Abstract
We recently reported that blockade of the CD40–CD154 ligand interaction with the cross-reacting mouse anti-human CD154 antibody, 5c8, together with donor-specific transfusion led to enhanced but not completely successful engraftment in a canine model of DLA-identical marrow transplantation after 100cGy total body irradiation (TBI). In order to improve transplantation outcomes, we sought to develop a canine-specific reagent. To that end, we fused the extracellular domain of the canine CD40 with a mouse IgG2a Fc tail and tested the immunosuppressive effectiveness of the fusion protein in mixed leukocyte reactions. The extracellular domain of canine CD40 was fused with the Fc portion of mouse IgG2a in a pcDNA3.1+ vector. Dhfr-deficient CHO cells were co-transfected with the CD40-Ig vector and a dhfr-containing vector. Stable, high producing clones were selected under increasing methotrexate concentrations. The fusion protein was purified, tested in mixed leukocyte reactions, and its immunosuppressive effect compared to that of the anti-CD154 antibody 5c8. The transfected cell line produced a CD40-Ig dimer whose identity was confirmed by mass spectroscopy. The purified canine CD40-Ig blocked mixed leukocyte reactions at a concentration of 1nM, which was more than 10 times effective than the anti-CD154 antibody. Canine CD40-Ig is more immunosuppressive than the anti-human CD154 antibody 5c8 in canine mixed leukocyte reactions and may be more effective in vivo in a model of marrow transplantation.
1. Introduction
Sustained engraftment of DLA-identical marrow was consistently observed in dogs conditioned with a nonmyeloablative dose of 2 Gy total body irradiation (TBI) and given postgrafting immunosuppression with short courses cyclosporine (CSP) along with either mycophenolate mofetil (MMF) or rapamycin (Storb et al., 1997; Hogan et al., 2003). However, when TBI conditioning was decreased to 1 Gy, all dogs eventually rejected their grafts. Extended and sustained engraftment was accomplished in most but not all dogs when 1 Gy TBI was preceded by intravenous injections of both peripheral blood mononuclear cells (PBMC) from the marrow donor and the T-cell costimulatory blockers recombinant human (rh) CTLA4-Ig or cross-reacting mouse anti-human CD154 antibody 5c8 (Storb et al., 1999; Jochum et al., 2007). One possible explanation for the lack of uniform success might be reduced affinity of these cross-reacting anti-human products for canine cell surface determinants.
Therefore, we focused on developing a canine specific reagent to block the CD40–CD154 interaction. Instead of generating an anti-CD154 monoclonal antibody, we developed a canine specific fusion protein, CD40-Ig. In other similar studies, CD40-Ig has been shown to be active in vitro with human (McLellan et al., 1996) cells and in vivo in rodent models of liver (Nomura et al., 2002), heart (Guillot et al., 2002), and other organ transplantation models (Jin and Xie, 2003; Kanaya et al., 2003; Yamashita et al., 2003).
2. Materials and Methods
2.1. Experimental animals and blood cell preparations
Beagles, mini-mongrel, basenji, and golden retriever crossbreeds used for all experiments were raised at the Fred Hutchinson Cancer Research Center (Seattle, WA, USA) or purchased from commercial kennels. PBMC were isolated on Ficoll-Hypaque (density 1.074). Lymph node and tonsil cells were obtained from dogs, which were euthanized for other reasons.
2.2. Cloning of the extra cellular domain of canine CD40
Oligonucleotides were custom-made by Invitrogen (Carlsbad, CA, USA). Total RNA was isolated from the lymph node, tonsil, and thymus using TRIzol reagent (Invitrogen). cDNA was synthesized using M-MLV reverse transcriptase (Invitrogen) and oligo (dT) primer (Promega, Madison, WI, USA). The cDNA of CD40 was synthesized by RT-PCR using Platinum PCR Supermix (Invitrogen) and a forward primer (CGGGAATATTACGGGGAACT) and a reverse primer (CCACTGAATCACAAACAATGCC) based on the GenBank sequence (AY333789) of canis familiaris CD40 mRNA. The PCR product was isolated from an agarose gel using QIAquick Gel Extraction kit (Qiagen, Valencia, CA) and ligated into the pGEM-T Easy vector (Promega, Madison, WI) for sequencing. DNA sequencing was performed with an automated sequencer by PCR amplification using BigDye terminator v3.1 reagents (Applied Biosystems, Foster City, CA) and T7 and SP6 promoter primers (Promega)‥
2.3. Cloning of murine IgG2a
The cDNA of murine IgG2a was isolated from the IgG2a-secreting mouse myeloma cell line RPC5.4 (ATCC, Manassas, VA) by RT-PCR using Platinum PCR Supermix and a forward primer (TAAAGAGCCCAGAGGGCCCACAATCAA) and a reverse primer (TCATTTACCCGGAGTCCGGGAGAA) based on the GenBank sequence (V00798) of mouse gamma 2a immunoglobulin heavy chain. The PCR product was isolated and ligated into the pGEM-T Easy vector (Promega, Madison, WI) for sequencing as outlined above.
2.4. Assembly of canine CD40 murine Ig fusion vector
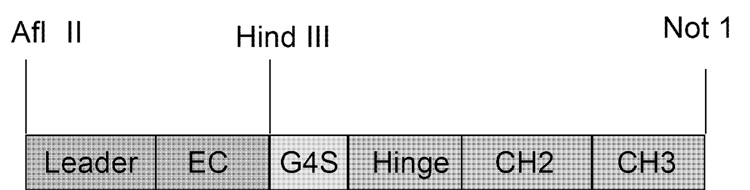
An AflII and HindIII restricted PCR product of the signal peptide and extracellular domain of CD40 was generated from CD40 cDNA using forward (CATTAGCTTAAGATGGTTCTCCTGCCTCTGCGC) and reverse (TCCGGGAAGCTT-GGCTCTTAACCGAGGCTGGGG) primers. A HindIII restriction site and a Gly4Ser linker were added at the 5′ end of the hinge region and a NotI restriction site was added at the 3′ end of the CH3 region of murine IgG2a using forward (ATAATTAAGCTTGGAGG-TGGAGGTAGTGAGCCCAGAGGGCCCACATC) and reverse (CCATTATAGCGGCCG-CTCATTTACCCGGAGTCCGGGA) primers, respectively (Figure 2). Following gel purification, PCR products were digested with the appropriate restriction enzymes and ligated into AflII and NotI digested pcDNA3.1 (+) (Invitrogen). Plasmids from DH5α (Invitrogen) transformants were sequenced with T7 forward and BGH reverse primers.
Figure 2.
Schematic diagram of CD40-Ig expression vector containing the leader and extracellular domain of canine CD40 fused to a Gly4Ser linker and the hinge through CH3 regions of murine IgG2a.
2.5. Cell culture and protein production
CHO cells deficient in the dhfr gene (CRL-9096; ATCC) were co-transfected with linearized canine CD40/murine Ig2a/pcDNA3.1 and pSV2-dhfr (ATCC) vectors using FuGENE®-6 reagent (Roche Applied Sciences, Indianapolis, IN) according to the manufacturer’s recommended protocol. Transfected cells were grown in selective medium containing 800 µg/mL G418 (Mediatech, Herndon, VA). The CD40 fusion protein was amplified by stepwise increases with methotrexate (Sigma) to a maximum of 5 µM and subjected to limiting dilution cloning after each increase.
2.6. ELISAs
Goat anti-mouse IgG2a and peroxidase-labeled goat anti-mouse IgG2a (SouthernBiotech, Birmingham, AL) were used in a sandwich ELISA as capture/detection antibodies for the quantitation of CD40 fusion protein according to established procedures. Regression analysis was performed using mouse IgG2a (SouthernBiotech) as a standard. Mouse anti-human CD40 (G28–5; ATCC) which cross-reacted with canine CD40 was used as a capture antibody to verify the presence of CD40.
2.7. Protein purification
The canine CD40-Ig was purified from serum-free culture supernatant on a protein A affinity column (Amersham Biosciences, Pittsburgh, PA) under aseptic conditions according to the manufacturer’s protocol. The protein-containing fractions were pooled and dialyzed overnight at 4°C against phosphate buffered saline and the concentration determined using the DC-protein assay (BioRad). Endotoxin levels were determined by the Limulus amoebocyte lysate assay. CD40-Ig preparations containing less than 0.05 EU of endotoxin were subsequently used.
2.8. Protein identification
Purity was determined on a Coomassie-stained SDS-PAGE gel according to the method of Laemmli (Laemmli, 1970). Western blotting with horseradish peroxidase-conjugated goat anti-mouse IgG2a (SouthernBiotech) confirmed the presence of fusion protein, and the corresponding band was cut from a Coomassie stained gel and digested with trypsin. The proteolytic peptides were subjected to tandem mass spectroscopy, and the resulting data were compared to a database of canine protein sequences.
2.9. Functional Assay
To test its immunosuppressive activity, canine CD40-Ig was added to a mixed leukocyte reaction (MLR) as described (Raff et al., 1983). Purified canine CD40-Ig was added to the medium at a concentration of 10 µg/mL or at concentrations increasing from 0.5 to 200 nM. Recombinant human (rh)CTLA4-Ig (courtesy of Richard Boismenu, Ph.D., Repligen Corporation, Waltham, MA) and the mouse anti-human CD154 antibody, 5c8, were used as positive controls, and the irrelevant mouse antibody 31A served as the negative control. The 5C8 antibody was added in doses equi-molar to the canine CD40-Ig.
2.10. Statistical analysis
Responses between canine CD40-Ig treated MLR cultures and controls were compared with a two sided paired Student‘s t-test.
3. Results
3.1. Expression, production, and purification of canine CD40-Ig
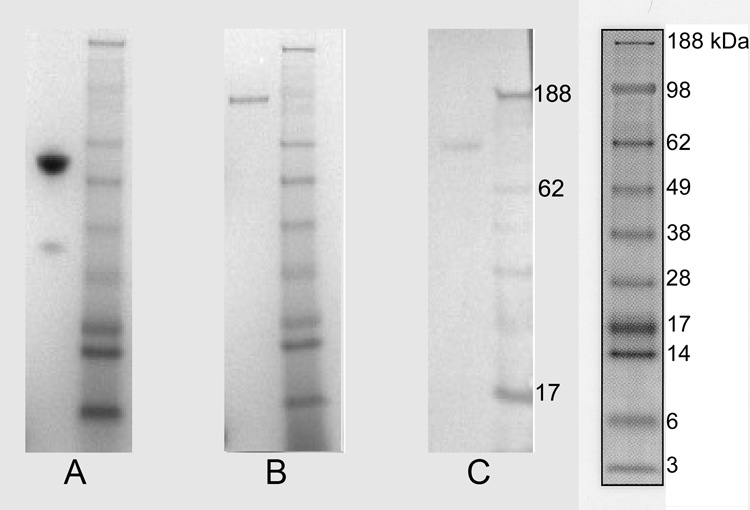
CD40-Ig was purified from CHO cell culture supernatants by Protein A affinity chromatography. The production rate for the transfected CHO cells exposed to 5 µM methotrexate was estimated at ~30 pg/cell/day. SDS-PAGE of the purified CD40-Ig in the presence of reducing agent showed a band between molecular weight marker 49000 Da and 62000 Da (Figure 3A). In the absence of a reducing agent, SDS-PAGE analysis of CD40-Ig revealed a single band at molecular weight marker 98000 Da (Figure 3B). This banding pattern indicated that CD40-Ig existed in solution as a disulfide-linked homodimer. The immunoblot with goat anti-mouse IgG2a antibody showed a reaction with this band (Figure 3C). Both bands were cut out and subjected to tandem mass spectroscopy. A comparison with the protein data bank showed 226 peptides, which matched canine CD40 and the mouse IgG2a constant region, and of these, 34 peptides were unique for these molecules. This indicated with a very high probability that the protein was canine CD40-Ig.
Figure 3.
SDS-PAGE and Immunoblot of rcCD40-Ig. rc CD40-Ig run under reducing conditions (A), and non-reducing conditions (B), and immunoblot of nonreduced rcCD40-Ig (C). Molecular weight standard “SeeBlue2” (Invitrogen) was used in all three experiments (right of each). The molecular weight marker is enlarged for technical reasons in Figure 3C. The monomer band between marker 49kDa and 62 kDa in (A) and the dimer band at marker 98kDa in (B) were proven to be canine CD40-Ig by tandem mass spectroscopy.
3.2. Effect of canine CD40-Ig on canine MLR
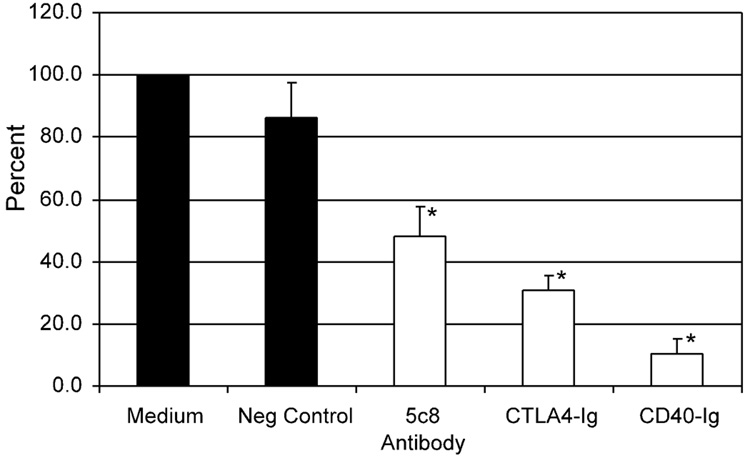
The results of MLRs using responding cells from four different dogs and a concentration of 10 µg/ml CD40-Ig are summarized in Figure 4. Canine CD40-Ig significantly suppressed the MLR to about 10% of the proliferation with medium alone (P = 0.00006). CD40-Ig was significantly more active than rhCTLA4-Ig and the mouse anti-human CD154 antibody 5c8 (P = 0.00098 and P = 0.00096), respectively.
Figure 4.
Canine CD40-Ig in canine allogenic MLR: Purified rcCD40-Ig was added on day 0 to the MLR at a dose of 10µg/mL. The irrelevant mouse antibody 31A was used as negative control. RhCTLA4-Ig and mouse anti-human CD154 antibody 5c8 served as positive controls (dose 10µg/mL). Four independent experiments were performed. All data points in each individual experiment were done in triplicate. The 3H-thymidine uptake with medium alone was set at 100%. The asterisks mark statistically significant reduction compared to medium alone (P< 0.05).
3.3. Dose escalation studies of CD40-Ig and antibody 5c8
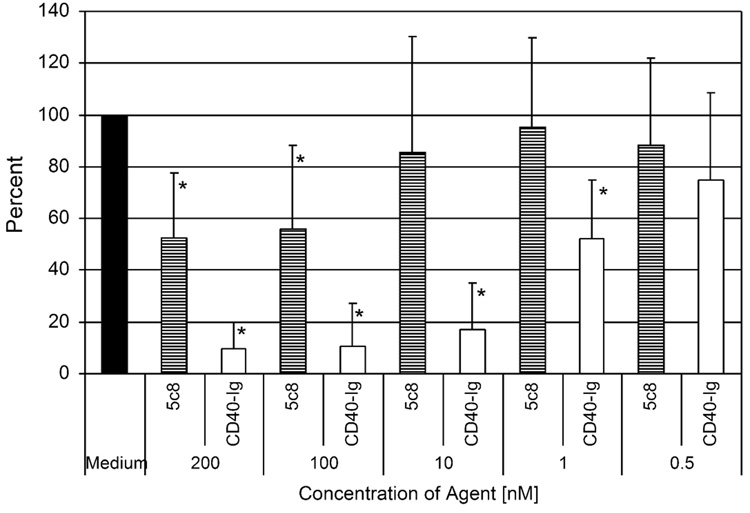
In order to establish the minimal active concentration of CD40-Ig compared to antibody 5c8, both agents were added to the MLR in equi-molar concentrations. Figure 4 summarizes the results of seven independent MLR. CD40-Ig significantly reduced the 3H-thymidine cellular uptake in the MLR at a concentration of 1 nM and blocked the MLR nearly completely at a concentration of 10 nM. There were no significant differences between effects of 200 nM, 100 nM or 10 nM concentrations of CD40-Ig. In contrast, antibody 5c8 blocked the MLR at a concentration of 100 nM and higher, while no blocking activity as detected at concentrations of 10 and 1 nM, respectively (Figure 5).
Figure 5.
Dose responses of rcCD40-Ig and mouse anti-human CD154 antibody 5c8 in canine MLR. Seven individual MLR were performed. All data points in each individual experiment were done in triplicate. The 3H-thymidine cellular uptake with medium alone was set at 100%. The asterisks mark statistically significant reductions compared to medium alone (P < 0.05).
4. Discussion
Blockade of the CD28/CD80–CD86 interactions with the fusion protein CTLA4-Ig and of the CD40–CD154 interaction with anti-CD154 antibodies or the fusion protein CD40-Ig have prolonged graft survival in different animal models of marrow or solid organ transplantation (McLellan et al., 1996; Nomura et al., 2002; Guillot et al., 2002; Fehr and Sykes, 2004; Kawai et al., 2004; Li et al., 2001; Lee et al., 2006; Wekerle et al., 2002; Yin et al., 2002; Wekerle et al., 2000; Wekerle et al., 1999; Kirk et al., 1997). We have used both human CTLA4-Ig and the monoclonal antibody 5C8, directed against human CD154, in our canine model of marrow transplantation and found both reagents only partially effective. This was possibly due to lack of complete specificity of these “human” reagents. In support of this conjecture, the current study showed canine CD40-Ig to be significantly more effective in suppressing MLR than equi-molar amounts of antibody 5C8.
The fusion construct of the extracellular domain of CD40 and the Fc portion of immunoglobulin has long been known to block T-cell activation (Fanslow et al., 1992). The Fc tail provided the dimerization of the molecule that was necessary for optimal CD40-Ig activity (Masunaga et al., 2005).
These current in vitro results suggested that the use of canine CD40-Ig in a canine model of marrow transplantation will likely be more successful in assuring sustained engraftment than the use of cross-reacting human reagents. These data suggest that the conditioning regimen intensity can be further decreased from the current nonmyeloablative dose of 2 Gy total body irradiation to 1 Gy or even lower.
Figure 1.
A 68% identity of amino acid sequences of the canine CD40 (GenBank Accession No. NM001002982) compared to human CD40 (Stamenkovic et al., 1989) The derived sequence of the extracellular domain of the canine CD40 is underscored with the first 20 amino acid representing the leader sequence. Matching amino acids are shown in black, non-matching in grey.
Acknowledgments
The authors would like to thank Michele Spector DVM; Alix Joslyn, and the technicians in the canine facilities of the Fred Hutchinson Cancer Research Center; Sam Shin, Patrice Stroup, Eustacia Zelmer and Erlinda Santos for technical assistance; Zejing Wang, MD and Gopalakrishnan Venkataraman, PhD for helpful discussions; and Phil Gafken and the Proteomics facility for mass spectroscopy and the Biologics production facility at the FHCRC for the endotoxin tests.
Supported in part by grant nos. CA78902, CA15704 and AI067770 awarded by the National Institutes of Health, Department of Health and Human Services, Bethesda, MD. Additional support was provided by the Laura Landro Salomon Endowment Fund (R.S.), the Jose Carreras International Leukemia Foundation (R.S.), the Lupin Foundation (R.S.), and grant no. BMBF-LPD 9901/8–63 of Deutsche Akademie der Naturforscher Leopoldina, Halle a.d. Saale, Germany (C.J.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Fanslow WC, Anderson DM, Grabstein KH, Clark EA, Cosman D, Armitage RJ. Soluble forms of CD40 inhibit biologic responses of human B cells. J. Immunol. 1992;149:655–660. [PubMed] [Google Scholar]
- Fehr T, Sykes M. Tolerance induction in clinical transplantation (Review) Transpl. Immunol. 2004;13:117–130. doi: 10.1016/j.trim.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Guillot C, Guillonneau C, Mathieu P, Gerdes CA, Menoret S, Braudeau C, Tesson L, Renaudin K, Castro MG, Lowenstein PR, Anegon I. Prolonged blockade of CD40-CD40 ligand interactions by gene transfer of CD40Ig results in long-term heart allograft survival and donor-specific hyporesponsiveness, but does not prevent chronic rejection. J. Immunol. 2002;168:1600–1609. doi: 10.4049/jimmunol.168.4.1600. [DOI] [PubMed] [Google Scholar]
- Hogan WJ, Little M-T, Zellmer E, Friedetzky A, Diaconescu R, Gisburne S, Lee R, Kuhr C, Storb R. Postgrafting immunosuppression with sirolimus and cyclosporine facilitates stable mixed hematopoietic chimerism in dogs given sublethal total body irradiation before marrow transplantation from DLA-identical littermates. Biol Blood Marrow Transplant. 2003;9:489–495. doi: 10.1016/s1083-8791(03)00148-4. [DOI] [PubMed] [Google Scholar]
- Jin YZ, Xie SS. Bicistronic adenovirus-mediated gene transfer of CTLA4Ig gene and CD40Ig gene result in indefinite survival of islet xenograft. Transplant. Proc. 2003;35:3165–3166. doi: 10.1016/j.transproceed.2003.10.064. [DOI] [PubMed] [Google Scholar]
- Jochum C, Beste M, Zellmer E, Graves SS, Storb R. CD154 blockade and donor-specific transfusions in DLA-identical marrow transplantation in dogs conditioned with 1-Gy total body irradiation. Biol Blood Marrow Transplant. 2007;13:164–171. doi: 10.1016/j.bbmt.2006.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaya K, Tsuchida Y, Inobe M, Murakami M, Hirose T, Kon S, Kawaguchi S, Wada T, Yamashita T, Ishii S, Uede T. Combined gene therapy with adenovirus vectors containing CTLA4Ig and CD40Ig prolongs survival of composite tissue allografts in rat model. Transplantation. 2003;75:275–281. doi: 10.1097/01.TP.0000046966.35399.75. [DOI] [PubMed] [Google Scholar]
- Kawai T, Sogawa H, Boskovic S, Abrahamian G, Smith RN, Wee SL, Andrews D, Nadazdin O, Koyama I, Sykes M, Winn HJ, Colvin RB, Sachs DH, Cosimi AB. CD154 blockade for induction of mixed chimerism and prolonged renal allograft survival in nonhuman primates. Am J Transplant. 2004;4:1391–1398. doi: 10.1111/j.1600-6143.2004.00523.x. [DOI] [PubMed] [Google Scholar]
- Kirk AD, Harlan DM, Armstrong NN, Davis TA, Dong Y, Gray GS, Hong X, Thomas D, Fechner JH, Jr, Knechtle SJ. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc. Natl. Acad. Sci. USA. 1997;94:8789–8794. doi: 10.1073/pnas.94.16.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee EN, Lee J, Kim EY, Park HJ, Chang CY, Jung DY, Choi SY, Lee SK, Kwon CH, Joh JW, Kim SJ. Tolerance induction through megadose bone marrow transplantation with two-signal blockade. J. Surg. Res. 2006;130:102–109. doi: 10.1016/j.jss.2005.07.042. [DOI] [PubMed] [Google Scholar]
- Li S, Thanikachalam M, Pang M, Carreno M, Aitouche A, Pham SM. Combined host-conditioning with CTLA4-Ig, tacrolimus, anti-lymphocyte serum, and low-dose radiation leads to stable mixed hematopoietic chimerism. Exp. Hematol. 2001;29:534–541. doi: 10.1016/s0301-472x(00)00685-8. [DOI] [PubMed] [Google Scholar]
- Masunaga T, Yamashita K, Sakihama H, Hashimoto T, Hua N, Imai A, Inobe M, Miyazaki T, Todo S, Uede T. Dimeric but not monomeric soluble CD40 prolongs allograft survival and generates regulatory T cells that inhibit CTL function. Transplantation. 2005;80:1614–1622. doi: 10.1097/01.tp.0000181093.50141.6c. [DOI] [PubMed] [Google Scholar]
- McLellan AD, Sorg RV, Williams LA, Hart DN. Human dendritic cells activate T lymphocytes via a CD40: CD40 ligand-dependent pathway. Eur. J. Immunol. 1996;26:1204–1210. doi: 10.1002/eji.1830260603. [DOI] [PubMed] [Google Scholar]
- Nomura M, Yamashita K, Murakami M, Takehara M, Echizenya H, Sunahara M, Kitagawa N, Fujita M, Furukawa H, Uede T, Todo S. Induction of donor-specific tolerance by adenovirus-mediated CD40Ig gene therapy in rat liver transplantation. Transplantation. 2002;73:1403–1410. doi: 10.1097/00007890-200205150-00008. [DOI] [PubMed] [Google Scholar]
- Raff RF, Deeg HJ, Farewell VT, DeRose S, Storb R. The canine major histocompatibility complex. Population study of DLA-D alleles using a panel of homozygous typing cells. Tissue Antigens. 1983;21:360–373. [PubMed] [Google Scholar]
- Stamenkovic I, Clark EA, Seed B. A B-lymphocyte activation molecule related to the nerve growth factor receptor and induced by cytokines in carcinomas. EMBO J. 1989;8:1403–1410. doi: 10.1002/j.1460-2075.1989.tb03521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storb R, Yu C, Wagner JL, Deeg HJ, Nash RA, Kiem H-P, Leisenring W, Shulman H. Stable mixed hematopoietic chimerism in DLA-identical littermate dogs given sublethal total body irradiation before and pharmacological immunosuppression after marrow transplantation. Blood. 1997;89:3048–3054. [PubMed] [Google Scholar]
- Storb R, Yu C, Zaucha JM, Deeg HJ, Georges G, Kiem H-P, Nash RA, McSweeney PA, Wagner JL. Stable mixed hematopoietic chimerism in dogs given donor antigen, CTLA4Ig, and 100 cGy total body irradiation before and pharmacologic immunosuppression after marrow transplant. Blood. 1999;94:2523–2529. [PubMed] [Google Scholar]
- Wekerle T, Blaha P, Langer F, Schmid M, Muehlbacher F. Tolerance through bone marrow transplantation with costimulation blockade (Review) Transpl. Immunol. 2002;9:125–133. doi: 10.1016/s0966-3274(02)00016-3. [DOI] [PubMed] [Google Scholar]
- Wekerle T, Kurtz J, Ito H, Ronquillo JV, Dong V, Zhao G, Shaffer J, Sayegh MH, Sykes M. Allogeneic bone marrow transplantation with co-stimulatory blockade induces macrochimerism and tolerance without cytoreductive host treatment. Nat. Med. 2000;6:464–469. doi: 10.1038/74731. [DOI] [PubMed] [Google Scholar]
- Wekerle T, Sayegh MH, Ito H, Hill J, Chandraker A, Pearson DA, Swenson KG, Zhao G, Sykes M. Anti-CD154 or CTLA4Ig obviates the need for thymic irradiation in a non-myeloablative conditioning regimen for the induction of mixed hematopoietic chimerism and tolerance. Transplantation. 1999;68:1348–1355. doi: 10.1097/00007890-199911150-00022. [DOI] [PubMed] [Google Scholar]
- Yamashita K, Masunaga T, Yanagida N, Takehara M, Hashimoto T, Kobayashi T, Echizenya H, Hua N, Fujita M, Murakami M, Furukawa H, Uede T, Todo S. Long-term acceptance of rat cardiac allografts on the basis of adenovirus mediated CD40Ig plus CTLA4Ig gene therapies. Transplantation. 2003;76:1089–1096. doi: 10.1097/01.TP.0000085651.20586.30. [DOI] [PubMed] [Google Scholar]
- Yin D, Ma L, Zeng H, Shen J, Chong AS. Allograft tolerance induced by intact active bone co-transplantation and anti-CD40L monoclonal antibody therapy. Transplantation. 2002;74:345–354. doi: 10.1097/00007890-200208150-00009. [DOI] [PubMed] [Google Scholar]