Abstract
Background
The seasonality of cholera is described in various study areas throughout the world. However, no study examines how temporal cycles of the disease vary around the world or reviews its hypothesized causes. This paper reviews the literature on the seasonality of cholera and describes its temporal cycles by compiling and analyzing 32 years of global cholera data. This paper also provides a detailed literature review on regional patterns and environmental and climatic drivers of cholera patterns.
Data, Methods, and Results
Cholera data are compiled from 1974 to 2005 from the World Health Organization Weekly Epidemiological Reports, a database that includes all reported cholera cases in 140 countries. The data are analyzed to measure whether season, latitude, and their interaction are significantly associated with the country-level number of outbreaks in each of the 12 preceding months using separate negative binomial regression models for northern, southern, and combined hemispheres. Likelihood ratios tests are used to determine the model of best fit. The results suggest that cholera outbreaks demonstrate seasonal patterns in higher absolute latitudes, but closer to the equator, cholera outbreaks do not follow a clear seasonal pattern.
Conclusion
The findings suggest that environmental and climatic factors partially control the temporal variability of cholera. These results also indirectly contribute to the growing debate about the effects of climate change and global warming. As climate change threatens to increase global temperature, resulting rises in sea levels and temperatures may influence the temporal fluctuations of cholera, potentially increasing the frequency and duration of cholera outbreaks.
Background
Introduction
This paper systematically describes the seasonality of cholera in different parts of the world and comprehensively reviews scientific literature that investigates why seasonal patterns exist. The causative agent of cholera, Vibrio cholerae (V. cholerae hereafter), is a water-borne bacterium that is a natural inhabitant of brackish aquatic environments. Cholera is an acute infection caused by the colonization and multiplication of V. cholerae O1 or O139 within the human small intestine. People contract cholera when they ingest an infective dose of V. cholerae from contaminated water or food. Many developing countries still endure frequent outbreaks due to the lack of basic sanitation services and clean water.
Previous studies describe the temporal variation of cholera in localized study areas [1-18]. Many investigators postulate that the temporal variation of the disease is due to environmental and climatic factors that affect the seasonal patterns of infection [1,9-13,19-25]. Despite this growing interest in the causes and patterns of cholera, no global review of cholera's seasonal variations or the hypothesized mechanisms causing these patterns has been published. This is partially due to the fact that there are only a small number of comprehensive cholera surveillance systems in affected areas. Thus, few empirical studies rigorously examine the seasonality of cholera, leaving our understanding of variations of cholera outbreaks in different regions of the world incomplete. To fill this gap, this paper explores the seasonal cycles of cholera around the world using 32 years of data compiled by the World Health Organization (WHO) in the Weekly Epidemiological Record [26]. The analysis includes all reported cholera cases by date and country between 1974 and 2005. While these data include only reported cases, and select countries such as Bangladesh do not regularly report cholera to the WHO, this global dataset is the only systematic source of its kind and offers a unique opportunity to analyze global and regional patterns of cholera.
Literature Review
Evidence of seasonality of cholera
Cholera infections vary greatly in frequency, severity, and duration, and the endemicity of cholera in different parts of the world is dynamic. Cholera is firmly endemic in some areas such as the South Asian countries of Bangladesh and India where cholera infections occur every year. In contrast, other regions such as parts of South America and Africa have historically had only sporadic epidemics. Yet, even in areas of persistent, endemic cholera, the magnitude of yearly epidemics varies dramatically from year to year.
In Bangladesh, several studies describe a regular seasonal cycle for cholera outbreaks, including specific studies on the different strains: classical, [5] El Tor, [27] and O139 [9]. While the symptoms of classical, El Tor, and O139 cholera are similar, some differences in their seasonal cycles are reported. Merson et al.[28], Glass et al. [29], and Samadi et al. [5] each describe a dominant seasonal cycle for classical cholera that is later than then peak of the newer strain, V. cholerae El Tor; El Tor is most incident from September to November, just after the monsoon. Several additional studies describe this pattern of two annual El Tor cholera peaks: a smaller spring outbreak in April before the monsoons followed by a larger fall outbreak from September to December after the monsoon [10,11,23,30-41]. Emch and Ali [42] describe a similar seasonal pattern for another new strain, V. cholerae 0139, when it first appeared in Bangladesh in 1993. These patterns are evident in other areas of the region as well. In Pakistan, classical cholera typically increases from November to January and from April to May [43,44] while in Kolkata, India, seasonal patterns of cholera cases peak in April, May, and June[35,37].
Few surveillance systems collect detailed cholera incidence data outside of South Asia; therefore, information on the seasonality of cholera outside of this region is more limited. In South America, seasonal peaks are reported in summer months, January to February [10,32,33,37,41] as well as with the rise in waters following the rainy season in Amazonia, Brazil [45]. Major cholera outbreaks are recorded in eastern African nations including Djibouti, Kenya, Mozambique, Somalia, Uganda and Tanzania where the majority of outbreaks occur following rainfall and/or floods [46]. These cholera peaks also coincide with the summer rains. Mhalu et al. [6] reports that from 1979 to 1983 there were two cholera peaks in Dar es Salaam, Tanzania from October to December and again from March to May, both coinciding with periods of increased rainfall. In rural southern Tanzania, the peak of the cholera epidemic is slightly later, in June and July [47]. Bateman [48] reported the 2002 cholera high season for the northern parts of southern Africa occurred from the last week of January to mid-March. This period of heightened cholera is slightly longer in Mozambique and occurs during the hot, rainy months from December to May [49,50].
Evidence of environmental drivers
V. cholerae inhabit seas, estuaries, brackish waters, rivers, and ponds of coastal areas of the tropical world [51,52]. They flourish in the dense organic matter, algae, and zooplankton of the Ganges delta and similar ecosystems. It is probable that primary transmission to humans is enabled by micro- and macro-level environmental factors such as temperature, salinity, nutrient concentrations, the number of available attachment sites (plankton), shellfish consumption, and contact with water [53]. These factors are, in turn, influenced by larger-scale climate variability [10].
Although the seasonality of cholera is apparent, until recently the reservoirs that enable the survival and multiplication of V. cholerae during interepidemic periods were unknown. During epidemics, V. cholerae are isolated from patients as well as from surface water; however, during inter-epidemic seasons, V. cholerae largely cannot be cultured from the environment [22,23,54-58]. Recent studies provide possible explanations of how seasonality and endemicity of cholera are maintained [59,60]. The seasonal cycles of cholera appear to be closely associated with changes in flora and fauna populations in the coastal environment [56].
Biotic and abiotic parameters of water appear to provide temporary and long-term biotic reservoirs for cholera. These relationships are examined in Asia, primarily in Bangladesh. The overall seasonal fluctuation in V. cholerae may be due to the seasonal variation in physical and nutritional aquatic parameters [17] including conditions in both oceans and the brackish ponds and canals of rural Bangladesh [61-63]. V. cholerae survival is also dependent on abiotic characteristics including alkalinity, salinity, and iron [20,24,54,55,59,64-67] that influence the expression of virulence genes and regulate the cholera toxin that causes watery diarrhea [10,68]. Salinity may also partially explain the seasonal variation of cholera [66,67,69]. V. cholerae may be unable to persist in winter with colder water temperatures, but aquatic reservoirs with salinities of 0.25 to 3.0% and temperatures consistently above 5°C may maintain cholera in endemic areas [70].
Microbiological studies in Bangladesh reveal additional details about the cycle of dormancy and activity of V. cholerae within environmental reservoirs [1,23,56,71,72]. V. cholerae secrete an enzyme called mucinase that degrades mucin in the environment [73]. As mucin is present in plant cell walls, early studies suggest the association between V. cholerae and aquatic plants [21,74]. Additional studies on V. cholerae O1 confirm this hypothesis and find that various aquatic plants (e.g., water hyacinth, marine algae, duck weed, cyanobacteria) can act as a temporary reservoir [22,24,55] while blue green algae (Anabaena spp.) can act as a long-term reservoir [1,21]. During interepidemic periods, toxigenic V. cholerae residing within aquatic plants do not lose their pathogenic properties [22,23,75] but become largely nonculturable [22,54,76-78]. It is likely that during cholera epidemics the bio-physicochemical parameters of estuaries are ideal for the multiplication and transmission of V. cholerae, and as a result, these water sources will be heavily contaminated with V. cholerae [74,79].
Changes in the aquatic environment also affect the seasonality of cholera because the growth of the phytoplankton and aquatic plants provide food for zooplankton. Zooplankton and cyanobacteria are also potential reservoirs of V. cholerae [56,64,80,81]. Viable but noncultureable V. cholerae are detectable in association with both cyanobacteria and zooplankton from the aquatic environment in Bangladesh [56,72,77,82,83]. V. cholerae O1 secrete an enzyme called chitinase, which is associated with chitinous fauna, mainly zooplankton [84,85]. Zooplankton may provide attachment sites for V. cholerae to multiply and serve as a vector to transmit an infective dose to humans [10,61,86]. Others concur and demonstrate that the post-monsoon epidemic is associated with a heavy bloom of phytoplankton and zooplankton [59,63,86-88]. After an initial lag period, V. cholerae proliferate and are subsequently transmitted to humans [36]. A recent hypothesis about environmental controls of cholera suggests that vibriophages regulate epidemics. Faruque et al [17] show that the inverse correlation between vibriophages and susceptible V. cholerae strains implies that epidemics are more likely to begin in periods of low phage concentration (i.e. after floods and the monsoon season).
Macro-level factors
Macro-level associations and patterns between climate and cholera can be indirectly measured illustrating additional mechanisms that influence seasonality. Pascual et al.[15] posits that the temporal variability of cholera is associated with three interrelated climate variables that include upper troposphere humidity, cloud cover, and top-of-atmosphere absorbed solar radiation. Lipp et al.[10] adds that climate variability (i.e., climate change, El Nino-Southern Oscillation [ENSO], North Atlantic Oscillation), seasonal effects (i.e., sunlight, temperature, precipitation, monsoons), and human dimensions (i.e., socioeconomics, demographics, and sanitation) are also key drivers of outbreaks. Similarly, ENSO raises water temperature, bringing about increased zooplankton blooms [10] that may influence longer cycles in cholera periodicity, including a 4-year fluctuation pattern [89].
Using satellite imagery, Lobitz et al [90] find a correlation between sea surface temperature, sea surface height, and cholera in the Bay of Bengal in Dhaka, Bangladesh from 1992 to 1995. They suggest that sea surface height increases human-vibrio contact by transporting the bacteria into inland waters through the tidal intrusion of plankton [90]. Climate can affect the temperature of the sea surface as well as local ponds and rivers, possibly increasing the incidence of cholera through the faster growth rate of the pathogen in aquatic environments [16,36]. The heating of surface water may lead to an increase in phytoplankton blooms which feed zooplankton, encouraging the subsequent multiplication of commensal copepods that house V. cholerae [10,83].
Periodic climatic and temperature cycles such as ENSO have an effect on inter-annual cholera variability. To understand these seasonal and inter-annual patterns, analysis at larger spatial scales is necessary [14]. The periodic effects of ENSO, including the warming of the Pacific Ocean off the coast of South America, correlate with a 2001 outbreak of cholera in Peru after a century long hiatus [2,91,92]. Epstein [93] notes that warm El Niño events are linked to cholera outbreaks in Bangladesh and also to the emergence of new harmful algal blooms throughout Asia. Koelle et al [2] found that cholera outbreaks in Bangladesh between 1966 and 2002 demonstrate a nine to fourteen month lag between Indian Ocean sea surface temperatures and atmospheric temperature changes and subsequent cholera outbreaks (examples include '87-88 El Niño, '88-89 La Niña, and the '97-98 El Niño). Rodo et al [89] further support the role of ENSO by identifying the change in variability of cholera between the past (1893–1940) and present (1980–2001) as the result of a more prominent role of climate forcing by current ENSO effects. Overall, inter-annual cycles are important indicators of large scale patterns in cholera seasonality and of anomalous seasonal outbreaks [14,89]. Greater emphasis on seasonal anomalies in conjunction with yearly averages would aid understanding of these inter-annual outbreak patterns [13].
Secondary infections and human transmission
The seasonality of cholera outbreaks may also be explained by secondary transmission. Factors affecting secondary transmission mandate the extent to which the disease is present, i.e. whether it will reach epidemic proportions [7]. Several studies find that the severity of secondary transmission is associated with local environmental variables, predominantly water sources for household consumption. People who use contaminated surface water for drinking, cooking and bathing are more likely to contract cholera than those who do not [94,95]. In epidemiological work, studies identify an inverse relationship between diarrhea and access to tube well water [96]; positive associations with canal water compared with river or pond water [57]; and higher cholera incidence rates in villages that are not adjacent to rivers [29]. Other studies find associations between cholera and flood control [34,97]. While these studies show that the local environment (e.g., water and sanitation) facilitates secondary transmission of cholera, it cannot fully explain why the disease has such predictable temporal patterns.
Materials and methods
Data
This paper describes the seasonality of cholera throughout the world during a 32-year period using WHO Weekly Epidemiological Record of all reported cases by date from 1974 to 2005 [26]. The Weekly Record compiles data from the World Health Organization's global cholera surveillance system. Each Weekly Record provides information about outbreaks in each country and including the number of reported cases, deaths, and the cholera serotype, when available. The Weekly Record does not consistently include within-country details such as the specific region of the outbreak. A project database is compiled that includes the number of cholera outbreaks for 140 countries for each of the 12 calendar months. An outbreak is defined as at least one reported cholera case in any calendar month. Multiple outbreaks during the same calendar month of the same year are treated as one outbreak. Outbreaks are used instead of disease rates due to the differences in surveillance systems. Some countries report cases more accurately than others, and there are always more cholera cases than those that are reported.
Methods
Dichotomous season variables are developed using WHO cholera case data. In the northern hemisphere, season one is December through May and season two is June through November; the seasons are reversed for the southern hemisphere. The latitude for each country is recorded using the centroid (i.e., the geographic center) of each country, assigning one latitude value to each. To ensure adequate model fit, the latitude variable is considered to be an absolute value, representing the distance to the equator. In addition, an ordinal variable is created to categorize individual countries within a geographic range represented by latitude values (0°–4°, 5°–9°, 10°–19°, 20°–29°, 30°–39°, 40°–49°, 50°–59°, and 60°+).
The outcome variable is the country-level number of outbreaks in each of the 12 preceding months. Because this variable represents a count over a fixed time period, a Poisson loglinear regression is initially considered using the model, ln(μ) = β0 + β1[abs(latitude)] + β2(season). However, the χ2/df (Pearson's chi-squared divided by the degrees of freedom) far exceeds one (approximately 4.3), providing evidence of overdispersion. Therefore, the Poisson model would be ineffective since the mean does not equal the variance. Instead, we use the negative binomial. The negative binomial distribution is robust to overdispersion and allows the variance to equal μ + k* μ2, where k is the overdispersion factor and μ the mean. The negative binomial output in SAS provides an ancillary parameter, α, which is an estimate of the degree of overdispersion. If overdispersion is present, α will be greater than one.
For the combined hemisphere model, the model of best fit is the saturated model with season, latitude, and interaction of season and latitude. To reach this conclusion, and test whether certain parameters in a model are zero, likelihood-ratio tests are conducted. To test the significance of the interaction term, the saturated model is compared to a model without the interaction term; the test result has an associated p-value of < .0001, providing evidence that the interaction term is non-zero. An overall test to see whether the saturated model, including season and latitude with interaction, fits better than the simple intercept model yields a p-value of < .0001 showing at least one, non-zero parameter. For the northern hemisphere model, the model of best fit also contains the saturated model of season and latitude with interaction. Likelihood-ratio tests are used to examine the intercept versus the saturated model as well as the model with just season and latitude versus the saturated model. These tests provide evidence that the saturated model is the model of best fit. Lastly, for the southern hemisphere model, the model of best fit includes only latitude. Because the seasons are reversed, the estimates confidence interval for latitude would be the negative of the value listed. The p-value would remain the same. A likelihood-ratio test is conducted to determine if the interaction term is zero: the resulting chi-square value of 0.36 with 1 degree of freedom and p-value > 0.25 provides evidence that the parameter is zero and should be dropped from the model. To determine if further simplification is possible, a likelihood-ratio test between the model with season and latitude is compared to the model with only latitude. The resulting test (p-value ~ 0.1) provides evidence that season can be dropped from the model. Lastly, a likelihood-ratio test between the intercept model and a model with only latitude has an associated p-value of < .0001. Therefore, we conclude that the model of best fit for our data is one containing only the parameter latitude.
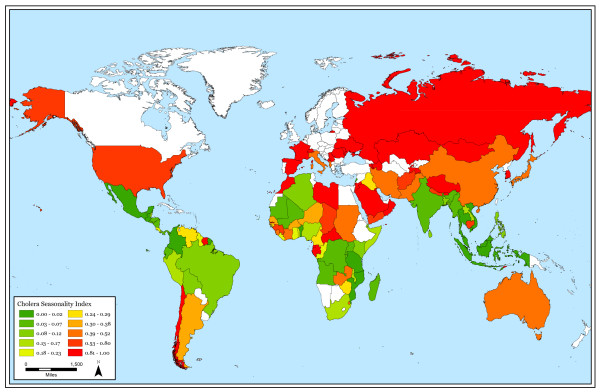
To map these results by country, we create a cholera seasonality index. To create this index, the total number of June to November outbreaks are subtracted from the total number of December to May outbreaks for each country, yielding a positive number, a negative number, or a zero. Subsequently, that number is divided by the total number of outbreaks (December to May outbreaks + June to November outbreaks), yielding a positive or negative number, ranging from -1 to 1, for each country. The absolute value of this number shows seasonality, regardless of hemisphere. These values are broken into 8 categories of increasing seasonality. A number close to 1 indicates strong seasonality. A number close to 0 indicates no cholera seasonality.
Results
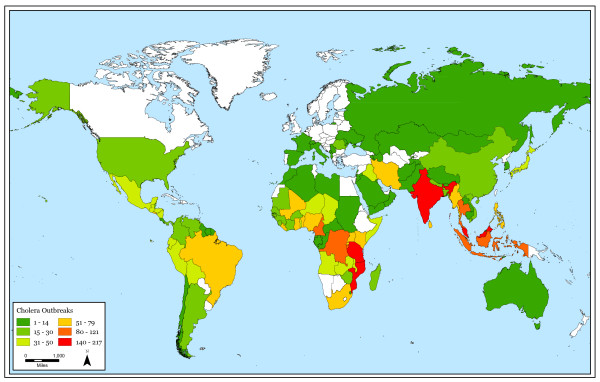
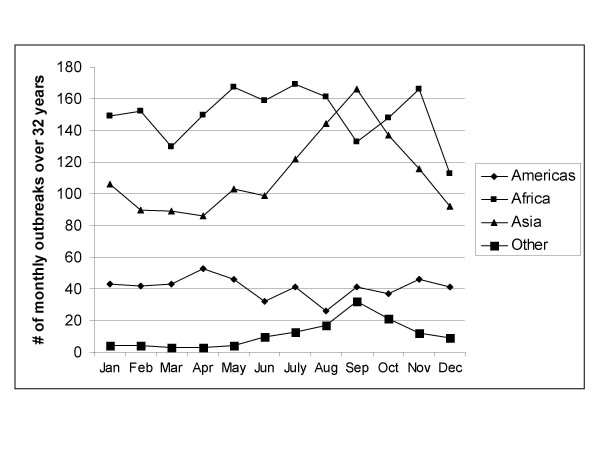
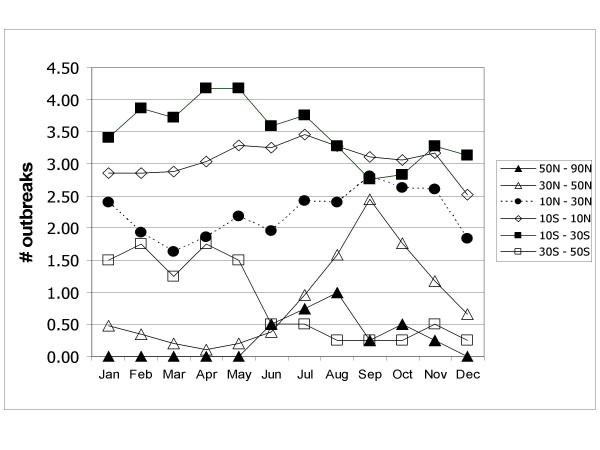
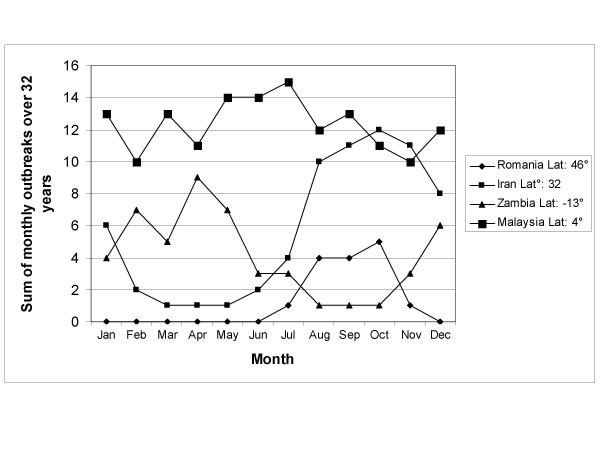
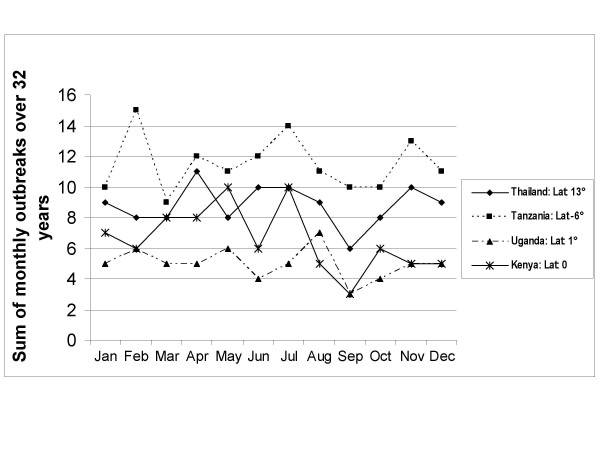
Seasonal patterns persist in higher absolute latitudes but cholera outbreaks do not follow a clear seasonal pattern near the equator. Also, cholera outbreaks occur more often closer to the equator than at higher latitudes. Figure 1 shows the total number of cholera outbreaks by country for the 32-year study period. Although there are exceptions, this figure illustrates that there are more outbreaks in countries near the equator in comparison to countries in higher absolute latitudes. Figure 2 displays the cholera seasonality index to show the strength of cholera seasonality in each country. The graphic shows that stronger seasonal patterns (in red) appear more prevalent in countries further from the equator. Figure 3 shows the total number of outbreaks per region over 32 years. Although annual peaks are evident, it is difficult to determine distinct seasonal patterns in cholera outbreaks across regions. However, grouping countries by latitude range, rather than region, makes these seasonal peaks more obvious. Figure 4 groups countries within absolute latitude ranges showing the average number of outbreaks per month over the 32-year period. Figure 5 illustrates seasonal cholera patterns in four countries, Romania, Iran, Zambia, and Malaysia. These four countries are purposefully selected because they represent different latitude ranges, including one from the southern hemisphere, and demonstrate clear seasonal patterns. The 10 to 30 north range has a similar pattern to the 30 to 50 north range, but the 30 to 50 range shows a dramatic seasonal peak. The peaks become more obvious moving from the 10 to 30 south range to the 30 to 50 south range as well. The seasonal cycles are especially clear when comparing the 30 to 50 north and south latitude ranges: they have the exact opposite seasonal cycles. Among lower latitude ranges, seasonal cycles are either non-existent or show few clear patterns. Figure 6 illustrates the total number of cholera outbreaks by month for four countries within 15 degrees of the equator, Thailand, Tanzania, Uganda, and Kenya. These countries are purposefully selected to represent equatorial countries with sizeable cholera cases that do not show dramatic seasonal variation.
Figure 1.
Total number of cholera outbreaks, 1974–2005.
Figure 2.
Cholera outbreak seasonality, 1974–2005.
Figure 3.
Monthly cholera outbreaks by region.
Figure 4.
Average # of monthly cholera outbreaks by latitude.
Figure 5.
Distinct seasonal cholera patterns in four countries.
Figure 6.
Cholera patterns in four countries within 15 degrees of the equator.
The results from the statistical models confirm these visible seasonal patterns. Table 1 shows that for the combined hemispheres statistical model, both latitude and latitude*season interaction are significantly associated with the number of cholera outbreaks in the preceding 12 months. For season one (December-May), the multiplicative effect on the number of expected outbreaks for each 1-unit increase in latitude is 0.94. There is a 6% decrease in the expected number of outbreaks for each 1-unit increase in latitude in season 1. This conclusion is reached by using the following equation, and adding the common exponentiated factors: e-.12*1e-.09 [latitude]e.03*1*latitude = e-.12e-.06 [latitude]. For example, for a latitude of 20°, the number of expected outbreaks for season two (June-November) is e0.47 = 1.61 times the expected number for season one. In other words, for latitude of 20°, there is a 61% increase in the number of expected outbreaks for season two compared to season one according to the equation: e-.12[season]e-.09*20e.03*season*20 = e-1.76 e0.47*season.
Table 1.
Influences on Cholera Seasonality: Combined Hemisphere Model
| Variables | Model-Season | Model-Latitude | Combined Model | Combined Model with Interaction |
| Season | 0.29* | 0.34* | -0.12 | |
| Latitude | 0.04* | -.04* | -0.09* | |
| Season*Latitude | 0.03* | |||
| Over-dispersion | 1.777 | 1.482 | 1.444 | 1.418 |
| Deviance | 1714 | 1713 | 1706 | 1693 |
| Pearson Chi-Square | 1633 | 1892 | 1966 | 2069 |
| Log-Likelihood | 676.8 | 767 | 783.1 | 798.1 |
| N | 1678 | 1678 | 1678 | 1678 |
*p < .01
For the model including only countries in the northern hemisphere, both latitude and seasonality are important (Table 2). For season one, the multiplicative effect on the number of expected outbreaks for each 1-unit increase in latitude is e-.058 = 0.94; therefore, there is a 6% decrease in the expected number of outbreaks for each 1-unit increase in latitude. For latitude of 20°, the number of expected outbreaks for season two is e0.47 = 1.61 times the expected number for season one. For 20° latitude, there is a 61% increase in the number of expected outbreaks for season two compared to season one
Table 2.
Influences on Cholera Seasonality: Northern Hemisphere Model
| Variables | Model-Season | Model-Latitude | Combined Model | Combined Model with Interaction |
| Season | 0.35* | 0.47* | -0.15 | |
| Latitude | -0.04* | -0.04* | -0.09* | |
| Season*Latitude | 0.03* | |||
| Over-dispersion | 1.921 | 1.636 | 1.582 | 1.551 |
| Deviance | 1267 | 1266 | 1259 | 1248 |
| Pearson Chi-Square | 1362 | 1563 | 1648 | 1760 |
| Log-Likelihood | 196.1 | 254.4 | 269.7 | 281.9 |
| N | 1282 | 1282 | 1282 | 1282 |
*p < .01
For the southern hemisphere only model, latitude is the most important variable (Table 3). Season is not significant in the model, and the season*latitude interaction is only marginally significant at the 0.1% level. The multiplicative effect on the number of expected outbreaks for each 1-unit increase in latitude is e-.036 = 0.96; therefore, there is a 4% decrease in the expected number of outbreaks for each 1-unit increase in latitude in the southern hemisphere.
Table 3.
Influences on Cholera Seasonality: Southern Hemisphere Model
| Variables | Model-Season | Model-Latitude | Combined Model | Combined Model with Interaction |
| Season | 0.18 | 0.22** | -0.03 | |
| Latitude | -0.04* | -0.04* | -0.07* | |
| Season*Latitude | 0.02** | |||
| Over-dispersion | 1.229 | 1.096 | 1.083 | 1.074 |
| Deviance | 441.1 | 440.7 | 440.3 | 439.7 |
| Pearson Chi-Square | 329.1 | 327.3 | 334.8 | 337.0 |
| Log-Likelihood | 506.8 | 521.8 | 523.8 | 524.0 |
| N | 394 | 394 | 394 | 394 |
*p < .01, **p < .10
Discussion
The results suggest the existence of seasonal patterns of cholera outbreaks over the last 32 years and demonstrate that these patterns differ by latitude. The patterns are evident in visual representation and confirmed by statistical analysis. In higher latitudes in both hemispheres, cholera outbreaks exhibit seasonal patterns while seasonal patterns do not persist near the equator. Cholera outbreaks are also more common near the equator. These findings suggest that larger climactic factors may be at play in the appearance of V. cholerae. These macro-level climatic factors are certainly related to a combination of complex local-level parameters that are described in the literature review section of this paper. This paper reviews many of the postulated reasons for temporal fluctuations of cholera, including both seasonal cycles and interannual variability. Although it is still difficult to determine whether the models linking cholera incidence to environmental parameters are valid outside of particular study areas, this paper reflects global trends. The body of evidence suggests that cholera is tied to environmental and temporal parameters ranging from local to global scales. Our study complements previous research by showing that cholera fluctuations are linked to latitude, and this confirms our expectation of seasonal cholera patterns over time. These findings are also consistent with a disease that is linked to climate, and it appears that there is an association between cholera and both micro- and macro-level environmental parameters. Ongoing research investigates the relationship between cholera and both environmental and climate variables in several local sites around the world where there is detailed cholera and population data [60].
There are several limitations to this study. Primarily, the WHO cholera surveillance database is incomplete. Not all cholera outbreaks are reported to the WHO, and some countries have better reporting systems than others. Other countries, such as Bangladesh, do not regularly report to the WHO, so a complete record of all global cholera outbreaks does not exist. Additionally, the WHO Weekly Record does not always provide details on the specific location or serotype of the outbreak, so it is not possible to examine within-country variation in cholera seasonality or differences in seasonal patterns by cholera serotype. However, the dataset is adequate to show general patterns around the world despite missing data. There are other very important local environment and climate parameters (rainfall, temperature, regional differences, etc.) that require further consideration in future studies. Despite the limitations, this study is a unique opportunity to gain insight into cholera patterns around the globe. The authors believe that the trends presented in this analysis would hold if additional data were included.
Conclusion
These results indirectly contribute to the growing debate about the effects of climate change and global warming. As climate change threatens to increase global temperature, resulting rises in sea levels and temperatures may influence the temporal fluctuations of cholera, potentially increasing the frequency and duration of cholera outbreaks. As found by this study, countries near the equator, which generally have higher and more constant temperatures, have a greater and more constant level of cholera outbreaks. Countries further from the equator in higher absolute latitudes typically have seasonal cholera outbreaks, mostly in the warmer months, and they have lower overall outbreak levels. However, climate change may influence the strength, duration, or appearance of these annual seasonal patterns. The potential alterations in seasonal cholera outbreaks may leave some countries unprepared for outbreaks outside of previously-recorded seasonal patterns. Greater numbers of cholera outbreaks and more unpredictability may increase both morbidity and mortality.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
ME conceived of the study and supervised all aspects of its implementation, CF and ME wrote the paper and MSI helped write the background section, MA helped with the study design and statistical analysis.
Acknowledgments
Acknowledgements
This work was supported by a grant from the NOAA Oceans and Human Health Program.
Contributor Information
Michael Emch, Email: emch@email.unc.edu.
Caryl Feldacker, Email: caryl@unc.edu.
M Sirajul Islam, Email: sislam@icddrb.org.
Mohammad Ali, Email: mali@IVI.INT.
References
- Islam MS, Drasar BS, Sack RB. Probable Role of Blue-Green-Algae in Maintaining Endemicity and Seasonality of Cholera in Bangladesh – a Hypothesis. Journal of Diarrhoeal Diseases Research. 1994;12:245–256. [PubMed] [Google Scholar]
- Koelle K, Rodo X, Pascual M, Yunus M, Mostafa G. Refractory periods and climate forcing in cholera dynamics. Nature. 2005;436:696–700. doi: 10.1038/nature03820. [DOI] [PubMed] [Google Scholar]
- Koelle K, Pascual M. Disentangling extrinsic from intrinsic factors in disease dynamics: A nonlinear time series approach with an application to cholera. American Naturalist. 2004;163:901–913. doi: 10.1086/420798. [DOI] [PubMed] [Google Scholar]
- Bouma MJ, Pascual M. Seasonal and interannual cycles of endemic cholera in Bengal 1891–1940 in relation to climate and geography. Hydrobiologia. 2001;460:147–156. doi: 10.1023/A:1013165215074. [DOI] [Google Scholar]
- Samadi AR, Chowdhury MK, Huq MI, Khan MU. Seasonality of classical and El Tor cholera in Dhaka, Bangladesh: 17-year trends. Trans R Soc Trop Med Hyg. 1983;77:853–856. doi: 10.1016/0035-9203(83)90306-1. [DOI] [PubMed] [Google Scholar]
- Mhalu FS, Mntenga WM, Mtango FD. Seasonality of cholera in Tanzania: possible role of rainfall in disease transmission. East Afr Med J. 1987;64:378–387. [PubMed] [Google Scholar]
- Miller CJ, Feachem RG, Drasar BS. Cholera epidemiology in developed and developing countries: new thoughts on transmission, seasonality, and control. Lancet. 1985;1:261–262. doi: 10.1016/S0140-6736(85)91036-0. [DOI] [PubMed] [Google Scholar]
- Heidelberg JF, Heidelberg KB, Colwell RR. Seasonality of Chesapeake Bay bacterioplankton species. Appl Environ Microbiol. 2002;68:5488–5497. doi: 10.1128/AEM.68.11.5488-5497.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam M, Hasan NA, Sadique A, Bhuiyan NA, Ahmed KU, Nusrin S, Nair GB, Siddique AK, Sack RB, Sack DA, et al. Seasonal cholera caused by Vibrio cholerae serogroups O1 and O139 in the coastal aquatic environment of Bangladesh. Appl Environ Microbiol. 2006;72:4096–4104. doi: 10.1128/AEM.00066-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipp EK, Huq A, Colwell RR. Effects of global climate on infectious disease: the cholera model. Clinical Microbiology Reviews. 2002;15:757. doi: 10.1128/CMR.15.4.757-770.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack RB, Siddique AK, Longini IM, Nizam A, Yunus M, Islam MS, Morris JG, Ali A, Huq A, Nair GB, et al. A 4-year study of the epidemiology of Vibrio cholerae in four rural areas of Bangladesh. Journal of Infectious Diseases. 2003;187:96–101. doi: 10.1086/345865. [DOI] [PubMed] [Google Scholar]
- Colwell R, Huq A. Marine ecosystems and cholera. Hydrobiologia. 2001;460:141–145. doi: 10.1023/A:1013111016642. [DOI] [Google Scholar]
- Pascual M, Dobson A. Seasonal patterns of infectious diseases. Plos Medicine. 2005;2:18–20. doi: 10.1371/journal.pmed.0020005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Bouma MJ, Dobson AP. Cholera and climate: revisiting the quantitative evidence. Microbes Infect. 2002;4:237–245. doi: 10.1016/S1286-4579(01)01533-7. [DOI] [PubMed] [Google Scholar]
- Pascual M, Rodo X, Ellner SP, Colwell R, Bouma MJ. Cholera dynamics and El Nino-Southern Oscillation. Science. 2000;289:1766–1769. doi: 10.1126/science.289.5485.1766. [DOI] [PubMed] [Google Scholar]
- Kiorboe T, Nielsen TG. Regulation of Zooplankton Biomass and Production in a Temperate, Coastal Ecosystem 1. Copepods. Limnology and Oceanography. 1994;39:493–507. [Google Scholar]
- Faruque SM, Naser IB, Islam MJ, Faruque ASG, Ghosh AN, Nair GB, Sack DA, Mekalanos JJ. Seasonal epidemics of cholera inversely correlate with the prevalence of environmental cholera phages. Proc Natl Acad Sci U S A. 2005;102:1702–1707. doi: 10.1073/pnas.0408992102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zo YG, Rivera IN, Russek-Cohen E, Islam MS, Siddique AK, Yunus M, Sack RB, Huq A, Colwell RR. Genomic profiles of clinical and environmental isolates of Vibrio cholerae O1 in cholera-endemic areas of Bangladesh. Proc Natl Acad Sci USA. 2002;99:12409–12414. doi: 10.1073/pnas.192426499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huq A, Colwell RR. Environmental factors associated with emergence of disease with special reference to cholera. Eastern Mediterraneal Health Journal. 1996;2:37–45. [Google Scholar]
- Huq A, Sack RB, Nizam A, Longini IM, Nair GB, Ali A, Morris JG, Khan MNH, Siddique AK, Yunus M, et al. Critical factors influencing the occurrence of Vibrio cholerae in the environment of Bangladesh. Appl Environ Microbiol. 2005;71:4645–4654. doi: 10.1128/AEM.71.8.4645-4654.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MS. Effect of various biophysicochemical conditions on toxigenicity of Vibrio cholerae 01 during survival with a green alga, Rhizoclonium fontanum, in an artificial aquatic environment. Can J Microbiol. 1990;36:464–468. doi: 10.1139/m90-081. [DOI] [PubMed] [Google Scholar]
- Islam MS, Drasar BS, Bradley DJ. Survival of toxigenic Vibrio cholerae O1 with a common duckweed, Lemna minor, in artificial aquatic ecosystems. Trans R Soc Trop Med Hyg. 1990;84:422–424. doi: 10.1016/0035-9203(90)90345-F. [DOI] [PubMed] [Google Scholar]
- Islam MS, Drasar BS, Sack RB. The aquatic environment as a reservoir of Vibrio cholerae: a review. J Diarrhoeal Dis Res. 1993;11:197–206. [PubMed] [Google Scholar]
- Islam MS, Rahim Z, Alam MJ, Begum S, Moniruzzaman SM, Umeda A, Amako K, Albert MJ, Sack RB, Huq A, et al. Association of Vibrio cholerae O1 with the cyanobacterium, Anabaena sp., elucidated by polymerase chain reaction and transmission electron microscopy. Trans R Soc Trop Med Hyg. 1999;93:36–40. doi: 10.1016/S0035-9203(99)90171-2. [DOI] [PubMed] [Google Scholar]
- Islam MS, Talukder KA, Khan NH, Mahmud ZH, Rahman MZ, Nair GB, Siddique AK, Yunus M, Sack DA, Sack RB, et al. Variation of toxigenic Vibrio cholerae O1 in the aquatic environment of Bangladesh and its correlation with the clinical strains. Microbiol Immunol. 2004;48:773–777. doi: 10.1111/j.1348-0421.2004.tb03604.x. [DOI] [PubMed] [Google Scholar]
- The Weekly Epidemiological Record (WER) http://www.who.int/wer/en/
- Khan MU, Samadi AR, Huq MI, Yunus M, Eusof A. Simultaneous classical and El Tor cholera in Bangladesh. J Diarrhoeal Dis Res. 1984;2:13–18. [PubMed] [Google Scholar]
- Merson MH, Black RE, Khan MU, Huq I. Enterotoxigenic Escherichia coli diarrhea: acquired immunity and transmission in an endemic area. Cholera and related diarrheas: molecular basis of a global health problem 43rd Nobel Symposium S Karger, Basel, Switzerland. 1980. pp. 34–45.
- Glass RI, Becker S, Huq MI, Stoll BJ, Khan MU, Merson MH, Lee JV, Black RE. Endemic cholera in rural Bangladesh, 1966–1980. American Journal of Epidemiology. 1982;116:959–970. doi: 10.1093/oxfordjournals.aje.a113498. [DOI] [PubMed] [Google Scholar]
- Baqui AH, Sack RB, Black RE, Haider K, Hossain A, Alim AR, Yunus M, Chowdhury HR, Siddique AK. Enteropathogens associated with acute and persistent diarrhea in Bangladeshi children less than 5 years of age. J Infect Dis. 1992;166:792–796. doi: 10.1093/infdis/166.4.792. [DOI] [PubMed] [Google Scholar]
- Black RE, Merson MH, Rahman AS, Yunus M, Alim AR, Huq I, Yolken RH, Curlin GT. A two-year study of bacterial, viral, and parasitic agents associated with diarrhea in rural Bangladesh. J Infect Dis. 1980;142:660–664. doi: 10.1093/infdis/142.5.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn TA, Cassanos JG. Epidemiology of endemic cholera. Public Health Rep. 1960;75:791–803. [PMC free article] [PubMed] [Google Scholar]
- Drasar BS. Pathogenesis and ecology: the case of cholera. J Trop Med Hyg. 1992;95:365–372. [PubMed] [Google Scholar]
- Emch M. Diarrheal disease risk in Matlab, Bangladesh. Soc Sci Med. 1999;49:519–530. doi: 10.1016/S0277-9536(99)00146-X. [DOI] [PubMed] [Google Scholar]
- Gangarosa EJ, Mosley WH. Epidemiology and surveillance of cholera. Cholera Philadelphia: WB Saunders. 1974. pp. 381–403.
- Huq A, Colwell RR. Vibrios in the marine and estuarine environment: Tracking Vibrio Cholerae. Ecosystem Health. 1996;2:198–214. [Google Scholar]
- Kaper JB, Morris JG, Jr, Levine MM. Cholera. Clinical Microbiology Reviews. 1995;8:48–86. doi: 10.1128/cmr.8.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelle K, Pascual M, Yunus M. Pathogen adaptation to seasonal forcing and climate change. Proc Biol Sci. 2005;272:971–977. doi: 10.1098/rspb.2004.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longini IM, Jr, Yunus M, Zaman K, Siddique AK, Sack RB, Nizam A. Epidemic and Endemic Cholera Trends over a 33-Year Period in Bangladesh. Journal of Infectious Diseases. 2002;186:246–251. doi: 10.1086/341206. [DOI] [PubMed] [Google Scholar]
- Siddique AK, Zaman K, Baqui AH, Akram K, Mutsuddy P, Eusof A, Haider K, Islam S, Sack RB. Cholera epidemics in Bangladesh: 1985–1991. J Diarrhoeal Dis Res. 1992;10:79–86. [PubMed] [Google Scholar]
- Tauxe RV, Mintz ED, Quick RE. Epidemic cholera in the new world: translating field epidemiology into new prevention strategies. Emerg Infect Dis. 1995;1:141–146. doi: 10.3201/eid0104.950408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emch M, Ali M. Spatial and temporal patterns of diarrheal disease in Matlab, Bangladesh. Environment and Planning A. 2001;33:339–350. doi: 10.1068/a33132. [DOI] [Google Scholar]
- Martin AR, Mosely WH, Sau BB, Ahmed S, Huq I. Epidemiologic analysis of endemic cholera in urban East Pakistan, 1964–1966. American Journal of Epidemiology. 1969;89:572. doi: 10.1093/oxfordjournals.aje.a120970. [DOI] [PubMed] [Google Scholar]
- McCormack WM, Mosley WH, Fahimuddin M, Benenson AS. Endemic Cholera in Rural East Pakistan. American Journal of Epidemiology. 1969;89:393. doi: 10.1093/oxfordjournals.aje.a120953. [DOI] [PubMed] [Google Scholar]
- Codeço T. Endemic and epidemic dynamics of cholera: the role of the aquatic reservoir. BMC Infect Dis. 2001;1:1471–2334. doi: 10.1186/1471-2334-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Cholera in 1997. Wkly Epidemiol Rec. 1998;73:201–208. [PubMed] [Google Scholar]
- Acosta CJ, Galindo CM, Kimario J, Senkoro K, Urassa H, Casals C, Corachan M, Eseko N, Tanner M, Mshinda H. Cholera outbreak in southern Tanzania: risk factors and patterns of transmission. Emerging Infectious Diseases. 2001;7:583–587. doi: 10.3201/eid0707.010741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman C. Mozambique cholera will affect region. S Afr Med J. 2002;92:104–106. [PubMed] [Google Scholar]
- Aragón MBA, Tabbard P, Chambule J, Santos C, Noya A. Epidemiologia da cólera em Moçambique no período de 1973–1992. Revista Saúde Pública. 1994;28:332–336. doi: 10.1590/s0034-89101994000500004. [DOI] [PubMed] [Google Scholar]
- Folgosa E, Mastrandrea S, Cappuccinelli P, Uzzau S, Rappelli P, Brian MJ, Colombo MM. Molecular identification of pathogenicity genes and ERIC types in Vibrio cholerae O1 epidemic strains from Mozambique. Epidemiol Infect. 2001;127:17–25. doi: 10.1017/S0950268801005623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell RR, Kaper J, Joseph SW. Vibrio cholerae, Vibrio parahaemolyticus, and other vibrios: occurrence and distribution in Chesapeake Bay. Science. 1977;198:394. [PubMed] [Google Scholar]
- Colwell RR, Tamplin ML, Brayton PR, Gauzens AL, Tall BD, Herrington D, Levine MM, Hall S, Huq A, Sack DA. Environmental aspects of Vibrio cholerae in transmission of cholera. Advances in research on cholera and related diarrhoea, 7th ed Tokyo, KTK Scientific Publishers. 1990. pp. 327–343.
- Colwell RR, Spira WM. The ecology of Vibrio cholerae. Cholera: Current Topics in Infections Disease. 1993. pp. 3–60.
- Colwell RR, Huq A. Disease in Evolution. Vol. 740. New York: New York Acad Sciences; 1994. Environmental Reservoir of Vibrio-Cholerae – the Causative Agent of Cholera; pp. 44–54. [DOI] [PubMed] [Google Scholar]
- Islam MS, Drasar BS, Bradley DJ. Attachment of toxigenic Vibrio cholerae 01 to various freshwater plants and survival with a filamentous green alga, Rhizoclonium fontanum. J Trop Med Hyg. 1989;92:396–401. [PubMed] [Google Scholar]
- Islam MS, Drasar BS, Sack RB. The aquatic flora and fauna as reservoirs of Vibrio cholerae: a review. J Diarrhoeal Dis Res. 1994;12:87–96. [PubMed] [Google Scholar]
- Khan MU. Scientific Report 45. Dhaka, Bangladesh.: International Centre for Diarrhoeal Disease Research; 1981. Role of water supply and sanitation in the incidence of cholera in refugee camps. [Google Scholar]
- Franco AA, Fix AD, Prada A, Paredes E, Palomino JC, Wright AC, Johnson JA, McCarter R, Guerra H, Morris JG., Jr Cholera in Lima, Peru, correlates with prior isolation of Vibrio cholerae from the environment. American Journal of Epidemiology. 1997;146:1067–1075. doi: 10.1093/oxfordjournals.aje.a009235. [DOI] [PubMed] [Google Scholar]
- Colwell RR. Viable but nonculturable bacteria: a survival strategy. J Infect Chemother. 2000;6:121–125. doi: 10.1007/PL00012151. [DOI] [PubMed] [Google Scholar]
- Emch M, Feldacker C, Yunus M, Streatfield PK, DinhThiem V, Canh DG, Ali M. Local Environmental Predictors of Cholera in Bangladesh and Vietnam. The American Journal of Tropical Medicine and Hygiene. 2008;78:823–832. [PubMed] [Google Scholar]
- Huq A, Xu B, Chowdhury MAR, Islam MS, Montilla R, Colwell RR. A simple filtration method to remove plankton-associated Vibrio cholerae in raw water supplies in developing countries. Appl Environ Microbiol. 1996;62:2508–2512. doi: 10.1128/aem.62.7.2508-2512.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty S, Mukhopadhyay AK, Bhadra RK, Ghosh AN, Mitra R, Shimada T, Yamasaki S, Faruque SM, Takeda Y, Colwell RR, et al. Virulence genes in environmental strains of Vibrio cholerae. Appl Environ Microbiol. 2000;66:4022–4028. doi: 10.1128/AEM.66.9.4022-4028.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell RR, Spira WM, Barua D. Cholera. Cholera. 1992;12 [Google Scholar]
- Huq A, West PA, Small EB, Huq MI, Colwell RR. Influence of water temperature, salinity, and pH on survival and growth of toxigenic Vibrio cholerae serovar 01 associated with live copepods in laboratory microcosms. Appl Environ Microbiol. 1984;48:420–424. doi: 10.1128/aem.48.2.420-424.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MS, Ahsan S, Khan SI, Ahmed QS, Rashid MH, Islam KM, Sack RB. Virulence properties of rough and smooth strains of Vibrio cholerae O1. Microbiol Immunol. 2004;48:229–235. doi: 10.1111/j.1348-0421.2004.tb03518.x. [DOI] [PubMed] [Google Scholar]
- Miller CJ, Drasar BS, Feachem RG. Cholera and estuarine salinity in Calcutta and London. Lancet. 1982;1:1216–1218. doi: 10.1016/S0140-6736(82)92340-6. [DOI] [PubMed] [Google Scholar]
- Venkateswaran K, Nakano H, Okabe T, Takayama K, Matsuda O, Hashimoto H. Occurrence and distribution of Vibrio spp., Listonella spp., and Clostridium botulinum in the Seto Inland Sea of Japan. Appl Environ Microbiol. 1989;55:559–567. doi: 10.1128/aem.55.3.559-567.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faruque SM, Albert MJ, Mekalanos JJ. Epidemiology, Genetics, and Ecology of Toxigenic Vibrio cholerae. Microbiol Mol Biol Rev. 1998;62:1301–1314. doi: 10.1128/mmbr.62.4.1301-1314.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis VR, Russek-Cohen E, Choopun N, Rivera ING, Gangle B, Jiang SC, Rubin A, Patz JA, Huq A, Colwell RR. Predictability of Vibrio cholerae in Chesapeake Bay. Appl Environ Microbiol. 2003;69:2773–2785. doi: 10.1128/AEM.69.5.2773-2785.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CJ, Drasar BS, Feachem RG. Response of toxigenic Vibrio cholerae 01 to physico-chemical stresses in aquatic environments. J Hyg (Lond) 1984;93:475–495. doi: 10.1017/s0022172400065074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MS, Hasan MK, Miah MA, Huq A, Bardhan PK, Sack RB, Albert MJ. Specificity of Cholera Screen test during an epidemic of cholera-like disease due to Vibrio cholerae O139 synonym Bengal. Trans R Soc Trop Med Hyg. 1994;88:424–425. doi: 10.1016/0035-9203(94)90413-8. [DOI] [PubMed] [Google Scholar]
- Islam MS, Hasan MK, Miah MA, Yunus M, Zaman K, Albert MJ. Isolation of Vibrio-Cholerae O139 Synonym Bengal from the Aquatic Environment in Bangladesh – Implications for Disease Transmission. Applied and Environmental Microbiology. 1994;60:1684–1686. doi: 10.1128/aem.60.5.1684-1686.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider DR, Parker CD. Purification and characterization of the mucinase of Vibrio cholerae. J Infect Dis. 1982;145:474–482. doi: 10.1093/infdis/145.4.474. [DOI] [PubMed] [Google Scholar]
- Islam MS, Aziz KMS. Association of vibrios with some hydrophytic plants. Bangladesh J Microbiol. 1981;1:70–72. [Google Scholar]
- Islam MS, Hasan MK, Miah MA, Qadri F, Yunus M, Sack RB, Albert MJ. Isolation of Vibrio cholerae O139 Bengal from water in Bangladesh. Lancet. 1993;342:430. doi: 10.1016/0140-6736(93)92840-P. [DOI] [PubMed] [Google Scholar]
- Colwell RR, Brayton P, Herrington D, Tall B, Huq A, Levine MM. Viable but non culturable Vibrio cholerae 01 revert to a cultivable state in the human intestine. World Journal of Microbiology & Biotechnology. 1996;12:28–31. doi: 10.1007/BF00327795. [DOI] [PubMed] [Google Scholar]
- Huq A, Colwell RR, Rahman R, Ali A, Chowdhury MA, Parveen S, Sack DA, Russek-Cohen E. Detection of Vibrio cholerae O1 in the aquatic environment by fluorescent-monoclonal antibody and culture methods. Appl Environ Microbiol. 1990;56:2370–2373. doi: 10.1128/aem.56.8.2370-2373.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MS, Drasar BS, Sack RB. Probable role of blue-green algae in maintaining endemicity and seasonality of cholera in Bangladesh: a hypothesis. J Diarrhoeal Dis Res. 1994;12:245–256. [PubMed] [Google Scholar]
- Islam MS, Alam MJ, Begum A, Rahim Z, Felsenstein A, Albert MJ. Occurrence of culturable Vibrio cholerae O139 with ctx gene in various components of the aquatic environment in Bangladesh. Trans R Soc Trop Med Hyg. 1996;90:128–128. doi: 10.1016/S0035-9203(96)90110-8. [DOI] [PubMed] [Google Scholar]
- Huq A, Small EB, West PA, Huq MI, Rahman R, Colwell RR. Ecological relationships between Vibrio cholerae and planktonic crustacean copepods. Appl Environ Microbiol. 1983;45:275–283. doi: 10.1128/aem.45.1.275-283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein P, Ford TE, Colwell RR. Health and Climate Change: Marine ecosystems. The Lancet. 1993;342:1216–1219. doi: 10.1016/0140-6736(93)92191-U. [DOI] [PubMed] [Google Scholar]
- Alam M, Sultana M, Nair GB, Sack RB, Sack DA, Siddique AK, Ali A, Huq A, Colwell RR. Toxigenic Vibrio cholerae in the aquatic environment of Mathbaria, Bangladesh. Applied and Environmental Microbiology. 2006;72:2849–2855. doi: 10.1128/AEM.72.4.2849-2855.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell RR. A voyage of discovery: cholera, climate and complexity. Environ Microbiol. 2002;4:67–69. doi: 10.1046/j.1462-2920.2002.00270.x. [DOI] [PubMed] [Google Scholar]
- Nalin DR. Cholera, copepods, and chitinase. Lancet. 1976;2:958. doi: 10.1016/S0140-6736(76)90915-6. [DOI] [PubMed] [Google Scholar]
- Nalin DR, Daya V, Reid A, Levine MM, Cisneros L. Adsorption and growth of Vibrio cholerae on chitin. Infection and Immunity. 1979;25:768–770. doi: 10.1128/iai.25.2.768-770.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huq A, Colwell RR, Chowdhury MA, Xu B, Moniruzzaman SM, Islam MS, Yunus M, Albert MJ. Coexistence of Vibrio cholerae O1 and O139 Bengal in plankton in Bangladesh. Lancet. 1995;345:1249. doi: 10.1016/S0140-6736(95)92038-2. [DOI] [PubMed] [Google Scholar]
- Epstein PR. Algal blooms in the spread and persistence of cholera. Biosystems. 1993;31:209–221. doi: 10.1016/0303-2647(93)90050-M. [DOI] [PubMed] [Google Scholar]
- Oppenheimer JR, Ahmad MG, Huq A, Haque KA, Alam A, Aziz KMS, Ali S, Haque ASM. Limnological studies in three ponds in Dhaka, Bangladesh. Bangladesh J Fisher. 1978;1:1–28. [Google Scholar]
- Rodo X, Pascual M, Fuchs G, Faruque ASG. ENSO and cholera: A nonstationary link related to climate change? Proceedings of the National Academy of Sciences of the United States of America. 2002;99:12901–12906. doi: 10.1073/pnas.182203999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobitz B, Beck L, Huq A, Wood B, Fuchs G, Faruque ASG, Colwell R. Climate and infectious disease: Use of remote sensing for detection of Vibrio cholerae by indirect measurement. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:1438–1443. doi: 10.1073/pnas.97.4.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speelmon EC, Checkley W, Gilman RH, Patz J, Calderon M, Manga S. Cholera Incidence and El Nino-Related Higher Ambient Temperature. JAMA. 2000;283:3072–3074. doi: 10.1001/jama.283.23.3072-a. [DOI] [PubMed] [Google Scholar]
- Seas C, Miranda J, Gil AI, Leon-Barua R, Patz J, Huq A, Colwell RR, Sack RB. New insights on the emergence of cholera in Latin America during 1991: the Peruvian experience. American Journal of Tropical Medicine and Hygiene. 2000;62:513–517. doi: 10.4269/ajtmh.2000.62.513. [DOI] [PubMed] [Google Scholar]
- Epstein PR. Health applications of remote sensing and climate modeling. People and pixels: Linking remote sensing and social science. 1998.
- Hughes JM, Boyce JM, Levine RJ, Khan M, Aziz KM, Huq MI, Curlin GT. Epidemiology of eltor cholera in rural Bangladesh: importance of surface water in transmission. Bull World Health Organ. 1982;60:395–404. [PMC free article] [PubMed] [Google Scholar]
- Birmingham ME, Lee LA, Ndayimirije N, Nkurikiye S, Hersh BS, Wells JG, Deming MS. Epidemic cholera in Burundi: patterns of transmission in the Great Rift Valley Lake region. Lancet. 1997;349:981–985. doi: 10.1016/S0140-6736(96)08478-4. [DOI] [PubMed] [Google Scholar]
- Sommer A, Woodward WE. The influence of protected water supplies on the spread of classical-Inaba and El Tor-Ogawa cholera in rural East Bengal. Lancet. 1972;2:985–987. doi: 10.1016/S0140-6736(72)92401-4. [DOI] [PubMed] [Google Scholar]
- Emch M. Relationships between flood control, kala-azar, and diarrheal disease in Bangladesh. ENVIRONMENT AND PLANNING A. 2000;32:1051–1064. doi: 10.1068/a32193. [DOI] [Google Scholar]