Abstract
Rates of serotonin synthesis were measured in the human brain using positron emission tomography. The sensitivity of the method is indicated by the fact that measurements are possible even after a substantial lowering of synthesis induced by acute tryptophan depletion. Unlike serotonin levels in human brain, which vary greatly in different brain areas, rates of synthesis of the indolamine are rather uniform throughout the brain. The mean rate of synthesis in normal males was found to be 52% higher than in normal females; this marked difference may be a factor relevant to the lower incidence of major unipolar depression in males.
Keywords: tryptophan depletion, major depression/α-methyl-l-tryptophan/positron emission tomography imaging
Low brain serotonin (5-HT) levels or function have been implicated in various types of psychopathology, including depression, suicide, aggression, anxiety, and bulimia (for reviews see refs. 1–3). Until recently, the principal methods for studying serotonin metabolism in human brain were determination of the metabolite of serotonin 5-hydroxyindole-3-acetic acid (5-HIAA) in cerebrospinal fluid (CSF) and postmortem measurements of brain serotonin and 5-HIAA. Both methods have limitations. In particular, neither provides a direct measure of serotonin synthesis in the living brain. Recently, a method for measuring serotonin synthesis in the brain of living mammals has been developed (4–5) and tested successfully in dogs (6). The method uses positron emission tomography (PET) and α-[11C]methyl-l-tryptophan as a tracer. The tracer is converted in part to α-[11C]methylserotonin, which accumulates in serotonin neurons, because it is not a substrate for monoamine oxidase and does not cross the blood–brain barrier.
We report here in vivo measurements of serotonin synthesis in the brain of healthy volunteers. Both male and female subjects were studied because CSF studies suggest that the rate of brain serotonin metabolism is higher in females than in males (7–8), and because the incidence of major unipolar depression is higher in women (9). We measured rates of serotonin synthesis under two conditions: at baseline and after acute tryptophan depletion (ATD). For ATD, subjects ingest a tryptophan-free mixture of all the essential amino acids. This induces protein synthesis, which incorporates body stores of free tryptophan into protein, thus reducing the level of this amino acid in tissues, including brain (10). Because tryptophan is the precursor of serotonin, its reduction is thought to lower the rate of serotonin synthesis in brain (11–12). ATD was used in the present study for several reasons. First, low serotonin levels have been proposed to relate to various types of psychopathology (for reviews see refs. 1–3), so any useful method of in vivo measurements of the serotonin synthesis rate must have a demonstrated capability for measuring low rates of serotonin synthesis. Second, ATD induces a transient reappearance of depressive symptoms in patients under treatment with antidepressants (13). Moreover, ATD also produces a mild lowering of mood in normal subjects with a family history of depression (14). It is therefore of interest to determine the degree of reduction in serotonin synthesis associated with changes in mood. Data obtained in rats suggest that lowering brain tryptophan causes a compensatory increase in tryptophan hydroxylase (15), which might offset the extent to which ATD lowers serotonin synthesis.
METHODS
Selection of Subjects.
Eight male and seven female subjects, aged from 18 to 35 years old, were recruited through newspaper advertisements. Inclusion criteria for all subjects included willingness to participate, good physical and mental health, and a knowledge of the psychiatric health of their first-degree relatives. Exclusion criteria included evidence of a past or present axis-I or axis-II DSM-III-R (Diagnostic Statistical Manual) diagnosis in the subject or first-degree relatives, and any significant medical illness. Psychiatric evaluations were conducted using the Structured Clinical Interview for DSM-III-R, nonpatient version (16). All subjects who participated in the study gave written informed consent. The study was approved by the Research and Ethics Committee of the Montreal Neurological Institute and Hospital and the Ethics Committee of McGill University.
Overview.
On the test day, the subject arrived at 7 a.m. for a MRI of the head, after which mood was rated (see below), a venous blood sample taken, and a PET scan performed. The subjects then ingested a tryptophan-deficient amino acid mixture (see below). Five hours later, mood was reevaluated, and a second PET scan performed.
ATD.
ATD was performed as described previously (14). The day before the test day, subjects ingested a low-protein diet. Prepacked, precooked meals, delivered to the subject’s home, contained 160 mg/24 h of tryptophan, 22.6 g/24 h protein, and 2,212 kcal/24 h. Subjects were instructed to eat at regular hours and were allowed ad libitum water and up to 3 cups of coffee or tea per day. On the test day the subject arrived, having fasted since the previous evening. After the first PET scan, the subject ingested a tryptophan-free amino acid mixture, containing 100 g of amino acids, consisting of 15 amino acids in 200 ml, as used by Young et al. (17). The amino acids mixture consisted of l-alanine 5.5 g, l-arginine 4.9 g, l-cysteine 2.7 g, glycine 3.2 g, l-histidine 3.2 g, l-isoleucine 8 g, l-leucine 13.5 g, l-lysine monohydrochloride 11 g, l-methionine 3 g, l-phenylalanine 5.7 g, l-proline 12.2 g, l-serine 6.9 g, l-threonine 6.9 g, l-tyrosine, 6.9 g, and l-valine 8.9 g. This is approximately the amount of amino acids in a 500-g steak. Because of the unpleasant taste of methionine, cysteine, and arginine, these amino acids were put into capsules and administered separately. The rest of the amino acids were mixed with 150 ml of water, 40–50 ml of chocolate syrup, and 0.5 g of sodium cyclamate. Subjects were asked to swallow the suspension in as short time as possible because of its somewhat unpalatable taste. For the next 5 hr, subjects stayed in a single room and were entertained by being shown one or two of three movies (the same video movies for all subjects) and allowed to read magazines. The movies and reading material were chosen to be relatively affectively neutral. Five hours after the ingestion of amino acids, rating scales were again administered, and a second blood sample drawn. At the completion of the session, each subject was given a high-protein snack and was administered a 1-g l-tryptophan tablet to restore their tryptophan levels. The tryptophan preparation used is available on prescription in Canada and has not been associated with any cases of eosinophilia myalgia syndrome (18).
Possible mood changes were evaluated with objective and subjective rating scales: the Hamilton Depression Rating Scale (19), the Bipolar Profile of Mood States (POMS) (20, 21), and the Visual Analogue Mood Scale (VAMS) (22). The bipolar form of the POMS, the principal measure of mood used in these studies, is composed of six bipolar scales: agreeable–hostile, composed–anxious, elated–depressed, confident–unsure, energetic–tired, and clearheaded–confused, and is highly sensitive to nonclinical changes in mood states. The VAMS consists of 16 100-mm horizontal lines, each representing a bipolar dimension of a mood state, on which the subject is instructed to place a perpendicular mark that best describes his mood state.
PET Scanning.
The radiopharmaceutical, α-[11C]methyl-l-tryptophan, was prepared by the method reported by Mzengeza et al. (23). The authenticity and stereochemical configuration of the final compound was confirmed by its coelution with a known sample of α-methyl-l-tryptophan. After injection of about 20 mCi of tracer over 2 min, venous blood samples (13 samples) were drawn at progressively longer time intervals to obtain the plasma input function. The main reason for using venous blood was ethical, because otherwise it would have been necessary to keep an arterial catheter in for about 8 hr, or two catheters would have had to be inserted in the same day. In another group of subjects scanned with 6-[18F]fluorodopa, the concentrations of amino acids as well as that of plasma-free tryptophan were found to be similar in arterial and venous plasma (M.D. et al., unpublished work). In an attempt to correct for a possible bias in using the venous input function in the calculation of K*(ml/g per min), we have evaluated influence of the venous plasma curve on the calculation of K*, in a separate group of subjects, in which we sampled venous and arterial blood simultaneously to determined both input functions. These two input functions were different during the first 15–20 min, but after 20 min the values of both curves coincided. From this experiment, we were able to calculate a correction factor that was used to normalize venous input function (actual data not given). In this correction, the venous input function is normalized to the exposure time (θ; min) at 20 min [θ(20) = 33.5 min; unpublished data]. After venous curves were corrected, the calculated values of K* agreed within a few percent (the average difference was less than one) of those calculated by using the arterial input function. This correction factor also was applied to the data analysis of subjects reported here. These experiments also showed that the venous input function in the first 20 min could be corrected by the sagittal sinus curve from individual subjects (unpublished data). Therefore, on the basis of the above-mentioned data and consideration of the well-being of the subjects, it was decided that it was valid to use the venous blood for the input function. The plasma free tryptophan was measured in the ultrafiltrate of plasma (10,000 molecular weight cut-off; centrifuged for 5 min at 8,000 × g at 4°C).
PET and MRI images were coregistered and superimposed to help in the delineation of the brain structures on PET images (24). The anatomical regions were identified in the MRI, from which outlines were transformed onto PET images. The MRI and PET images were coregistered using a program developed at the McConnel Brain Imaging Center, Montreal Neurological Institute (24). The PET images were acquired by the Scanditronix PC-2048 15B scanner with 6.5-mm intervals between slices. The T1-weighted MRI images of 2-mm thickness were used for the detailed anatomical identification and the coregistration of the PET images. For the coregistration from each dynamic study, two sets of cumulative images were generated. The first set represented a sum of images acquired between 3 and 8 min after tracer injection, and the second set by summing images acquired between 20 and 60 min after tracer injection. The coregistration of the MRI and PET images was carried out by selecting between 15 and 20 anatomical landmarks. Some of the points used for the alignment of images were: the sinus confluence, the junction of the transverse and sigmoid sinus, a center of the cavernous sinus, anterior tip of the straight sinus (early images), the center of the orbit, the petrous pyramid and frontal sinus, frontal and occipital poles of the brain, the genu of the corpus callosum, the midline of the thalamus, and the base of the frontal and temporal lobes. The tracer concentrations read from these regions were used for further calculations.
The measurements of serotonin synthesis rates are based on the unidirectional trapping of α-methyltryptophan in the brain (4, 6). The method is similar to the deoxyglucose method of Sokoloff et al. (25). We wish to emphasize that it has been shown before that this tracer is not incorporated into proteins (4), is not a substrate for tyrosine hydroxylase (26), and there is no appreciable amount of a metabolite present in the plasma, so that the plasma level of radioactivity (M.D. et al., unpublished data), at all times, relates directly to the amount of tracer present. The PET approach used here is based on the serotonin synthesis measurements in the dog brain (6). In short, the tissue unidirectional uptake of tracer (K*; ml⋅g−1⋅min−1) or the slope of the linear portion (4, 6) of the tracer distribution volume (DV; ml/g) as a function of the exposure time [θ = ∫0T Cp*(t) dt/Cp*(T); note that θ was normalized as described above] was calculated by the least-squares method. The slopes (K*) were individually calculated for 10 brain structures in each subject, using the tissue uptake curve between 20 and 60 min (exposure time θ being between approximately 35 and 100 min). The calculation was done on the tissue time–activity curves before (baseline) and after ATD. From the slope K*, the plasma free tryptophan (Cp; pmol⋅g−1⋅min−1), and the in vivo measured lumped constant (LC = 0.42) (5), regional serotonin synthesis rates (R; pmol⋅g−1⋅min−1) were calculated as R = Cp K*/LC (6). The LC is defined in one formulation as the ratio of the ratios of the Michaelis–Menten constants and the tissue volume of distribution for tracer and tracee. It can also be defined as the ratio of the tissue uptake of tracer and tracee (5). The regional LC was measured in vivo by a direct comparison of the unidirectional tissue uptake of tryptophan and α-methyltryptophan in the rat brain (5). There is no evidence that the LC in human and rat brain are the same. However, the LC consists of the ratio of constants for α-methyltryptophan and tryptophan. Thus, it is likely that the ratio does not vary much between different species, nor between different brain regions (5, 25). For example the LC for 2-deoxy-d-glucose in human brain is similar to the one in rat (27). It is not known whether the LC is the same in the subject’s brain before and after ATD. However, rat protein synthesis in the brain does not change when an amino acid is increased, in contrast to some other organs (e.g., liver; refs. 28, 29). At any rate, if there were a change in the LC, it would probably be the same in males and females rendering sex comparison valid.
The reduction in serotonin synthesis (ratio of values before and after ATD) in the individual subjects was calculated as an antilogarithm of the differences of the log-transformed synthesis rates. Because in the log-transformed data the ratios represent differences between the logarithms, calculation of the SD of the ratio was simplified. The SD of the mean was calculated as the square root of the sum of squares of the relative errors of the individual serotonin synthesis rates. The log transformation of the data yielded normal distribution of data meeting the requirements for the statistical analysis of the ratios. Statistical analysis of all data was done using bmdp statistical programs (BMDP, Los Angeles, 1993).
RESULTS
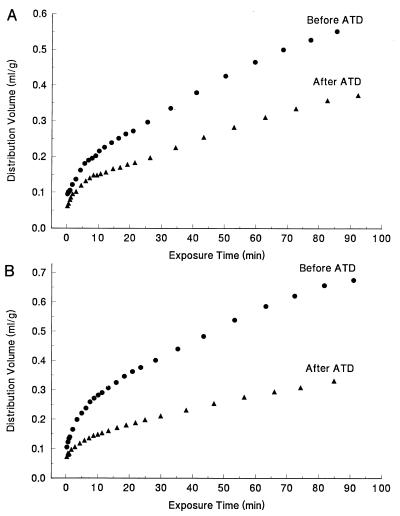
Examples of the plots of the tissue distribution volume (DV; ml/g) as a function of the exposure time (θ; min) exemplifying the shape of the curve, and the existence of a linear portion suggesting that the biological system achieved an apparent steady state, are shown in Fig. 1 for male (Fig. 1A) and female (Fig. 1B) subjects. In Fig. 1, the curves obtained at baseline (upper curves) and after tryptophan depletion (lower curves) are shown. The analysis of these curves was also carried out by fitting them to the full operation equation (4) in an attempt to compare values of K* obtained using a sinus corrected venous curve as input function. There was an excellent agreement between values of K* calculated from the linear portion of the graph and those obtained from the fit to the full-operation equation, indicating further that the biological system came to or close to an apparent steady-state. The latter are requirements for the validity of the use of the approach described by Patlak et al. (30).
Figure 1.
A set of representative plots exemplifying the tissue distribution volume (ml/g) in the frontal cortex of a male (A) and female (B) subjects as a function of the exposure time θ [θ = ∫0T Cp*(t) dt/Cp*(T); min]. The upper curves in A and B were obtained at baseline, and the lower curves were obtained after tryptophan depletion.
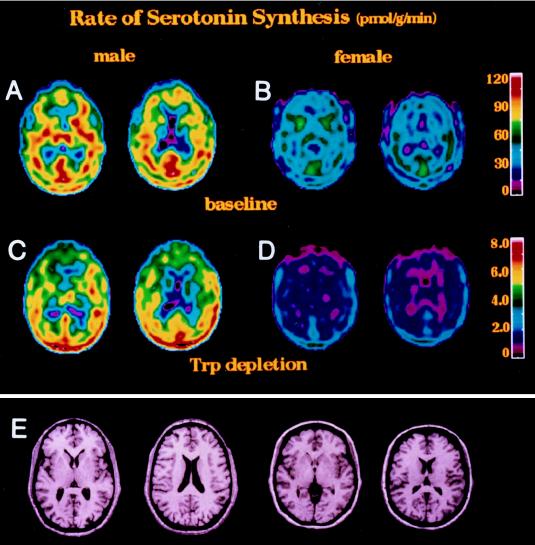
Examples of PET images obtained in a male and a female subject are provided in Fig. 2. Regional serotonin synthesis rates are color coded (vertical bar). Serotonin synthesis rates used to construct these pictorial presentations were calculated from the brain radioactivity distribution with certain approximations, because serotonin synthesis rates are related to the slope of the curves, which cannot be visualized in a static image. The images collected between 30 and 60 min after tracer injection were summed to obtain a better visual representation for regional serotonin synthesis rates. Briefly, the brain radioactivity (nCi/g) was converted into DV(T) (ml/g) by dividing it with the plasma tracer concentration [Cp*(T); nCi/ml]. From these plots, the K* (ml⋅g−1⋅min−1), and the apparent volume of the precursor (Vapp; ml/g) were calculated for each brain structure as described for the dog brain before (6). The average value of Vapp (ml/g) was calculated from the values for the individual brain structures in a particular subject, and this average value of Vapp was subtracted from the DV images on pixel by pixel bases. DV images were then converted into serotonin synthesis rate images by multiplying them by Cp (plasma free tryptophan; pmol/ml) and dividing them by LC. This conversion is also supported by our recently reported experiments in rat brain (31), where we showed that the Vapp is almost uniform through the rat brain.
Figure 2.
Representative PET images obtained from a male and a female subject are shown. The serotonin synthesis rates for this representation were calculated as described (see text). Images are shown before (A and B) and after (C and D) depletion of the plasma tryptophan. A and B were obtained from a male and a female subject before tryptophan depletion, respectively. The color bar on the right in the same row gives an indication of synthesis. C and D were obtained from a male and female subject after tryptophan depletion, respectively. The color bar on the right in the same row cross-references colors in the images and the synthesis rates. E shows MRI images obtained at the same level as the PET images. Images in male and female subjects are at the same level.
The average rates of serotonin synthesis obtained in male (n = 7) and female (n = 7) subjects before and after ATD are given in Table 1. These rates at baseline were about 75 and 50 pmol⋅g−1⋅min−1 in males and females, respectively (Table 1). After the ATD the rates of synthesis were about 9.5 and 1.5 pmol⋅g−1⋅min−1 in males and females, respectively. The rate of serotonin synthesis was reduced by ATD by a factor of about 9.5 in males and of about 40 in females. One male was excluded from these calculations on the basis of the χ2 statistic, (P < 0.001), indicating that he was an outlier. The rates in this subject were rather high and although they would not affect the mean of the male subjects significantly, they would substantially increase the SDs. However, the inclusion of this subject’s values did not change the main effects observed.
Table 1.
Serotonin synthesis rates in male and female subjects before and after tryptophan depletion
| Brain area | Male subjects
|
Female subjects
|
||||
|---|---|---|---|---|---|---|
| Synthesis rate, pmol⋅g−1⋅min−1
|
Ratio†‡ | Synthesis rate, pmol·g−1·min−1
|
Ratio†‡ | |||
| Before* | After | Before* | After | |||
| Frontal cortex | 70 ± 19 | 9 ± 6 | 9.2 ± 1.3 | 43 ± 20 | 1.4 ± 1.0 | 38 ± 8 |
| Parietal cortex | 73 ± 19 | 10 ± 7 | 9.0 ± 1.3 | 44 ± 21 | 1.4 ± 0.9 | 38 ± 8 |
| Temporal cortex | 74 ± 19 | 10 ± 6 | 8.9 ± 1.4 | 44 ± 21 | 1.5 ± 1.0 | 34 ± 7 |
| Occipital cortex | 68 ± 15 | 9 ± 6 | 9.3 ± 1.5 | 40 ± 19 | 1.3 ± 0.9 | 38 ± 8 |
| Caudate | 64 ± 15 | 8 ± 5 | 9.7 ± 1.6 | 42 ± 19 | 1.3 ± 0.7 | 37 ± 12 |
| Putamen | 80 ± 19 | 9 ± 6 | 10.5 ± 1.7 | 49 ± 22 | 1.3 ± 0.8 | 36 ± 9 |
| Globus palidus | 68 ± 18 | 8 ± 6 | 10.4 ± 2.9 | 43 ± 20 | 1.2 ± 1.0 | 38 ± 8 |
| Thalamus | 70 ± 19 | 9 ± 6 | 9.1 ± 1.6 | 47 ± 21 | 1.5 ± 0.9 | 42 ± 10 |
| Hypothalamus | 63 ± 22 | 8 ± 5 | 9.7 ± 2.8 | 42 ± 22 | 1.3 ± 1.0 | 39 ± 12 |
| Amygdala and hippocampus | 65 ± 14 | 8 ± 5 | 9.8 ± 2.0 | 42 ± 20 | 1.3 ± 0.9 | 43 ± 12 |
Data are presented as the mean ± SD with seven subjects of each sex. All subjects were scanned before and after plasma tryptophan depletion.
Synthesis rates in male subjects were statistically different (P < 0.05; ANOVA; analysis of variance), at baseline, from those in female subjects.
Ratios were calculated as antilogarithms of differences of the serotonin synthesis rates logarithms. The SDs for the ratios were calculated as the square roots of the sum of squares of the relative SD in each structure and subject. The SDs of individual serotonin synthesis measurements were calculated by adding uncertainty from the plasma free tryptophan and that obtained from the least-squares fits of the volume of distribution data to a straight line.
Significant difference (P < 0.05; two-way repeated measure ANOVA) between the degree of reductions (ratios) in male from that in female subjects.
The rates of serotonin synthesis (Table 1) were rather uniform throughout the terminal fields of serotonergic neurons of the human brain, as is the case in the rat brain (5). The resolution of our scanner did not permit determination of serotonin synthesis rates in the serotonergic cell body areas of the brain stem.
Male subjects had higher levels of plasma free tryptophan than female subjects (Table 2), but there was no significant correlation between plasma free or total tryptophan levels and rates of serotonin synthesis in either group of subjects (Tables 1 and 2). There was no significant difference in total plasma tryptophan in male and female subjects either at baseline or after ATD (Table 2). The use of the ratios of free or total plasma tryptophan to the plasma levels of other large neutral amino acids as covariate in the ANOVA analysis did not affect the degree of significance of differences between serotonin synthesis rates in male and female subjects. ATD induced a significant reduction (P < 0.05 for the sex–time interaction; two-way repeated measures ANOVA on the log transformed data) of serotonin synthesis, as indicated by the ratios of synthesis rates before and after ATD. ATD did not have a statistically significant (P > 0.05; two-tailed paired t test) effect on mood ratings overall, though one of seven female subjects (but none of the male subjects) showed signs of distress, low mood, and a crying spell by the end of the second scan.
Table 2.
Plasma total and free tryptophan in male and female subjects before and after depletion
| Plasma tryptophan | Plasma tryptophan conc., nmol⋅ml−1
|
|||
|---|---|---|---|---|
| Male subjects
|
Female subjects
|
|||
| Before | After | Before | After | |
| Free* | 12 ± 6 | 3.4 ± 3.0 | 6.1 ± 1.8 | 0.66 ± 0.32 |
| Total† | 64 ± 16 | 11 ± 6 | 52 ± 16 | 7.8 ± 4.6 |
Data are presented as the mean ± SD with seven subjects of each sex. All subjects were scanned before and after plasma tryptophan depletion.
The plasma free tryptophan in male subjects was significantly different (P < 0.05; ANOVA) from that in female subjects at the baseline, but not after ATD. There was no significant correlation (P > 0.05; Pearson rank correlation) between plasma free tryptophan levels and rate of serotonin synthesis shown in Table 1.
No significant difference (P > 0.05; ANOVA) between plasma total tryptophan levels at the baseline or after ATD in male and female subjects. There was no significant correlation between plasma total tryptophan levels and the serotonin synthesis rates shown in Table 1.
DISCUSSION
Until recently, the principal method for estimating the rate of serotonin metabolism in the human central nervous system has been the measurement of 5-HIAA in CSF. However, such measurements in lumbar CSF may reflect, in part, spinal cord metabolism of serotonin, and also can be influenced by factors such as the transport of 5-HIAA into and out of the CSF, mixing of CSF, as well as the rate of serotonin catabolism (32). Thus, CSF 5-HIAA level is a poor index of dynamic changes in serotonin synthesis in brain tissue. The advantages of the PET method are substantial: (i) it measures serotonin synthesis directly in various brain regions; (ii) it can be repeated after a short time interval; (iii) it is less invasive than a lumbar puncture; and (iv) the results are not influenced by a wide variety of factors unrelated to the rate of serotonin synthesis that can alter CSF values. Our method, like other PET methods, involves certain assumptions, as detailed in previous papers (4, 6). The main disadvantages of our method are cost and availability.
The mean rates of serotonin synthesis determined in the present study range from 66 to 85 pmol⋅g−1⋅min−1 for different brain areas in male subjects and 47 to 55 pmol⋅g−1⋅min−1 in female subjects. A surprising uniformity in the different areas studied, contrasting with the variable serotonin levels measured in different areas of postmortem brains. For instance, the ratio of serotonin levels in a region with high content, such as caudate, to an area with a low content, such as frontal cortex, is of about 15 (33, 34). In the present study, the ratio for the rate of synthesis in these two areas was 0.9 for male and 1.0 for female subjects. The density of serotonergic innervation of different areas can be estimated by measuring the number of serotonin uptake sites. In postmortem studies on the density of re-uptake sites measured by the binding 3H-labeled imipramine, cyanoimipramine, or paroxetine in human brain, ratios of the number of binding sites in the caudate to that in the frontal cortex were in the range between 1.2 and 3.3 (35–38). This suggests that the serotonergic innervation of the frontal cortex is less dense than that of the caudate. However, this difference is substantially less than that of serotonin levels. The uniformity observed in the present study in different brain areas suggests that the rate of serotonin synthesis depends on factors other than the density of innervation. Using the postmortem concentrations of serotonin reported by Young et al. (34) for the putamen (466 ng/g) and temporal cortex (11 ng/g), and the present data for the rates of serotonin synthesis, the time required to synthesize an amount of serotonin equal to the tissue content is 31 and 48 min for the putamen of males and females, respectively, and 0.8 and 1.3 min, respectively for the temporal cortex. Thus, for yet unidentified reasons, the storage of serotonin is very much less, in relation to its rate of synthesis, in cortex than in basal ganglia. One could speculate that this seemingly redundant capacity to synthesize serotonin in cortical areas could be related to the ability of the serotonergic system to provide rapidly enough of its neurotransmitter in situations where increased availability is required.
The marked difference in the rates of serotonin synthesis between male and female subjects is, to our knowledge, a new finding. In the few postmortem studies where male-female differences in the brain serotonin levels were examined, no significant differences were found (38, 39). Moreover, no differences have been found between the number of serotonin re-uptake sites in the brains of male and female subjects (35, 39). However, two CSF studies have suggested a higher rate of serotonin synthesis in female than male subjects (7, 8), opposite to the results of the present study. One possible explanation for this apparent discrepancy could be that the system transporting 5-HIAA out of CSF is less active in female than male subjects.
The rate of serotonin synthesis will depend on numerous factors including the free plasma tryptophan levels, the plasma levels of tryptophan relative to the other large neutral amino acids, the activity of the system that transports the large neutral amino acids into brain, the gene expression of tryptophan hydroxylase, degradation of tryptophan hydroxylase, compartmentalization of tryptophan and tryptophan hydroxylase in brain cells, as well as probably numerous other factors. Any difference in the level to which plasma CpTrp is decreased in males and females is in part related to the metabolic differences of their bodies, and as suggested by data presented in this manuscript, possibly could have influence, by rather complex and not yet well understood processes, on the brain biology. If one accepts the biological model derivation, which is based on the plasma input function, then it must be also accepted that the rate of serotonin synthesis must be somehow related to the plasma CpTrp, but the relationship is not necessarily linear. Indeed, the presented data suggest that despite reduction in the CpTrp of 10 and 3.5 times in females and males, respectively, the rate of serotonin synthesis was reduced 40 and 10 times in females and males, respectively. From this large difference between reduction in CpTrp and the serotonin synthesis rate it is obvious that substantially more complex mechanism(s) controlling brain serotinin synthesis is (are) involved here.
Averaging over the different brain areas, the rate of serotonin synthesis is 52% greater in male than in female subjects. This is one of the largest differences between the brains of males and females that is not related to hormone binding sites. The reason for this difference is not clear at this time. Tryptophan is taken up into the brain by a transport system that is common to all the large neutral amino acids. There is competition between the large neutral amino acids for this system, and the plasma ratio of tryptophan to the other large neutral amino acids best explains its brain level, at least in laboratory animals (41). In the present study, using ratios of the plasma level of free tryptophan to the levels of the other large neutral amino acids as a covariant in the ANOVA analysis did not alter the statistical significance of this difference. This suggests that differences in peripheral tryptophan availability do not explain the sex difference in brain serotonin synthesis, unless there is a large component for the entry of tryptophan into human brain that is not affected by the other large neutral amino acids. In rat brain under steady-state conditions, we reported a substantial diffusion component for the blood–brain transfer of tryptophan (42). What evidence there is from human CSF studies suggests that other large neutral amino acids reduce, as in experimental animals, tryptophan uptake into human brain (43–45).
The possible association between serotonin and major depression suggests that the rate of serotonin synthesis in women may be related to the higher incidence of major unipolar depression. Animal data indicate that female rats adapt less readily than male rats to stress in an animal model of depression, and that serotonin may play a role in this difference (46). Human males and females seem to have similar stores of brain serotonin, but, if there were increased utilization of serotonin during stressful situations, a lower rate of synthesis in the females may not be as efficient in maintaining adequate stores of the neurotransmitter. Thus, in such situations, serotonin levels would decline more in female than in male subjects, possibly increasing vulnerability to depression.
The effect of ATD in the present study (Table 1) show that (i) the PET method is capable of measuring rates of serotonin synthesis considerably below baseline rates (e.g. before ATD), and is therefore a suitable method for studies of patients with low rates of serotonin synthesis, and (ii) ATD causes a marked lowering of brain serotonin synthesis in all brain regions examined. The magnitude of the effect in the brain was somewhat greater than the decline in free plasma tryptophan. While the effect of ATD on serotonin synthesis was uniform throughout the brain, the effects on serotonin levels (as opposed to serotonin synthesis) are unlikely to be uniform. For instance, in the cortex, where the rate of serotonin synthesis is large compared to its level, ATD-induced decline in levels of serotonin are likely to occur more rapidly than in other structures.
The reason for the greater biochemical effect of ATD in women than in men is not known. We have reported that healthy women were more susceptible than healthy men to a lowering of mood after ATD (47). The present results suggest that this might be related to ATD causing a larger decrease in the rate of serotonin synthesis in women.
The results of this study raise a number of questions. First, why are rates of serotonin synthesis so similar in different brain areas, when the density of innervation varies more, and serotonin levels vary even more? Second, what are the causes and implications of the higher rates of serotonin synthesis in male brains? Gender-related differences in serotonin synthesis could be related to early serotonergic events in the brain organization and/or effects of circulating gonadal hormones. A better understanding of the gender differences reported here might be provided by studies of individuals with pathologically altered levels of gonadal hormones. The possible role of serotonin synthesis in susceptibility to depression could be investigated by studying subjects who are at elevated risk for depression and depressed patients presenting with a major depressive episode.
Acknowledgments
We thank the staff of the Medical Cyclotron and PET Units of the McConnell Brain Imaging Centre, Montreal Neurological Institute. This research was supported by grants from the U.S. Public Health Service (M.D., RO1-NS-29629) and the Medical Research Council of Canada (S.N.Y. and C.B.). P.B. is a recipient of a Medical Research Council Scientist Award.
ABBREVIATIONS
- CSF
cerebrospinal fluid
- 5-HIAA
5-hydroxyindole-3-acetic acid
- PET
positron emission tomography
- ATD
acute tryptophan depletion
- LC
lumped constant
- DV
distribution volume
References
- 1.van Praag H M. In: Current Therapeutic Approaches to Panic and Other Anxiety Disorders. Mendlewicz J, Racagni G, Brunello N, editors. Basel: Karger; 1994. pp. 144–150. [Google Scholar]
- 2.Brewerton T D. Psychoneuroendocrinology. 1995;20:561–590. doi: 10.1016/0306-4530(95)00001-5. [DOI] [PubMed] [Google Scholar]
- 3.Heninger G R. In: Psychopharmacology: The Fourth Generation of Progress. Bloom F E, Kupfer D J, editors. New York: Raven; 1995. pp. 471–482. [Google Scholar]
- 4.Diksic M, Nagahiro S, Sourkes T L, Yamamoto Y L. J Cereb Blood Flow Metab. 1990;10:1–12. doi: 10.1038/jcbfm.1990.1. [DOI] [PubMed] [Google Scholar]
- 5.Vanier M, Tsuiki K, Grdiša M, Worsley K, Diksic M. J Neurochem. 1995;64:624–635. doi: 10.1046/j.1471-4159.1995.64020624.x. [DOI] [PubMed] [Google Scholar]
- 6.Diksic M, Nagahiro S, Chaly T, Sourkes T L, Yamamoto Y L, Feindel W. J Neurochem. 1991;56:153–162. doi: 10.1111/j.1471-4159.1991.tb02575.x. [DOI] [PubMed] [Google Scholar]
- 7.Young S N, Gauthier S, Anderson G M, Purdy W C. J Neurol Neurosurg Psychiatry. 1980;43:438–445. doi: 10.1136/jnnp.43.5.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ågren H, Mefford I N, Rudorfer M V, Linnoila M, Potter W Z. J Psychiat Res. 1986;20:175–193. doi: 10.1016/0022-3956(86)90002-6. [DOI] [PubMed] [Google Scholar]
- 9.Weissman M M, Olfson M. Science. 1995;269:799–801. doi: 10.1126/science.7638596. [DOI] [PubMed] [Google Scholar]
- 10.Moja E A, Restani P, Corsini E, Stacchezzini M C, Assereto R, Galli C L. Life Sci. 1991;49:1121–1128. doi: 10.1016/0024-3205(91)90600-g. [DOI] [PubMed] [Google Scholar]
- 11.Biggio G, Fadda F, Fanni P, Tagliamonte A, Gessa G L. Life Sci. 1974;14:1321–1329. doi: 10.1016/0024-3205(74)90440-8. [DOI] [PubMed] [Google Scholar]
- 12.Young S N, Ervin F R, Pihl R O, Finn P. Psychopharmacology. 1989;98:508–511. doi: 10.1007/BF00441950. [DOI] [PubMed] [Google Scholar]
- 13.Delgado P L, Charney D S, Price L H, Aghajanian G K, Landis H, Heninger G R. Arch Gen Psychiatry. 1990;47:411–418. doi: 10.1001/archpsyc.1990.01810170011002. [DOI] [PubMed] [Google Scholar]
- 14.Benkelfat C, Ellenbogen M A, Dean P, Palmour R M, Young S N. Arch Gen Psychiatry. 1994;51:687–697. doi: 10.1001/archpsyc.1994.03950090019003. [DOI] [PubMed] [Google Scholar]
- 15.Neckers L M, Biggio G, Moja E, Meek J L. J Pharmacol Exp Ther. 1977;201:110–116. [PubMed] [Google Scholar]
- 16.Spitzer R L. Structured Clinical Interview for DSM-IIIR, Biometrics Research Department. New York: New York State Psychiatric Institute; 1987. [Google Scholar]
- 17.Young S N, Smith S E, Pihl R O, Ervin F R. Psychopharmacology. 1985;87:173–177. doi: 10.1007/BF00431803. [DOI] [PubMed] [Google Scholar]
- 18.Wilkins K. Can Med Assoc J. 1990;142:1265–1266. [PMC free article] [PubMed] [Google Scholar]
- 19.Hamilton M. J Clin Psychiatry. 1960;41:21–24. [PubMed] [Google Scholar]
- 20.Lorr M, McNair D M, Fisher S. J Pers Assess. 1982;46:432–436. doi: 10.1207/s15327752jpa4604_16. [DOI] [PubMed] [Google Scholar]
- 21.McNair D M, Lorr M, Droppleman L F. Manual for the Profile of Mood States. San Diego: Educational and Industrial Testing Service; 1988. [Google Scholar]
- 22.Bond A, Lader M. Br J Med Psychol. 1974;47:211–218. [Google Scholar]
- 23.Mzengeza S, Venkatachalam T K, Diksic M. Nucl Med Biol. 1995;22:303–307. doi: 10.1016/0969-8051(94)00116-2. [DOI] [PubMed] [Google Scholar]
- 24.Evans A C, Thompson C J, Marrett S, Mazza M. IEEE Trans Med Imaging. 1991;10:90–98. doi: 10.1109/42.75615. [DOI] [PubMed] [Google Scholar]
- 25.Sokoloff L, Reivich M, Kennedy C, Rosiers H D, Patlak C S, Pettigrew K D, Sakurada O, Shinohara M. J Neurochem. 1977;28:897–910. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- 26.Sourkes T L. Aromatic Amino Acids in the Brain, Ciba Foundation Symposium 22, New Series. New York: Elsevier; 1974. pp. 361–378. [Google Scholar]
- 27.Nakai H, Matsuda H, Diksic M, Takara E, Meyer E, Redies C, Kirikae M, Yamamoto Y L. Neurol Med Chir (Tokyo) 1988;28:1–10. doi: 10.2176/nmc.28.1. [DOI] [PubMed] [Google Scholar]
- 28.Smith C B, Sun Y, Deibler G E, Sokoloff L. J Neurochem. 1991;57:1540–1547. doi: 10.1111/j.1471-4159.1991.tb06349.x. [DOI] [PubMed] [Google Scholar]
- 29.Dunlop D S, Yang X-R, Lajtha A. Biochem J. 1994;302:601–610. doi: 10.1042/bj3020601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patlak C S, Blasberg R G, Fenstermacher J D. J Cereb Blood Flow Metab. 1983;3:1–7. doi: 10.1038/jcbfm.1983.1. [DOI] [PubMed] [Google Scholar]
- 31.Diksic M, Grdiša M. Neurochem Res. 1995;20:1353–1360. doi: 10.1007/BF00992511. [DOI] [PubMed] [Google Scholar]
- 32.Garelis E, Young S N, Lal S, Sourkes T L. Brain Res. 1974;79:1–8. doi: 10.1016/0006-8993(74)90562-9. [DOI] [PubMed] [Google Scholar]
- 33.Cheetham S C, Crompton M R, Czudek C, Horton R W, Katona C L E, Reynolds G P. Brain Res. 1989;502:332–340. doi: 10.1016/0006-8993(89)90629-x. [DOI] [PubMed] [Google Scholar]
- 34.Young L T, Warsh J J, Kish S J, Shannak K, Hornykiewicz O. Biol Psychiatry. 1994;35:121–127. doi: 10.1016/0006-3223(94)91201-7. [DOI] [PubMed] [Google Scholar]
- 35.Cortés R, Soriano E, Pazos A, Probst A, Palacios J M. Neuroscience. 1988;27:473–496. doi: 10.1016/0306-4522(88)90282-5. [DOI] [PubMed] [Google Scholar]
- 36.Laruelle M, Vanisberg M A, Maloteaux J M. Biol Psychiatry. 1988;24:299–309. doi: 10.1016/0006-3223(88)90198-9. [DOI] [PubMed] [Google Scholar]
- 37.Joyce J N, Shane A, Lexow N, Winokur A, Casanova M F, Kleinman J E. Neuropsychopharmacology. 1993;8:315–336. doi: 10.1038/npp.1993.32. [DOI] [PubMed] [Google Scholar]
- 38.Dean B, Opeskin K, Pavey G, Naylor L, Hill C, Keks N, Copolov D L. J Neurochem. 1995;64:1197–1202. doi: 10.1046/j.1471-4159.1995.64031197.x. [DOI] [PubMed] [Google Scholar]
- 39.Arato M, Frecska E, MacCrimmon D J, Guscott R, Saxena B, Tekes K, Tothfalusi L. Prog Neuropsychopharmacol Biol Psychiatry. 1991;15:759–764. doi: 10.1016/0278-5846(91)90004-k. [DOI] [PubMed] [Google Scholar]
- 40.Mackay A V P, Yates C M, Wright A, Hamilton P, Davies P. J Neurochem. 1978;30:841–848. doi: 10.1111/j.1471-4159.1978.tb10792.x. [DOI] [PubMed] [Google Scholar]
- 41.Wurtman R J, Hefti F, Melamed E. Pharmacol Rev. 1981;32:315–335. [PubMed] [Google Scholar]
- 42.Takada A, Grdiša M, Diksic M, Gjedde A, Yamamoto Y L. Neurochem Int. 1993;23:351–359. doi: 10.1016/0197-0186(93)90079-k. [DOI] [PubMed] [Google Scholar]
- 43.Pérez-Cruet J, Chase T N, Murphy D L. Nature (London) 1974;248:693–695. doi: 10.1038/248693a0. [DOI] [PubMed] [Google Scholar]
- 44.Gillman P K, Bartlett J R, Bridges P K, Hunt A, Patel A J, Kantamaneni B D, Curzon G. J Neurochem. 1981;37:410–417. doi: 10.1111/j.1471-4159.1981.tb00470.x. [DOI] [PubMed] [Google Scholar]
- 45.Martinez M, Arnalich F, Vazquez J J, Hernanz A. Life Sci. 1993;53:1643–1650. doi: 10.1016/0024-3205(93)90188-9. [DOI] [PubMed] [Google Scholar]
- 46.Kennett G A, Chaouloff F, Marcou M, Curzon G. Brain Res. 1986;382:416–421. doi: 10.1016/0006-8993(86)91355-7. [DOI] [PubMed] [Google Scholar]
- 47.Ellenbogen M A, Young S N, Dean P, Palmour R M, Benkelfat C. Neuropsychopharmacology. 1996;15:465–474. doi: 10.1016/S0893-133X(96)00056-5. [DOI] [PubMed] [Google Scholar]