Additionally, bitter melon is able to inhibit glucose absorption in intestine, reduce hepatic gluconeogenesis, and increase glucose uptake, glucose oxidation and hepatic glycogen content.
CONCLUSION
TCM is an excellent system in complementary and alternative medicine. It holds great and unique potential in the management of metabolic syndrome, especially in the control of glucose and lipid metabolism. Its potential in the treatment of diabetic complications is not reviewed, but is promising to provide new effective therapies. The metabolic activities of many Chinese herbal medicines have been proved in well-designed animal experiments. The mechanism of action remains to be investigated, and large-scale clinical trials have not been conducted for these Chinese herbal medicines.
Acknowledgments
This study is partially supported by NIH grant 1P50AT002776-010002 and DK68036 to J Ye.
References
- 1.Day C. Metabolic syndrome, or What you will: definitions and epidemiology. Diab Vasc Dis Res. 2007;4:32–38. doi: 10.3132/dvdr.2007.003. [DOI] [PubMed] [Google Scholar]
- 2.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. Jama. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 3.E K. Studien ueber das Hyertonie-Hyperglykamie-Hyperurika miesyndrom. Zentralblatt Fuer Innere Medizin. 1923;44:105–127. [Google Scholar]
- 4.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 5.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 6.Balkau B, Charles MA. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR) Diabet Med. 1999;16:442–443. doi: 10.1046/j.1464-5491.1999.00059.x. [DOI] [PubMed] [Google Scholar]
- 7.Gogia A, Agarwal PK. Metabolic syndrome. Indian J Med Sci. 2006;60:72–81. [PubMed] [Google Scholar]
- 8.Wang B. Huangdi Neijing. 1. Beijing: Ancient Books of Traditional Chinese Medicine Press; 2003. [Google Scholar]
- 9.Tang X. Basic Theroies of Traditional Chinese Medicine. 1. Shanghai: Shanghai University of Traditional Chinese Medicine Press; 2006. [Google Scholar]
- 10.Wang QQ. Experience in clinical practice of doctrines in Huangdi Neijing: Part 1. Zhong Xi Yi Jie He Xue Bao. 2005;3:486–488. doi: 10.3736/jcim20050618. [DOI] [PubMed] [Google Scholar]
- 11.Yao S, Liu A. Analyze the content of Xiaoke and its complications. Neijing Journal of Beijing University of TCM. 2000;23 [Google Scholar]
- 12.Li WL, Zheng HC, Bukuru J, De Kimpe N. Natural medicines used in the traditional Chinese medical system for therapy of diabetes mellitus. J Ethnopharmacol. 2004;92:1–21. doi: 10.1016/j.jep.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 13.Qu R. Herbology. 1. Shanghai: Shanghai University of Traditional Chinese Medicine Press; 2006. [Google Scholar]
- 14.Renshen . In: Chinese Materia Medica Dictionary. 1. School JNM, editor. Shanghai: Shanghai Scientific & Technical Publishers; 1986. pp. 29–36. [Google Scholar]
- 15.Vuksan V, Sievenpiper JL. Herbal remedies in the management of diabetes: lessons learned from the study of ginseng. Nutr Metab Cardiovasc Dis. 2005;15:149–160. doi: 10.1016/j.numecd.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Xie JT, McHendale S, Yuan CS. Ginseng and diabetes. Am J Chin Med. 2005;33:397–404. doi: 10.1142/S0192415X05003004. [DOI] [PubMed] [Google Scholar]
- 17.Fen Y. Radix ginseng. In: Xiao P, editor. Modern Chinese Materia Medica. 1. Beijing: Chemical Industry Press; 2002. pp. 1–12. [Google Scholar]
- 18.Ng TB, Yeung HW. Hypoglycemic constituents of Panax ginseng. Gen Pharmacol. 1985;16:549–552. doi: 10.1016/0306-3623(85)90140-5. [DOI] [PubMed] [Google Scholar]
- 19.Attele AS, Zhou YP, Xie JT, Wu JA, Zhang L, Dey L, Pugh W, Rue PA, Polonsky KS, Yuan CS. Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes. 2002;51:1851–1858. doi: 10.2337/diabetes.51.6.1851. [DOI] [PubMed] [Google Scholar]
- 20.Xie JT, Zhou YP, Dey L, Attele AS, Wu JA, Gu M, Polonsky KS, Yuan CS. Ginseng berry reduces blood glucose and body weight in db/db mice. Phytomedicine. 2002;9:254–258. doi: 10.1078/0944-7113-00106. [DOI] [PubMed] [Google Scholar]
- 21.Dey L, Xie JT, Wang A, Wu J, Maleckar SA, Yuan CS. Anti-hyperglycemic effects of ginseng: comparison between root and berry. Phytomedicine. 2003;10:600–605. doi: 10.1078/094471103322331908. [DOI] [PubMed] [Google Scholar]
- 22.Xie JT, Mehendale SR, Wang A, Han AH, Wu JA, Osinski J, Yuan CS. American ginseng leaf: ginsenoside analysis and hypoglycemic activity. Pharmacol Res. 2004;49:113–117. doi: 10.1016/j.phrs.2003.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Yun SN, Moon SJ, Ko SK, Im BO, Chung SH. Wild ginseng prevents the onset of high-fat diet induced hyperglycemia and obesity in ICR mice. Arch Pharm Res. 2004;27:790–796. doi: 10.1007/BF02980150. [DOI] [PubMed] [Google Scholar]
- 24.Yun SN, Ko SK, Lee KH, Chung SH. Vinegar-processed ginseng radix improves metabolic syndrome induced by a high fat diet in ICR mice. Arch Pharm Res. 2007;30:587–595. doi: 10.1007/BF02977653. [DOI] [PubMed] [Google Scholar]
- 25.Sotaniemi EA, Haapakoski E, Rautio A. Ginseng therapy in non-insulin-dependent diabetic patients. Diabetes Care. 1995;18:1373–1375. doi: 10.2337/diacare.18.10.1373. [DOI] [PubMed] [Google Scholar]
- 26.Vuksan V, Sung MK, Sievenpiper JL, Stavro PM, Jenkins AL, Di Buono M, Lee KS, Leiter LA, Nam KY, Arnason JT, Choi M, Naeem A. Nutr Metab Cardiovasc Dis. 2006. Korean red ginseng (Panax ginseng) improves glucose and insulin regulation in well-controlled, type 2 diabetes: Results of a randomized, double-blind, placebo-controlled study of efficacy and safety. [DOI] [PubMed] [Google Scholar]
- 27.Vuksan V, Sievenpiper JL, Koo VY, Francis T, Beljan-Zdravkovic U, Xu Z, Vidgen E. American ginseng (Panax quinquefolius L) reduces postprandial glycemia in nondiabetic subjects and subjects with type 2 diabetes mellitus. Arch Intern Med. 2000;160:1009–1013. doi: 10.1001/archinte.160.7.1009. [DOI] [PubMed] [Google Scholar]
- 28.Vuksan V, Stavro MP, Sievenpiper JL, Beljan-Zdravkovic U, Leiter LA, Josse RG, Xu Z. Similar postprandial glycemic reductions with escalation of dose and administration time of American ginseng in type 2 diabetes. Diabetes Care. 2000;23:1221–1226. doi: 10.2337/diacare.23.9.1221. [DOI] [PubMed] [Google Scholar]
- 29.Vuksan V, Stavro MP, Sievenpiper JL, Koo VY, Wong E, Beljan-Zdravkovic U, Francis T, Jenkins AL, Leiter LA, Josse RG, Xu Z. American ginseng improves glycemia in individuals with normal glucose tolerance: effect of dose and time escalation. J Am Coll Nutr. 2000;19:738–744. doi: 10.1080/07315724.2000.10718073. [DOI] [PubMed] [Google Scholar]
- 30.Vuksan V, Sievenpiper JL, Wong J, Xu Z, Beljan-Zdravkovic U, Arnason JT, Assinewe V, Stavro MP, Jenkins AL, Leiter LA, Francis T. American ginseng (Panax quinquefolius L.) attenuates postprandial glycemia in a time-dependent but not dose-dependent manner in healthy individuals. Am J Clin Nutr. 2001;73:753–758. doi: 10.1093/ajcn/73.4.753. [DOI] [PubMed] [Google Scholar]
- 31.Sievenpiper JL, Sung MK, Di Buono M, Seung-Lee K, Nam KY, Arnason JT, Leiter LA, Vuksan V. Korean red ginseng rootlets decrease acute postprandial glycemia: results from sequential preparation- and dose-finding studies. J Am Coll Nutr. 2006;25:100–107. doi: 10.1080/07315724.2006.10719519. [DOI] [PubMed] [Google Scholar]
- 32.Qureshi AA, Abuirmeileh N, Din ZZ, Ahmad Y, Burger WC, Elson CE. Suppression of cholesterogenesis and reduction of LDL cholesterol by dietary ginseng and its fractions in chicken liver. Atherosclerosis. 1983;48:81–94. doi: 10.1016/0021-9150(83)90019-9. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto M, Uemura T, Nakama S, Uemiya M, Kumagai A. Serum HDL-cholesterol-increasing and fatty liver-improving actions of Panax ginseng in high cholesterol diet-fed rats with clinical effect on hyperlipidemia in man. Am J Chin Med. 1983;11:96–101. doi: 10.1142/S0192415X83000161. [DOI] [PubMed] [Google Scholar]
- 34.Kim SH, Park KS. Effects of Panax ginseng extract on lipid metabolism in humans. Pharmacol Res. 2003;48:511–513. doi: 10.1016/s1043-6618(03)00189-0. [DOI] [PubMed] [Google Scholar]
- 35.Yang Y, Wu T, He K, Fu ZG. Effect of aerobic exercise and ginsenosides on lipid metabolism in diet-induced hyperlipidemia mice. Zhongguo Yao Li Xue Bao. 1999;20:563–565. [PubMed] [Google Scholar]
- 36.Karu N, Reifen R, Kerem Z. Weight gain reduction in mice fed Panax ginseng saponin, a pancreatic lipase inhibitor. J Agric Food Chem. 2007;55:2824–2828. doi: 10.1021/jf0628025. [DOI] [PubMed] [Google Scholar]
- 37.Kim JH, Hahm DH, Yang DC, Kim JH, Lee HJ, Shim I. Effect of crude saponin of Korean red ginseng on high-fat diet-induced obesity in the rat. J Pharmacol Sci. 2005;97:124–131. doi: 10.1254/jphs.fp0040184. [DOI] [PubMed] [Google Scholar]
- 38.Yoon M, Lee H, Jeong S, Kim JJ, Nicol CJ, Nam KW, Kim M, Cho BG, Oh GT. Peroxisome proliferator-activated receptor alpha is involved in the regulation of lipid metabolism by ginseng. Br J Pharmacol. 2003;138:1295–1302. doi: 10.1038/sj.bjp.0705169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kimura M, Waki I, Chujo T, Kikuchi T, Hiyama C, Yamazaki K, Tanaka O. Effects of hypoglycemic components in ginseng radix on blood insulin level in alloxan diabetic mice and on insulin release from perfused rat pancreas. J Pharmacobiodyn. 1981;4:410–417. doi: 10.1248/bpb1978.4.410. [DOI] [PubMed] [Google Scholar]
- 40.Lee WK, Kao ST, Liu IM, Cheng JT. Increase of insulin secretion by ginsenoside Rh2 to lower plasma glucose in Wistar rats. Clin Exp Pharmacol Physiol. 2006;33:27–32. doi: 10.1111/j.1440-1681.2006.04319.x. [DOI] [PubMed] [Google Scholar]
- 41.Waki I, Kyo H, Yasuda M, Kimura M. Effects of a hypoglycemic component of ginseng radix on insulin biosynthesis in normal and diabetic animals. J Pharmacobiodyn. 1982;5:547–554. doi: 10.1248/bpb1978.5.547. [DOI] [PubMed] [Google Scholar]
- 42.Li Q. Effect of hyperglycemia on insulin release from isolated rat pancreatic islets. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 1993;15:187–192. [PubMed] [Google Scholar]
- 43.Su CF, Cheng JT, Liu IM. Increase of acetylcholine release by Panax ginseng root enhances insulin secretion in Wistar rats. Neurosci Lett. 2007;412:101–104. doi: 10.1016/j.neulet.2006.10.044. [DOI] [PubMed] [Google Scholar]
- 44.Luo JZ, Luo L. American Ginseng Stimulates Insulin Production and Prevents Apoptosis through Regulation of Uncoupling Protein-2 in Cultured beta Cells. Evid Based Complement Alternat Med. 2006;3:365–372. doi: 10.1093/ecam/nel026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim HY, Kim K. Protective effect of ginseng on cytokine-induced apoptosis in pancreatic beta-cells. J Agric Food Chem. 2007;55:2816–2823. doi: 10.1021/jf062577r. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Z, Li X, Lv W, Yang Y, Gao H, Yang J, Shen Y, Ning G. Ginsenoside Re Reduces Insulin Resistance through Inhibition of JNK and NF-κB. Mol Endocrinol. 2007 doi: 10.1210/me.2007-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ye J. Role of insulin in the pathogenesis of free fatty acid-induced insulin resistance in skeletal muscle. Endocr Metab Immune Disord Drug Targets. 2007;7:65–74. doi: 10.2174/187153007780059423. [DOI] [PubMed] [Google Scholar]
- 48.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 49.Huang lian. In: Chinese Materia Medica Dictionary. 1. School JNM, editor. Shanghai: Shanghai Scientific & Technical Publishers; 1986. pp. 2022–2030. [Google Scholar]
- 50.Berberine Bureau CMMICoNMM. Handbook of Effective Compositions in Plants. 1. Beijing: People’s Medical Publishing House; 1991. pp. 12–18. [Google Scholar]
- 51.Yin J, Xing H, Ye J. Efficacy of berberine in patients with type 2 diabetes. Metabolism. 2008;57 doi: 10.1016/j.metabol.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kong W, Wei J, Abidi P, Lin M, Inaba S, Li C, Wang Y, Wang Z, Si S, Pan H, Wang S, Wu J, Wang Y, Li Z, Liu J, Jiang JD. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004;10:1344–1351. doi: 10.1038/nm1135. [DOI] [PubMed] [Google Scholar]
- 53.Yin J, Gao Z, Liu D, Liu Z, Ye J. Berberine improves glucose metabolism through induction of glycolysis. Am J Physiol Endocrinol Metab. 2008;294:E148–156. doi: 10.1152/ajpendo.00211.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ, Shen Y, Ye JM, Lee CH, Oh WK, Kim CT, Hohnen-Behrens C, Gosby A, Kraegen EW, James DE, Kim JB. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 2006;55:2256–2264. doi: 10.2337/db06-0006. [DOI] [PubMed] [Google Scholar]
- 55.Yin J, Chen M, Tang J, Li F, Zhou L, Yang Y, Chen J. Effects of berberine on glucose and lipid metabolism in animal experiment. Chinese Journal of Diabetes. 2004;12:215–218. [Google Scholar]
- 56.Leng SH, Lu FE, Xu LJ. Therapeutic effects of berberine in impaired glucose tolerance rats and its influence on insulin secretion. Acta Pharmacol Sin. 2004;25:496–502. [PubMed] [Google Scholar]
- 57.Tang LQ, Wei W, Chen LM, Liu S. Effects of berberine on diabetes induced by alloxan and a high-fat/high-cholesterol diet in rats. J Ethnopharmacol. 2006 doi: 10.1016/j.jep.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 58.Yin J, Chen M, Yang Y, Tang J, Li F. Effects of berberine on lipid metabolism in rats. Acta Universitatis Medicinalis Secondae Shanghai. 2003;23:28–30. [Google Scholar]
- 59.Cicero AF, Rovati LC, Setnikar I. Eulipidemic effects of berberine administered alone or in combination with other natural cholesterol-lowering agents. A single-blind clinical investigation. Arzneimittelforschung. 2007;57:26–30. doi: 10.1055/s-0031-1296582. [DOI] [PubMed] [Google Scholar]
- 60.Brusq JM, Ancellin N, Grondin P, Guillard R, Martin S, Saintillan Y, Issandou M. Inhibition of lipid synthesis through activation of AMP-kinase: An additional mechanism for the hypolipidemic effects of Berberine. J Lipid Res. 2006 doi: 10.1194/jlr.M600020-JLR200. [DOI] [PubMed] [Google Scholar]
- 61.Yin J, Hu R, Chen M, Tang J, Li F, Yang Y, Chen J. Effects of berberine on glucose metabolism in vitro. Metabolism. 2002;51:1439–1443. doi: 10.1053/meta.2002.34715. [DOI] [PubMed] [Google Scholar]
- 62.Cheng Z, Pang T, Gu M, Gao AH, Xie CM, Li JY, Nan FJ, Li J. Berberine-stimulated glucose uptake in L6 myotubes involves both AMPK and p38 MAPK. Biochim Biophys Acta. 2006;1760:1682–1689. doi: 10.1016/j.bbagen.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 63.Kim SH, Shin EJ, Kim ED, Bayaraa T, Frost SC, Hyun CK. Berberine Activates GLUT1-Mediated Glucose Uptake in 3T3-L1 Adipocytes. Biol Pharm Bull. 2007;30:2120–2125. doi: 10.1248/bpb.30.2120. [DOI] [PubMed] [Google Scholar]
- 64.Zhou L, Yang Y, Wang X, Liu S, Shang W, Yuan G, Li F, Tang J, Chen M, Chen J. Berberine stimulates glucose transport through a mechanism distinct from insulin. Metabolism. 2007;56:405–412. doi: 10.1016/j.metabol.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 65.Wang SH, Wang WJ, Wang XF, Chen W. Effect of Astragalus polysaccharides and berberine on carbohydrate metabolism and cell differentiation in 3T3-L1 adipocytes. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2004;24:926–928. [PubMed] [Google Scholar]
- 66.Ko BS, Choi SB, Park SK, Jang JS, Kim YE, Park S. Insulin sensitizing and insulinotropic action of berberine from Cortidis rhizoma. Biol Pharm Bull. 2005;28:1431–1437. doi: 10.1248/bpb.28.1431. [DOI] [PubMed] [Google Scholar]
- 67.Hardie DG. AMP-activated protein kinase: a master switch in glucose and lipid metabolism. Rev Endocr Metab Disord. 2004;5:119–125. doi: 10.1023/B:REMD.0000021433.63915.bb. [DOI] [PubMed] [Google Scholar]
- 68.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 69.Mikes V, Dadak V. Berberine derivatives as cationic fluorescent probes for the investigation of the energized state of mitochondria. Biochim Biophys Acta. 1983;723:231–239. doi: 10.1016/0005-2728(83)90122-6. [DOI] [PubMed] [Google Scholar]
- 70.Mikes V, Yaguzhinskij LS. Interaction of fluorescent berberine alkyl derivatives with respiratory chain of rat liver mitochondria. J Bioenerg Biomembr. 1985;17:23–32. doi: 10.1007/BF00744986. [DOI] [PubMed] [Google Scholar]
- 71.Barreto MC, Pinto RE, Arrabaca JD, Pavao ML. Inhibition of mouse liver respiration by Chelidonium majus isoquinoline alkaloids. Toxicol Lett. 2003;146:37–47. doi: 10.1016/j.toxlet.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 72.Kong LD, Cheng CH, Tan RX. Monoamine oxidase inhibitors from rhizoma of Coptis chinensis. Planta Med. 2001;67:74–76. doi: 10.1055/s-2001-10874. [DOI] [PubMed] [Google Scholar]
- 73.Iagodina OV, Nikol’skaia EB, Faddeeva MD. Inhibition of liver mitochondrial monoamine oxidase activity by alkaloids isolated from Chelidonium and Macleaya and by their derivative drugs. Tsitologiia. 2003;45:1032–1037. [PubMed] [Google Scholar]
- 74.Castillo J, Hung J, Rodriguez M, Bastidas E, Laboren I, Jaimes A. LED fluorescence spectroscopy for direct determination of monoamine oxidase B inactivation. Anal Biochem. 2005;343:293–298. doi: 10.1016/j.ab.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 75.Pospisilik JA, Knauf C, Joza N, Benit P, Orthofer M, Cani PD, Ebersberger I, Nakashima T, Sarao R, Neely G, Esterbauer H, Kozlov A, Kahn CR, Kroemer G, Rustin P, Burcelin R, Penninger JM. Targeted Deletion of AIF Decreases Mitochondrial Oxidative Phosphorylation and Protects from Obesity and Diabetes. Cell. 2007;131:476–491. doi: 10.1016/j.cell.2007.08.047. [DOI] [PubMed] [Google Scholar]
- 76.Pan GY, Wang GJ, Sun JG, Huang ZJ, Zhao XC, Gu Y, Liu XD. Inhibitory action of berberine on glucose absorption. Yao Xue Xue Bao. 2003;38:911–914. [PubMed] [Google Scholar]
- 77.Pan GY, Huang ZJ, Wang GJ, Fawcett JP, Liu XD, Zhao XC, Sun JG, Xie YY. The antihyperglycaemic activity of berberine arises from a decrease of glucose absorption. Planta Med. 2003;69:632–636. doi: 10.1055/s-2003-41121. [DOI] [PubMed] [Google Scholar]
- 78.Huang C, Zhang Y, Gong Z, Sheng X, Li Z, Zhang W, Qin Y. Berberine inhibits 3T3-L1 adipocyte differentiation through the PPARgamma pathway. Biochem Biophys Res Commun. 2006;348:571–578. doi: 10.1016/j.bbrc.2006.07.095. [DOI] [PubMed] [Google Scholar]
- 79.Choi BH, Ahn IS, Kim YH, Park JW, Lee SY, Hyun CK, Do MS. Berberine reduces the expression of adipogenic enzymes and inflammatory molecules of 3T3-L1 adipocyte. Exp Mol Med. 2006;38:599–605. doi: 10.1038/emm.2006.71. [DOI] [PubMed] [Google Scholar]
- 80.Zhou LB, Chen MD, Wang X, Song HD, Yang Y, Tang JF, Li FY, Xu MY, Chen JL. Effect of berberine on the differentiation of adipocyte. Zhonghua Yi Xue Za Zhi. 2003;83:338–340. [PubMed] [Google Scholar]
- 81.Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 82.Abidi P, Zhou Y, Jiang JD, Liu J. Extracellular signal-regulated kinase-dependent stabilization of hepatic low-density lipoprotein receptor mRNA by herbal medicine berberine. Arterioscler Thromb Vasc Biol. 2005;25:2170–2176. doi: 10.1161/01.ATV.0000181761.16341.2b. [DOI] [PubMed] [Google Scholar]
- 83.Lee S, Lim HJ, Park HY, Lee KS, Park JH, Jang Y. Berberine inhibits rat vascular smooth muscle cell proliferation and migration in vitro and improves neointima formation after balloon injury in vivo. Berberine improves neointima formation in a rat model. Atherosclerosis. 2006;186:29–37. doi: 10.1016/j.atherosclerosis.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 84.Liang KW, Ting CT, Yin SC, Chen YT, Lin SJ, Liao JK, Hsu SL. Berberine suppresses MEK/ERK-dependent Egr-1 signaling pathway and inhibits vascular smooth muscle cell regrowth after in vitro mechanical injury. Biochem Pharmacol. 2006;71:806–817. doi: 10.1016/j.bcp.2005.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee S, Lim HJ, Park JH, Lee KS, Jang Y, Park HY. Berberine-induced LDLR up-regulation involves JNK pathway. Biochem Biophys Res Commun. 2007;362:853–857. doi: 10.1016/j.bbrc.2007.08.060. [DOI] [PubMed] [Google Scholar]
- 86.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 87.Ku gua. In: Chinese Materia Medica Dictionary. 1. School JNM, editor. Shanghai: Shanghai Scientific & Technical Publishers; 1986. p. 1281. [Google Scholar]
- 88.Khanna P, Jain SC, Panagariya A, Dixit VP. Hypoglycemic activity of polypeptide-p from a plant source. J Nat Prod. 1981;44:648–655. doi: 10.1021/np50018a002. [DOI] [PubMed] [Google Scholar]
- 89.Day C, Cartwright T, Provost J, Bailey CJ. Hypoglycaemic effect of Momordica charantia extracts. Planta Med. 1990;56:426–429. doi: 10.1055/s-2006-961003. [DOI] [PubMed] [Google Scholar]
- 90.Abd El Sattar El Batran S, El-Gengaihi SE, El Shabrawy OA. Some toxicological studies of Momordica charantia L. on albino rats in normal and alloxan diabetic rats. J Ethnopharmacol. 2006;108:236–242. doi: 10.1016/j.jep.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 91.Rathi SS, Grover JK, Vikrant V, Biswas NR. Prevention of experimental diabetic cataract by Indian Ayurvedic plant extracts. Phytother Res. 2002;16:774–777. doi: 10.1002/ptr.1064. [DOI] [PubMed] [Google Scholar]
- 92.Miura T, Itoh C, Iwamoto N, Kato M, Kawai M, Park SR, Suzuki I. Hypoglycemic activity of the fruit of the Momordica charantia in type 2 diabetic mice. J Nutr Sci Vitaminol (Tokyo) 2001;47:340–344. doi: 10.3177/jnsv.47.340. [DOI] [PubMed] [Google Scholar]
- 93.Miura T, Itoh Y, Iwamoto N, Kato M, Ishida T. Suppressive activity of the fruit of Momordica charantia with exercise on blood glucose in type 2 diabetic mice. Biol Pharm Bull. 2004;27:248–250. doi: 10.1248/bpb.27.248. [DOI] [PubMed] [Google Scholar]
- 94.Srivastava Y, Venkatakrishna-Bhatt H, Verma Y. Effect of Momordica charantia Linn. pomous aqueous extract on cataractogenesis in murrin alloxan diabetics. Pharmacol Res Commun. 1988;20:201–209. doi: 10.1016/s0031-6989(88)80041-9. [DOI] [PubMed] [Google Scholar]
- 95.Singh N, Tyagi SD, Agarwal SC. Effects of long term feeding of acetone extract of Momordica charantia (whole fruit powder) on alloxan diabetic albino rats. Indian J Physiol Pharmacol. 1989;33:97–100. [PubMed] [Google Scholar]
- 96.Shibib BA, Khan LA, Rahman R. Hypoglycaemic activity of Coccinia indica and Momordica charantia in diabetic rats: depression of the hepatic gluconeogenic enzymes glucose-6-phosphatase and fructose-1,6-bisphosphatase and elevation of both liver and red-cell shunt enzyme glucose-6-phosphate dehydrogenase. Biochem J. 1993;292(Pt 1):267–270. doi: 10.1042/bj2920267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Virdi J, Sivakami S, Shahani S, Suthar AC, Banavalikar MM, Biyani MK. Antihyperglycemic effects of three extracts from Momordica charantia. J Ethnopharmacol. 2003;88:107–111. doi: 10.1016/s0378-8741(03)00184-3. [DOI] [PubMed] [Google Scholar]
- 98.Sarkar S, Pranava M, Marita R. Demonstration of the hypoglycemic action of Momordica charantia in a validated animal model of diabetes. Pharmacol Res. 1996;33:1–4. doi: 10.1006/phrs.1996.0001. [DOI] [PubMed] [Google Scholar]
- 99.Ali L, Khan AK, Mamun MI, Mosihuzzaman M, Nahar N, Nur-e-Alam M, Rokeya B. Studies on hypoglycemic effects of fruit pulp, seed, and whole plant of Momordica charantia on normal and diabetic model rats. Planta Med. 1993;59:408–412. doi: 10.1055/s-2006-959720. [DOI] [PubMed] [Google Scholar]
- 100.Karunanayake EH, Jeevathayaparan S, Tennekoon KH. Effect of Momordica charantia fruit juice on streptozotocin-induced diabetes in rats. J Ethnopharmacol. 1990;30:199–204. doi: 10.1016/0378-8741(90)90008-h. [DOI] [PubMed] [Google Scholar]
- 101.Platel K, Srinivasan K. Effect of dietary intake of freeze dried bitter gourd (Momordica charantia) in streptozotocin induced diabetic rats. Nahrung. 1995;39:262–268. doi: 10.1002/food.19950390403. [DOI] [PubMed] [Google Scholar]
- 102.Chaturvedi P, George S, Milinganyo M, Tripathi YB. Effect of Momordica charantia on lipid profile and oral glucose tolerance in diabetic rats. Phytother Res. 2004;18:954–956. doi: 10.1002/ptr.1589. [DOI] [PubMed] [Google Scholar]
- 103.Fernandes NP, Lagishetty CV, Panda VS, Naik SR. An experimental evaluation of the antidiabetic and antilipidemic properties of a standardized Momordica charantia fruit extract. BMC Complement Altern Med. 2007;7:29. doi: 10.1186/1472-6882-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ahmed I, Lakhani MS, Gillett M, John A, Raza H. Hypotriglyceridemic and hypocholesterolemic effects of anti-diabetic Momordica charantia (karela) fruit extract in streptozotocin-induced diabetic rats. Diabetes Res Clin Pract. 2001;51:155–161. doi: 10.1016/s0168-8227(00)00224-2. [DOI] [PubMed] [Google Scholar]
- 105.Yadav UC, Moorthy K, Baquer NZ. Combined treatment of sodium orthovanadate and Momordica charantia fruit extract prevents alterations in lipid profile and lipogenic enzymes in alloxan diabetic rats. Mol Cell Biochem. 2005;268:111–120. doi: 10.1007/s11010-005-3703-y. [DOI] [PubMed] [Google Scholar]
- 106.Chaturvedi P. Role of Momordica charantia in maintaining the normal levels of lipids and glucose in diabetic rats fed a high-fat and low-carbohydrate diet. Br J Biomed Sci. 2005;62:124–126. doi: 10.1080/09674845.2005.11732698. [DOI] [PubMed] [Google Scholar]
- 107.Raza H, Ahmed I, John A. Tissue specific expression and immunohistochemical localization of glutathione S-transferase in streptozotocin induced diabetic rats: modulation by Momordica charantia (karela) extract. Life Sci. 2004;74:1503–1511. doi: 10.1016/j.lfs.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 108.Sathishsekar D, Subramanian S. Beneficial effects of Momordica charantia seeds in the treatment of STZ-induced diabetes in experimental rats. Biol Pharm Bull. 2005;28:978–983. doi: 10.1248/bpb.28.978. [DOI] [PubMed] [Google Scholar]
- 109.Sitasawad SL, Shewade Y, Bhonde R. Role of bittergourd fruit juice in stz-induced diabetic state in vivo and in vitro. J Ethnopharmacol. 2000;73:71–79. doi: 10.1016/s0378-8741(00)00282-8. [DOI] [PubMed] [Google Scholar]
- 110.Ahmed I, Adeghate E, Sharma AK, Pallot DJ, Singh J. Effects of Momordica charantia fruit juice on islet morphology in the pancreas of the streptozotocin-diabetic rat. Diabetes Res Clin Pract. 1998;40:145–151. doi: 10.1016/s0168-8227(98)00022-9. [DOI] [PubMed] [Google Scholar]
- 111.Cummings E, Hundal HS, Wackerhage H, Hope M, Belle M, Adeghate E, Singh J. Momordica charantia fruit juice stimulates glucose and amino acid uptakes in L6 myotubes. Mol Cell Biochem. 2004;261:99–104. doi: 10.1023/b:mcbi.0000028743.75669.ab. [DOI] [PubMed] [Google Scholar]
- 112.Ahmed I, Adeghate E, Cummings E, Sharma AK, Singh J. Beneficial effects and mechanism of action of Momordica charantia juice in the treatment of streptozotocin-induced diabetes mellitus in rat. Mol Cell Biochem. 2004;261:63–70. doi: 10.1023/b:mcbi.0000028738.95518.90. [DOI] [PubMed] [Google Scholar]
- 113.Kumar Shetty A, Suresh Kumar G, Veerayya Salimath P. Bitter gourd (Momordica charantia) modulates activities of intestinal and renal disaccharidases in streptozotocin-induced diabetic rats. Mol Nutr Food Res. 2005;49:791–796. doi: 10.1002/mnfr.200500035. [DOI] [PubMed] [Google Scholar]
- 114.Ahmad N, Hassan MR, Halder H, Bennoor KS. Effect of Momordica charantia (Karolla) extracts on fasting and postprandial serum glucose levels in NIDDM patients. Bangladesh Med Res Counc Bull. 1999;25:11–13. [PubMed] [Google Scholar]
- 115.Welihinda J, Karunanayake EH, Sheriff MH, Jayasinghe KS. Effect of Momordica charantia on the glucose tolerance in maturity onset diabetes. J Ethnopharmacol. 1986;17:277–282. doi: 10.1016/0378-8741(86)90116-9. [DOI] [PubMed] [Google Scholar]
- 116.Tongia A, Tongia SK, Dave M. Phytochemical determination and extraction of Momordica charantia fruit and its hypoglycemic potentiation of oral hypoglycemic drugs in diabetes mellitus (NIDDM) Indian J Physiol Pharmacol. 2004;48:241–244. [PubMed] [Google Scholar]
- 117.Dans AM, Villarruz MV, Jimeno CA, Javelosa MA, Chua J, Bautista R, Velez GG. The effect of Momordica charantia capsule preparation on glycemic control in type 2 diabetes mellitus needs further studies. J Clin Epidemiol. 2007;60:554–559. doi: 10.1016/j.jclinepi.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 118.Okyar A, Can A, Akev N, Baktir G, Sutlupinar N. Effect of Aloe vera leaves on blood glucose level in type I and type II diabetic rat models. Phytother Res. 2001;15:157–161. doi: 10.1002/ptr.719. [DOI] [PubMed] [Google Scholar]
- 119.Rajasekaran S, Sivagnanam K, Subramanian S. Modulatory effects of Aloe vera leaf gel extract on oxidative stress in rats treated with streptozotocin. J Pharm Pharmacol. 2005;57:241–246. doi: 10.1211/0022357055416. [DOI] [PubMed] [Google Scholar]
- 120.Rajasekaran S, Ravi K, Sivagnanam K, Subramanian S. Beneficial effects of aloe vera leaf gel extract on lipid profile status in rats with streptozotocin diabetes. Clin Exp Pharmacol Physiol. 2006;33:232–237. doi: 10.1111/j.1440-1681.2006.04351.x. [DOI] [PubMed] [Google Scholar]
- 121.Kannappan S, Jayaraman T, Rajasekar P, Ravichandran MK, Anuradha CV. Cinnamon bark extract improves glucose metabolism and lipid profile in the fructose-fed rat. Singapore Med J. 2006;47:858–863. [PubMed] [Google Scholar]
- 122.Mang B, Wolters M, Schmitt B, Kelb K, Lichtinghagen R, Stichtenoth DO, Hahn A. Effects of a cinnamon extract on plasma glucose, HbA, and serum lipids in diabetes mellitus type 2. Eur J Clin Invest. 2006;36:340–344. doi: 10.1111/j.1365-2362.2006.01629.x. [DOI] [PubMed] [Google Scholar]
- 123.Kim SH, Hyun SH, Choung SY. Anti-diabetic effect of cinnamon extract on blood glucose in db/db mice. J Ethnopharmacol. 2006;104:119–123. doi: 10.1016/j.jep.2005.08.059. [DOI] [PubMed] [Google Scholar]
- 124.Qin B, Nagasaki M, Ren M, Bajotto G, Oshida Y, Sato Y. Cinnamon extract prevents the insulin resistance induced by a high-fructose diet. Horm Metab Res. 2004;36:119–125. doi: 10.1055/s-2004-814223. [DOI] [PubMed] [Google Scholar]
- 125.Qin B, Nagasaki M, Ren M, Bajotto G, Oshida Y, Sato Y. Cinnamon extract (traditional herb) potentiates in vivo insulin-regulated glucose utilization via enhancing insulin signaling in rats. Diabetes Res Clin Pract. 2003;62:139–148. doi: 10.1016/s0168-8227(03)00173-6. [DOI] [PubMed] [Google Scholar]
- 126.Shanthi S, Parasakthy K, Deepalakshmi PD, Devaraj SN. Hypolipidemic activity of tincture of Crataegus in rats. Indian J Biochem Biophys. 1994;31:143–146. [PubMed] [Google Scholar]
- 127.Jouad H, Lemhadri A, Maghrani M, Burcelin R, Eddouks M. Hawthorn evokes a potent anti-hyperglycemic capacity in streptozotocin-induced diabetic rats. J Herb Pharmacother. 2003;3:19–29. [PubMed] [Google Scholar]
- 128.Jayaprakasam B, Olson LK, Schutzki RE, Tai MH, Nair MG. Amelioration of obesity and glucose intolerance in high-fat-fed C57BL/6 mice by anthocyanins and ursolic acid in Cornelian cherry (Cornus mas) J Agric Food Chem. 2006;54:243–248. doi: 10.1021/jf0520342. [DOI] [PubMed] [Google Scholar]
- 129.Qian DS, Zhu YF, Zhu Q. Effect of alcohol extract of Cornus officinalis Sieb et Zucc on GLUT4 expression in skeletal muscle in type 2 (non-insulin-dependent) diabetic mellitus rats. Zhongguo Zhong Yao Za Zhi. 2001;26:859–862. [PubMed] [Google Scholar]
- 130.Hsu JH, Wu YC, Liu IM, Cheng JT. Release of acetylcholine to raise insulin secretion in Wistar rats by oleanolic acid, one of the active principles contained in Cornus officinalis. Neurosci Lett. 2006;404:112–116. doi: 10.1016/j.neulet.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 131.Hao Z, Hang B, Wang Y. Hypoglycemic effect of fructus Ligustri Lucidi. Zhongguo Zhong Yao Za Zhi. 1992;17:429–5431. 447. [PubMed] [Google Scholar]
- 132.Li XM. Protective effect of Lycium barbarum polysaccharides on streptozotocin-induced oxidative stress in rats. Int J Biol Macromol. 2007;40:461–465. doi: 10.1016/j.ijbiomac.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 133.Luo Q, Cai Y, Yan J, Sun M, Corke H. Hypoglycemic and hypolipidemic effects and antioxidant activity of fruit extracts from Lycium barbarum. Life Sci. 2004;76:137–149. doi: 10.1016/j.lfs.2004.04.056. [DOI] [PubMed] [Google Scholar]
- 134.Xu M, Zhang H, Wang Y. The protective effects of Lycium barbarum polysaccharide on alloxan-induced isolated islet cells damage in rats. Zhong Yao Cai. 2002;25:649–651. [PubMed] [Google Scholar]
- 135.Chen WQ, Luo SH, Ll HZ, Yang H. Effects of ganoderma lucidum polysaccharides on serum lipids and lipoperoxidation in experimental hyperlipidemic rats. Zhongguo Zhong Yao Za Zhi. 2005;30:1358–1360. [PubMed] [Google Scholar]
- 136.Berger A, Rein D, Kratky E, Monnard I, Hajjaj H, Meirim I, Piguet-Welsch C, Hauser J, Mace K, Niederberger P. Cholesterol-lowering properties of Ganoderma lucidum in vitro, ex vivo, and in hamsters and minipigs. Lipids Health Dis. 2004;3:2. doi: 10.1186/1476-511X-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang HN, He JH, Yuan L, Lin ZB. In vitro and in vivo protective effect of Ganoderma lucidum polysaccharides on alloxan-induced pancreatic islets damage. Life Sci. 2003;73:2307–2319. doi: 10.1016/s0024-3205(03)00594-0. [DOI] [PubMed] [Google Scholar]
- 138.He CY, Li WD, Guo SX, Lin SQ, Lin ZB. Effect of polysaccharides from Ganoderma lucidum on streptozotocin-induced diabetic nephropathy in mice. J Asian Nat Prod Res. 2006;8:705–711. doi: 10.1080/10286020500289071. [DOI] [PubMed] [Google Scholar]
- 139.Zhang HN, Lin ZB. Hypoglycemic effect of Ganoderma lucidum polysaccharides. Acta Pharmacol Sin. 2004;25:191–195. [PubMed] [Google Scholar]
- 140.Hikino H, Ishiyama M, Suzuki Y, Konno C. Mechanisms of hypoglycemic activity of ganoderan B: a glycan of Ganoderma lucidum fruit bodies. Planta Med. 1989;55:423–428. doi: 10.1055/s-2006-962057. [DOI] [PubMed] [Google Scholar]
- 141.Al-Amin ZM, Thomson M, Al-Qattan KK, Peltonen-Shalaby R, Ali M. Anti-diabetic and hypolipidaemic properties of ginger (Zingiber officinale) in streptozotocin-induced diabetic rats. Br J Nutr. 2006;96:660–666. doi: 10.1079/bjn20061849. [DOI] [PubMed] [Google Scholar]
- 142.Akhani SP, Vishwakarma SL, Goyal RK. Anti-diabetic activity of Zingiber officinale in streptozotocin-induced type I diabetic rats. J Pharm Pharmacol. 2004;56:101–105. doi: 10.1211/0022357022403. [DOI] [PubMed] [Google Scholar]
- 143.Ojewole JA. Analgesic, antiinflammatory and hypoglycaemic effects of ethanol extract of Zingiber officinale (Roscoe) rhizomes (Zingiberaceae) in mice and rats. Phytother Res. 2006;20:764–772. doi: 10.1002/ptr.1952. [DOI] [PubMed] [Google Scholar]
- 144.Bhandari U, Kanojia R, Pillai KK. Effect of ethanolic extract of Zingiber officinale on dyslipidaemia in diabetic rats. J Ethnopharmacol. 2005;97:227–230. doi: 10.1016/j.jep.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 145.Kadnur SV, Goyal RK. Beneficial effects of Zingiber officinale Roscoe on fructose induced hyperlipidemia and hyperinsulinemia in rats. Indian J Exp Biol. 2005;43:1161–1164. [PubMed] [Google Scholar]
- 146.Fuhrman B, Rosenblat M, Hayek T, Coleman R, Aviram M. Ginger extract consumption reduces plasma cholesterol, inhibits LDL oxidation and attenuates development of atherosclerosis in atherosclerotic, apolipoprotein E-deficient mice. J Nutr. 2000;130:1124–1131. doi: 10.1093/jn/130.5.1124. [DOI] [PubMed] [Google Scholar]
- 147.Liu N, Huo G, Zhang L, Zhang X. Effect of Zingiber OfficinaleRosc on lipid peroxidation in hyperlipidemia rats. Wei Sheng Yan Jiu. 2003;32:22–23. [PubMed] [Google Scholar]
- 148.Bhandari U, Sharma JN, Zafar R. The protective action of ethanolic ginger (Zingiber officinale) extract in cholesterol fed rabbits. J Ethnopharmacol. 1998;61:167–171. doi: 10.1016/s0378-8741(98)00026-9. [DOI] [PubMed] [Google Scholar]
- 149.Mae T, Kishida H, Nishiyama T, Tsukagawa M, Konishi E, Kuroda M, Mimaki Y, Sashida Y, Takahashi K, Kawada T, Nakagawa K, Kitahara M. A licorice ethanolic extract with peroxisome proliferator-activated receptor-gamma ligand-binding activity affects diabetes in KK-Ay mice, abdominal obesity in diet-induced obese C57BL mice and hypertension in spontaneously hypertensive rats. J Nutr. 2003;133:3369–3377. doi: 10.1093/jn/133.11.3369. [DOI] [PubMed] [Google Scholar]
- 150.Aoki F, Honda S, Kishida H, Kitano M, Arai N, Tanaka H, Yokota S, Nakagawa K, Asakura T, Nakai Y, Mae T. Suppression by licorice flavonoids of abdominal fat accumulation and body weight gain in high-fat diet-induced obese C57BL/6J mice. Biosci Biotechnol Biochem. 2007;71:206–214. doi: 10.1271/bbb.60463. [DOI] [PubMed] [Google Scholar]
- 151.Nakagawa K, Kishida H, Arai N, Nishiyama T, Mae T. Licorice flavonoids suppress abdominal fat accumulation and increase in blood glucose level in obese diabetic KK-A(y) mice. Biol Pharm Bull. 2004;27:1775–1778. doi: 10.1248/bpb.27.1775. [DOI] [PubMed] [Google Scholar]
- 152.Ji W, Gong BQ. Hypolipidemic effects and mechanisms of Panax notoginseng on lipid profile in hyperlipidemic rats. J Ethnopharmacol. 2007;113:318–324. doi: 10.1016/j.jep.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 153.Cicero AF, Vitale G, Savino G, Arletti R. Panax notoginseng (Burk) effects on fibrinogen and lipid plasma level in rats fed on a high-fat diet. Phytother Res. 2003;17:174–178. doi: 10.1002/ptr.1262. [DOI] [PubMed] [Google Scholar]
- 154.Gong YH, Jiang JX, Li Z, Zhu LH, Zhang ZZ. Hypoglycemic effect of sanchinoside C1 in alloxan-diabetic mice. Yao Xue Xue Bao. 1991;26:81–85. [PubMed] [Google Scholar]
- 155.Wen C, Xu H, Huang QF. Effect of drugs for promoting blood circulation on blood lipids and inflammatory reaction of atherosclerotic plaques in ApoE gene deficiency mice. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2005;25:345–349. [PubMed] [Google Scholar]
- 156.Han LK, Zheng YN, Xu BJ, Okuda H, Kimura Y. Saponins from platycodi radix ameliorate high fat diet-induced obesity in mice. J Nutr. 2002;132:2241–2245. doi: 10.1093/jn/132.8.2241. [DOI] [PubMed] [Google Scholar]
- 157.Han LK, Xu BJ, Kimura Y, Zheng Y, Okuda H. Platycodi radix affects lipid metabolism in mice with high fat diet-induced obesity. J Nutr. 2000;130:2760–2764. doi: 10.1093/jn/130.11.2760. [DOI] [PubMed] [Google Scholar]
- 158.Zhao HL, Sim JS, Shim SH, Ha YW, Kang SS, Kim YS. Antiobese and hypolipidemic effects of platycodin saponins in diet-induced obese rats: evidences for lipase inhibition and calorie intake restriction. Int J Obes (Lond) 2005;29:983–990. doi: 10.1038/sj.ijo.0802948. [DOI] [PubMed] [Google Scholar]
- 159.Choi H, Eo H, Park K, Jin M, Park EJ, Kim SH, Park JE, Kim S. A water-soluble extract from Cucurbita moschata shows anti-obesity effects by controlling lipid metabolism in a high fat diet-induced obesity mouse model. Biochem Biophys Res Commun. 2007;359:419–425. doi: 10.1016/j.bbrc.2007.05.107. [DOI] [PubMed] [Google Scholar]
- 160.Quanhong L, Caili F, Yukui R, Guanghui H, Tongyi C. Effects of protein-bound polysaccharide isolated from pumpkin on insulin in diabetic rats. Plant Foods Hum Nutr. 2005;60:13–16. doi: 10.1007/s11130-005-2536-x. [DOI] [PubMed] [Google Scholar]
- 161.Xie JT, Wang CZ, Wang AB, Wu J, Basila D, Yuan CS. Antihyperglycemic effects of total ginsenosides from leaves and stem of Panax ginseng. Acta Pharmacol Sin. 2005;26:1104–1110. doi: 10.1111/j.1745-7254.2005.00156.x. [DOI] [PubMed] [Google Scholar]
- 162.Lu J, Zou D, Zhang J. Preventive effect of radix Astragali on insulin resistance caused by tumor necrosis factor-alpha. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1999;19:420–422. [PubMed] [Google Scholar]
- 163.Li RJ, Qiu SD, Chen HX, Tian H, Wang HX. The immunotherapeutic effects of Astragalus polysaccharide in type 1 diabetic mice. Biol Pharm Bull. 2007;30:470–476. doi: 10.1248/bpb.30.470. [DOI] [PubMed] [Google Scholar]
- 164.Wu Y, Cao Y, Shi X, Shi Y. Inhibitory effect of radix et rhizoma Rhei on the production of lipid peroxides (LPO) in mice liver. Zhongguo Zhong Yao Za Zhi. 1996;21:240–242. inside backcover. [PubMed] [Google Scholar]
- 165.Choi SB, Ko BS, Park SK, Jang JS, Park S. Insulin sensitizing and alpha-glucoamylase inhibitory action of sennosides, rheins and rhaponticin in Rhei Rhizoma. Life Sci. 2006;78:934–942. doi: 10.1016/j.lfs.2005.05.101. [DOI] [PubMed] [Google Scholar]
- 166.Zhang SP, Fang WJ, Lu JH, Tan HR, Pan JQ. The experimental study of Radix Puerariae inhibiting glycation in rats induced by D-galactose. Zhong Yao Cai. 2006;29:266–269. [PubMed] [Google Scholar]
- 167.Kang KA, Chae S, Koh YS, Kim JS, Lee JH, You HJ, Hyun JW. Protective effect of puerariae radix on oxidative stress induced by hydrogen peroxide and streptozotocin. Biol Pharm Bull. 2005;28:1154–1160. doi: 10.1248/bpb.28.1154. [DOI] [PubMed] [Google Scholar]
- 168.Xiong FL, Sun XH, Gan L, Yang XL, Xu HB. Puerarin protects rat pancreatic islets from damage by hydrogen peroxide. Eur J Pharmacol. 2006;529:1–7. doi: 10.1016/j.ejphar.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 169.Song CY, Bi HM. Effects of puerarin on plasma membrane GLUT4 content in skeletal muscle from insulin-resistant Sprague-Dawley rats under insulin stimulation. Zhongguo Zhong Yao Za Zhi. 2004;29:172–175. [PubMed] [Google Scholar]
- 170.Chen WC, Hayakawa S, Yamamoto T, Su HC, Liu IM, Cheng JT. Mediation of beta-endorphin by the isoflavone puerarin to lower plasma glucose in streptozotocin-induced diabetic rats. Planta Med. 2004;70:113–116. doi: 10.1055/s-2004-815486. [DOI] [PubMed] [Google Scholar]
- 171.Hsu FL, Liu IM, Kuo DH, Chen WC, Su HC, Cheng JT. Antihyperglycemic effect of puerarin in streptozotocin-induced diabetic rats. J Nat Prod. 2003;66:788–792. doi: 10.1021/np0203887. [DOI] [PubMed] [Google Scholar]
- 172.Wang JF, Guo YX, Niu JZ, Liu J, Wang LQ, Li PH. Effects of Radix Puerariae flavones on liver lipid metabolism in ovariectomized rats. World J Gastroenterol. 2004;10:1967–1970. doi: 10.3748/wjg.v10.i13.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yokozawa T, Kim HY, Yamabe N. Amelioration of diabetic nephropathy by dried Rehmanniae Radix (Di Huang) extract. Am J Chin Med. 2004;32:829–839. doi: 10.1142/S0192415X04002442. [DOI] [PubMed] [Google Scholar]
- 174.Kuang P, Tao Y, Tian Y. Radix Salviae miltiorrhizae treatment results in decreased lipid peroxidation in reperfusion injury. J Tradit Chin Med. 1996;16:138–142. [PubMed] [Google Scholar]
- 175.Li S, Wan L. Experimental study on the preventive mechanism of salviae miltiorrhizae against atherosclerosis in rabbits models. J Huazhong Univ Sci Technolog Med Sci. 2004;24:233–235. doi: 10.1007/BF02831998. [DOI] [PubMed] [Google Scholar]
- 176.Miura T, Kato A. The difference in hypoglycemic action between polygonati rhizoma and polygonati officinalis rhizoma. Biol Pharm Bull. 1995;18:1605–1606. doi: 10.1248/bpb.18.1605. [DOI] [PubMed] [Google Scholar]
- 177.Miura T, Kato A, Usami M, Kadowaki S, Seino Y. Effect of polygonati rhizoma on blood glucose and facilitative glucose transporter isoform 2 (GLUT2) mRNA expression in Wistar fatty rats. Biol Pharm Bull. 1995;18:624–625. doi: 10.1248/bpb.18.624. [DOI] [PubMed] [Google Scholar]
- 178.Kato A, Miura T, Yano H, Masuda K, Ishida H, Seino Y. Suppressive effects of polygonati rhizoma on hepatic glucose output, GLUT2 mRNA expression and its protein content in rat liver. Endocr J. 1994;41:139–144. doi: 10.1507/endocrj.41.139. [DOI] [PubMed] [Google Scholar]
- 179.Kato A, Miura T. Hypoglycemic activity of polygonati rhizoma in normal and diabetic mice. Biol Pharm Bull. 1993;16:1118–1120. doi: 10.1248/bpb.16.1118. [DOI] [PubMed] [Google Scholar]
- 180.Chen H, Feng R, Guo Y, Sun L, Jiang J. Hypoglycemic effects of aqueous extract of Rhizoma Polygonati Odorati in mice and rats. J Ethnopharmacol. 2001;74:225–229. doi: 10.1016/s0378-8741(00)00359-7. [DOI] [PubMed] [Google Scholar]
- 181.Choi SB, Park S. A steroidal glycoside from Polygonatum odoratum (Mill) Druce improves insulin resistance but does not alter insulin secretion in 90% pancreatectomized rats. Biosci Biotechnol Biochem. 2002;66:2036–2043. doi: 10.1271/bbb.66.2036. [DOI] [PubMed] [Google Scholar]
- 182.Han LK, Sumiyoshi M, Zhang J, Liu MX, Zhang XF, Zheng YN, Okuda H, Kimura Y. Anti-obesity action of Salix matsudana leaves (Part 1). Anti-obesity action by polyphenols of Salix matsudana in high fat-diet treated rodent animals. Phytother Res. 2003;17:1188–1194. doi: 10.1002/ptr.1404. [DOI] [PubMed] [Google Scholar]
- 183.Han LK, Sumiyoshi M, Zheng YN, Okuda H, Kimura Y. Anti-obesity action of Salix matsudana leaves (Part 2). Isolation of anti-obesity effectors from polyphenol fractions of Salix matsudana. Phytother Res. 2003;17:1195–1198. doi: 10.1002/ptr.1405. [DOI] [PubMed] [Google Scholar]
- 184.Li HB, Fang KY, Lu CT, Li XE. Study on lipid-regulating function for the extracts and their prescriptions from Semen Cassiae and fructus crataegi. Zhong Yao Cai. 2007;30:573–575. [PubMed] [Google Scholar]
- 185.Junbao Y, Long J, Jiangbi W, Yonghui D, Tianzhen Z, Songyi Q, Wei L. Inhibitive effect of Semen Cassiae on the weight gain in rats with nutritive obesity. Zhong Yao Cai. 2004;27:281–284. [PubMed] [Google Scholar]
- 186.Bryans JA, Judd PA, Ellis PR. The effect of consuming instant black tea on postprandial plasma glucose and insulin concentrations in healthy humans. J Am Coll Nutr. 2007;26:471–477. doi: 10.1080/07315724.2007.10719638. [DOI] [PubMed] [Google Scholar]
- 187.Khan SA, Priyamvada S, Arivarasu NA, Khan S, Yusufi AN. Influence of green tea on enzymes of carbohydrate metabolism, antioxidant defense, and plasma membrane in rat tissues. Nutrition. 2007;23:687–695. doi: 10.1016/j.nut.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 188.Islam MS, Choi H. Green tea, anti-diabetic or diabetogenic: a dose response study. Biofactors. 2007;29:45–53. doi: 10.1002/biof.5520290105. [DOI] [PubMed] [Google Scholar]
- 189.Zhou X, Wang D, Sun P, Bucheli P, Li L, Hou Y, Wang J. Effects of soluble tea polysaccharides on hyperglycemia in alloxan-diabetic mice. J Agric Food Chem. 2007;55:5523–5528. doi: 10.1021/jf070699t. [DOI] [PubMed] [Google Scholar]
- 190.Anandh Babu PV, Sabitha KE, Shyamaladevi CS. Green tea extract impedes dyslipidaemia and development of cardiac dysfunction in streptozotocin-diabetic rats. Clin Exp Pharmacol Physiol. 2006;33:1184–1189. doi: 10.1111/j.1440-1681.2006.04509.x. [DOI] [PubMed] [Google Scholar]
- 191.Shoji Y, Nakashima H. Glucose-lowering effect of powder formulation of African black tea extract in KK-A(y)/TaJcl diabetic mouse. Arch Pharm Res. 2006;29:786–794. doi: 10.1007/BF02974080. [DOI] [PubMed] [Google Scholar]
- 192.Babu PV, Sabitha KE, Shyamaladevi CS. Therapeutic effect of green tea extract on advanced glycation and cross-linking of collagen in the aorta of streptozotocin diabetic rats. Clin Exp Pharmacol Physiol. 2006;33:351–357. doi: 10.1111/j.1440-1681.2006.04374.x. [DOI] [PubMed] [Google Scholar]
- 193.Li RW, Douglas TD, Maiyoh GK, Adeli K, Theriault AG. Green tea leaf extract improves lipid and glucose homeostasis in a fructose-fed insulin-resistant hamster model. J Ethnopharmacol. 2006;104:24–31. doi: 10.1016/j.jep.2005.08.045. [DOI] [PubMed] [Google Scholar]
- 194.Shimada K, Kawarabayashi T, Tanaka A, Fukuda D, Nakamura Y, Yoshiyama M, Takeuchi K, Sawaki T, Hosoda K, Yoshikawa J. Oolong tea increases plasma adiponectin levels and low-density lipoprotein particle size in patients with coronary artery disease. Diabetes Res Clin Pract. 2004;65:227–234. doi: 10.1016/j.diabres.2004.01.003. [DOI] [PubMed] [Google Scholar]