Abstract
Background
Impaired coronary vasodilation to both endothelial-dependent and endothelial-independent stimuli have been associated with atherosclerosis. Direct measurement of coronary vasodilation using x-ray angiography or intravascular ultrasound is invasive and, thus, not appropriate for asymptomatic patients or for serial follow-up. In this study, high-resolution coronary cardiovascular magnetic resonance (CMR) was used to investigate the vasodilatory response to nitroglycerine (NTG) of asymptomatic patients at high risk for CAD.
Methods
A total of 46 asymptomatic subjects were studied: 13 high-risk patients [8 with diabetes mellitus (DM), 5 with end stage renal disease (ESRD)] and 33 age-matched controls. Long-axis and cross-sectional coronary artery images were acquired pre- and 5 minutes post-sublingual NTG using a sub-mm-resolution multi-slice spiral coronary CMR sequence. Coronary cross sectional area (CSA) was measured on pre- and post-NTG images and % coronary vasodilation was calculated.
Results
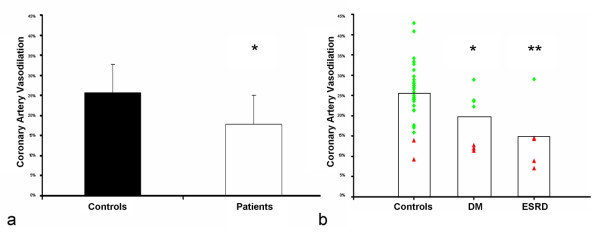
Patients with DM and ESRD had impaired coronary vasodilation to NTG compared to age-matched controls (17.8 ± 7.3% vs. 25.6 ± 7.1%, p = 0.002). This remained significant for ESRD patients alone (14.8 ± 7.7% vs. 25.6 ± 7.1%; p = 0.003) and for DM patients alone (19.8 ± 6.3% vs. 25.6 ± 7.1%; p = 0.049), with a non-significant trend toward greater impairment in the ESRD vs. DM patients (14.8 ± 7.7% vs. 19.8 ± 6.3%; p = 0.23).
Conclusion
Noninvasive coronary CMR demonstrates impairment of coronary vasodilation to NTG in high-risk patients with DM and ESRD. This may provide a functional indicator of subclinical atherosclerosis and warrants clinical follow up to determine prognostic significance.
Background
Impaired vasodilation is an early marker of atherosclerosis [1-4]. Although abnormal response to endothelial-dependent stimuli is more commonly associated with coronary artery disease (CAD) [3,5-8], several studies have associated impaired vasodilatory response to nitroglycerin (NTG) with risk factors for coronary artery disease [9] and increased future clinical events [6,10]. Previous studies, however, have used x ray angiography [3-8] and intravascular ultrasound [11] which are invasive and, thus, not appropriate for asymptomatic patients or serial follow-up. A non-invasive measure of subclinical coronary atherosclerosis may help identify patients who are at increased risk and guide therapy toward reducing morbidity and mortality. We and others have previously developed a non-invasive method to measure NTG-induced coronary vasodilation with coronary cardiovascular magnetic resonance (CMR) [12,13]. We hypothesize that impaired coronary vasodilation to NTG can be demonstrated noninvasively in asymptomatic patients at increased risk for coronary artery disease.
Methods
Subjects
Asymptomatic patients with diabetes mellitus (DM, N = 8) and end stage renal disease (ESRD, N = 5), as well as age-matched controls (N = 33) were recruited consecutively. Subjects were excluded if they had a history of chest pain, coronary artery disease, myocardial infarction, stroke or peripheral vascular disease. Vasoactive medications were discontinued 24 hours before the examination. All subjects provided written informed consent approved by the Human Subjects Committee at Stanford University.
CMR
A 1.5-T Signa MRI scanner (GE Healthcare, Milwaukee, Wisconsin) equipped with high-performance gradients (40 mT/m, 150 mt/m/ms) and a real-time interactive workstation were used. A commercial coil provided signal reception (5-inch General Purpose Coil, Model #2127316, GE Healthcare, Milwaukee, Wisconsin). Blood pressure and heart rate were monitored throughout the study (Omega 1400, In vivo Research, Inc., Orlando, Florida).
Protocol
NTG-induced coronary vasodilation was performed as previously described [13]. A real-time interactive MR system [13-15] was used to localize coronary arteries (16 frames/sec, TR 4.6 ms, flip 30, slice thickness 7 mm, FOV 24 cm, in-plane resolution 2.7 mm). High-resolution coronary MRA was then performed using a cardiac-gated, breath-held, multi-slice spiral sequence [13] (FOV = 20–28 cm, slice thickness = 5 mm, TR = 1 heartbeat, TE = 7 ms, in-plane spatial resolution = 0.62–0.99 mm, 14 to 20 interleaves, flip angle = 60 degrees, acquisition gated to diastole). In-plane and cross-sectional images were acquired before and then 5 minutes after 0.4 mg sublingual NTG, which was given while the subject was in the magnet. Images were reconstructed onto a 512 × 512 matrix, yielding a pixel size of 0.39 to 0.55 mm. Real-time short axis views of the left ventricle (LV) from the apex to base as well as 4-, 3- and 2-chamber views were also obtained to evaluate LV function.
Image Analysis
For quantitative analysis of coronary vasodilation, the cross-sectional right coronary artery (RCA) images were used, except in subjects with a small non-dominant RCA, where the cross-sectional left anterior descending artery (LAD) images (n = 5) were used. As described previously[13] the slice with the most circular cross-section was identified on the pre-NTG images and the corresponding post-NTG slice was carefully matched according to the surrounding cardiac and chest wall structures. These images were then pooled and randomized, with no patient information or NTG status provided on the images, and then analyzed independently and in a blinded fashion by one observer. A custom designed software program was used to analyze the cross sectional images: after images were magnified two-fold, an ovoid region of interest tool was used to trace around the RCA or LAD, yielding the cross-sectional area (CSA). This analysis has been shown previously to have a low intra- and inter-observer variability [12,13] and good correlation with x-ray coronary angiography [13].
Statistical Analysis
Data were expressed as mean values ± standard deviation. The difference in coronary artery size before and after NTG was compared by a paired two-sided t test. Differences in % coronary vasodilation between patients and controls were tested using an unpaired two-sided t test. Differences among subject groups were tested using a one-way analysis of variance (ANOVA) and Fisher exact test. All statistical analyses were performed with StatView (version 5, SAS Institute Inc., Cary, NC).
Results
Clinical characteristics of the patient and control groups are shown in Table 1. All 46 subjects completed the study without complications. NTG caused a small systemic effect: 9.1 ± 8.5% decrease in systolic blood pressure, 3.2 ± 6.7% decrease in diastolic blood pressure, and 5.4 ± 7.7% increase in heart rate, which was not different between patients and controls (Table 1). The mean age of the patients and controls was similar (patients: 55.3 ± 14.3 yrs, controls: 51.8 ± 10.4 yrs, p = 0.4); however, the sex difference was significant (patients: 92% male, controls: 48% male, p = 0.006). Patients with DM and ESRD had a higher incidence of hypertension and use of angiotensin converting enzyme inhibitors (p = 0.001). One control subject was excluded from analysis because of abnormal left ventricular function. No subjects needed to be excluded because of image quality.
Table 1.
Baseline characteristics of study subjects
| Characteristics | Controls (n = 33) | Patients with DM or ESRD (n = 13) | P value |
| Age (years) | 51.8 ± 10.4 | 55.3 ± 14.3 | 0.4 |
| Male | 16/33 | 12/13 | 0.006 |
| HTN | 9/33 | 9/13 | 0.02 |
| FH | 6/33 | 5/13 | 0.15 |
| Dyslipidemia | 4/33 | 4/13 | 0.13 |
| ACE-I | 2/33 | 6/13 | 0.001 |
| Current smoker | 1/33 | 1/13 | 0.49 |
| Decrease in SBP (mmHg) | 9.2 ± 7.0% | 9.0 ± 11.5% | 0.97 |
| Decrease in DBP (mmHg) | 3.7 ± 7.0% | 2.0 ± 5.5% | 0.45 |
| Increase in HR (beats/min) | 5.5 ± 7.9% | 5.2 ± 7.2% | 0.89 |
Results are given as mean ± standard deviation or proportions. HTN: hypertension; FH: family history; ACE-I: angiotensin converting enzyme inhibitor; SBP: systolic blood pressure; DBP: diastolic blood pressure; HR: heart rate
Coronary CMR detected a significant increase in coronary CSA with NTG in both patients and controls (p < 0.0001 for both), but the degree of coronary artery vasodilation to NTG was significant impaired in patients compared to controls (17.8 ± 7.3% vs. 25.6 ± 7.1%, p = 0.002, Figures 1, 2, 3). This % vasodilation in controls was similar to that of our prior study (24.0%) [13]. Analysis by patient subgroup was also significant (ANOVA p = 0.005, Figure 3b), with ESRD patient alone vs. controls (14.8 ± 7.7% vs. 25.6 ± 7.1%; Fisher exact test p = 0.003) and DM patients alone vs. controls (19.8 ± 6.3% vs. 25.6 ± 7.1%; Fisher exact test p = 0.049) showing impaired coronary vasodilation. There was a non-significant trend toward greater impairment in the ESRD vs. DM patients (14.8 ± 7.7% vs. 19.8 ± 6.3%; Fisher exact test p = 0.23).
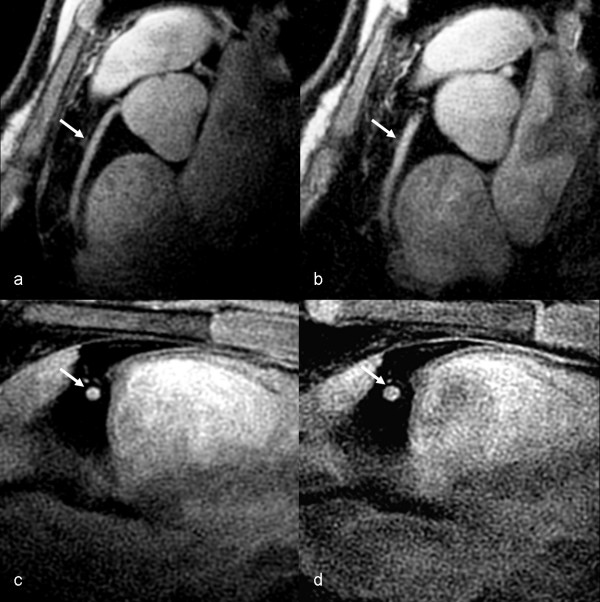
Figure 1.
Coronary CMR of a control subject showing coronary vasodilation: In plane pre-NTG (a) and post-NTG (b) and cross-sectional pre-NTG (c) and post-NTG (d) images of the proximal right coronary artery (arrows).
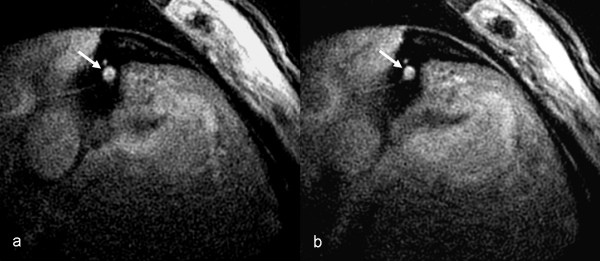
Figure 2.
Coronary CMR of a patient with end stage renal disease showing no significant coronary vasodilation: Cross-sectional pre-NTG (a) and post-NTG (b) images of the proximal right coronary artery.
Figure 3.
Comparison of the percent coronary vasodilation in all subjects: a) Compared to controls, patients had significantly impaired vasodilation. b) Patient subgroup analysis: Endstage renal disease (ESRD) patients and diabetes (DM) patients both had significantly impaired vasodilation compared to controls. Individual subjects with vasodilation < 15% indicated in red. * – p < 0.05 vs. controls, ** – p < 0.005 vs. controls.
Given the disparity in gender distribution between the patients and controls, we analyzed by gender. The impairment of coronary vasodilation remained significant in male patients vs. male controls (18.3 ± 7.4% vs. 27.6 ± 5.7 %, p = 0.002). The lack of females in the patient group precluded comparing female patients to controls.
Comparing RCA and LAD, there were not significant differences in baseline CSA (RCA: 16.3 ± 4.2 mm2 vs. LAD: 14.7 ± 2.6 mm2, p = 0.31) or coronary vasodilation (RCA: 23.5 ± 7.9% vs. LAD: 22.2 ± 8.2%, p = 0.77).
Discussion
In the present study, noninvasive coronary CMRA demonstrated impaired coronary artery vasodilation to NTG in a group of patients at increased risk for coronary artery disease. To our knowledge, this is the first study to use noninvasive imaging to directly assess impaired epicardial coronary vasodilation in this patient group.
Coronary Vasomotor Function and Atherosclerosis
Abnormal coronary vasomotor function occurs early in the development of atherosclerosis [16] and is typically assessed by studying endothelial-dependent epicardial coronary vasodilation by invasive methods [5,17]. However, several x-ray coronary angiography studies [6,10] have also found impairment of endothelial-independent coronary vasodilation to NTG in patients, with prognostic significance. In a study of 147 patients with risk factors for CAD (including 9% with diabetes and 84% with angiographic evidence of atherosclerosis) followed for a median 7.7 years, abnormal vasodilator response to acetycholine, cold pressor, and NTG were each independently associated with disease progression and increased cardiovascular events [6]. Consistent with these findings, a study of 163 women (including 26% with diabetes and 45% without angiographic evidence of CAD) found impaired reactivity to NTG and acetycholine in subjects who had future cardiovascular events [10].
Impaired Coronary Vasomotor Function and Patients with DM and ESRD
Prospective data on coronary vasomotor function are limited in patients with DM [18] and ESRD [19]. However, consistent with an increased cardiovascular risk, peripheral vasomotor dysfunction has been almost universally found in these patients [20-26]. Two studies in non-insulin-dependent DM have shown impaired brachial artery vasodilation to both endothelial-dependent and endothelial-independent stimuli [24,26]. Interestingly, in a study using brachial ultrasound in subjects with no documented CAD (including 13.1% with DM), the only risk factor independently associated with impaired NTG-induced vasodilation was DM [27]. Similar to findings in the peripheral vasculature, reduced coronary artery reactivity to NTG has been previously reported in patients with DM. In a study [9] of non-insulin-dependent DM using intravascular ultrasound, coronary artery distensibility and diastolic cross sectional luminal area after NTG were significantly lower in DM compared to controls.
Similar to patients with DM, patients with renal failure have impaired peripheral vasomotor function [19,28,29]. In a study of 28 patients with chronic renal failure (13 on hemodialysis), both flow-mediated and NTG-induced brachial artery vasodilation were impaired to comparable degrees [28]. To date, there are no known studies on coronary vasoreactivity in patients with ESRD.
Our data show that patients with DM and ESRD have impaired NTG-induced coronary vasodilation compared to age-matched controls. The individual data (Figure 3b) reveal that patients generally fell into two groups: those with normal coronary vasodilation (~25%) and those with low coronary vasodilation (< 15%). Eighty percent (80%) of ESRD and 38% of DM patients fell below the 15% threshold, compared to only 6% of the controls.
Noninvasive Coronary Imaging
CMR [30,31], computed tomography (CT) [32,33], ultrasound [34-36], and nuclear techniques [37,38] all offer alternative approaches to assess coronary artery disease noninvasively. By using sub-mm spatial resolution and analyzing the lumen cross-sectional area, CMR has been shown to have adequate resolution to detect coronary vasodilation to NTG in two prior studies [12,13]. CMR can also directly image the coronary wall, with increased wall thickness demonstrated in patients with Type I DM [39] and non-obstructive CAD [39,40]. CT can provide high-resolution structural imaging of the coronary lumen and wall [32], but the radiation and contrast involved make it suboptimal for serial imaging of coronary vasomotor changes. One recent study did look retrospectively at patients who had more than one coronary CT scan, where NTG was used in one and not in another, and did show significantly larger coronary diameter with NTG [41]. The feasibility of transthoracic echocardiography for measuring epicardial coronary vasodilation has recently been shown in healthy men [42]. The other main approach to assess coronary function noninvasively has been to measure coronary flow or perfusion reserve to a vasodilator stimulus (e.g., adenosine). This is primarily a measure of coronary microvascular function in the absence of epicardial stenoses. This can be performed by CMR [30,31,43], positron emission tomography (PET) [37,38], and transthoracic Doppler techniques [34-36] and has been shown to be impaired in patients with coronary risk factors [31,38], including DM[35,38]. More data comparing the prognostic significance of epicardial vs. microvascular vasomotor function are needed.
Study limitations
A major study limitation is that only endothelium-independent coronary vasodilation with NTG was evaluated. Endothelium-dependent vasomotor function has been shown to be an earlier marker of atherosclerosis and may be more sensitive in patients with subclinical disease [2,4,16]. Future studies should focus on overcoming the challenges of performing a more endothelial-dependent stimulus in the magnetic resonance environment. A preliminary report [44] and a case report [45] on using the cold pressor test show promise [44]. In addition, the size of the clinical cohort was small and recruitment was consecutive, which may have contributed to the significant differences in demographics (i.e., % female) between the two groups. This difference did not account for the finding of impaired vasodilation in the patients, as this finding remained significant even when the only males were analyzed. A study incorporating more female patients is needed to verify if the impaired coronary vasodilation applies to high-risk women. Finally, long-term clinical follow up is needed to determine the prognostic significance of these findings.
Conclusion
NTG-induced coronary vasodilation assessed noninvasively by CMR was significantly impaired in asymptomatic patients with DM and ESRD. This may provide an additional functional measure of subclinical coronary atherosclerosis in high-risk patients.
Abbreviations
GE: General Electric; CMR: cardiovascular magnetic resonance; NTG: nitroglycerine; DM: diabetes mellitus; ESRD: end stage renal disease; CSA: cross sectional area; CAD: coronary artery disease; LV: left ventricle; RCA: right coronary artery; LAD: left anterior descending coronary artery; ANOVA: analysis of variance; CT: computed tomography; PET: positron emission tomography
Authors' contributions
PKN contributed to the study in data collection, data analysis, and manuscript preparation. MT, PY and JE contributed to data collection and data analysis. CHM developed the MR sequences used in the study. MVMC contributed to the study design, data collection, data analysis, and manuscript preparation. All authors have contributed to the manuscript review and editing. All authors have read and approved the final manuscript.
Acknowledgments
Acknowledgements
We would like to acknowledge the National Institutes of Health, the American College of Cardiology/Merck, and General Electric (GE) Healthcare for support of this research.
Contributor Information
Patricia K Nguyen, Email: pnguyen@cvmed.stanford.edu.
Craig Meyer, Email: cmeyer@virginia.edu.
Jan Engvall, Email: janengvall@telia.com.
Phillip Yang, Email: pyang@cvmed.stanford.edu.
Michael V McConnell, Email: mcconnell@stanford.edu.
References
- Behrendt D, Ganz P. Endothelial function. From vascular biology to clinical applications. Am J Cardiol. 2002;90(10C):40L–48L. doi: 10.1016/S0002-9149(02)02963-6. [DOI] [PubMed] [Google Scholar]
- Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23(2):168–175. doi: 10.1161/01.ATV.0000051384.43104.FC. [DOI] [PubMed] [Google Scholar]
- Schindler TH, Hornig B, Buser PT, Olschewski M, Magosaki N, Pfisterer M, Nitzsche EU, Solzbach U, Just H. Prognostic value of abnormal vasoreactivity of epicardial coronary arteries to sympathetic stimulation in patients with normal coronary angiograms. Arterioscler Thromb Vasc Biol. 2003;23(3):495–501. doi: 10.1161/01.ATV.0000057571.03012.F4. [DOI] [PubMed] [Google Scholar]
- Vita JA, Treasure CB, Nabel EG, McLenachan JM, Fish RD, Yeung AC, Vekshtein VI, Selwyn AP, Ganz P. Coronary vasomotor response to acetylcholine relates to risk factors for coronary artery disease. Circulation. 1990;81(2):491–497. doi: 10.1161/01.cir.81.2.491. [DOI] [PubMed] [Google Scholar]
- Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr., Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101(9):948–954. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101(16):1899–1906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, Nour KR, Quyyumi AA. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106(6):653–658. doi: 10.1161/01.CIR.0000025404.78001.D8. [DOI] [PubMed] [Google Scholar]
- Quyyumi AA. Prognostic value of endothelial function. Am J Cardiol. 2003;91(12A):19H–24H. doi: 10.1016/S0002-9149(03)00430-2. [DOI] [PubMed] [Google Scholar]
- Vavuranakis M, Stefanadis C, Triandaphyllidi E, Toutouzas K, Toutouzas P. Coronary artery distensibility in diabetic patients with simultaneous measurements of luminal area and intracoronary pressure: evidence of impaired reactivity to nitroglycerin. J Am Coll Cardiol. 1999;34(4):1075–1081. doi: 10.1016/S0735-1097(99)00331-9. [DOI] [PubMed] [Google Scholar]
- von Mering GO, Arant CB, Wessel TR, McGorray SP, Bairey Merz CN, Sharaf BL, Smith KM, Olson MB, Johnson BD, Sopko G, Handberg E, Pepine CJ, Kerensky RA. Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: results from the National Heart, Lung, and Blood Institute-Sponsored Women's Ischemia Syndrome Evaluation (WISE) Circulation. 2004;109(6):722–725. doi: 10.1161/01.CIR.0000115525.92645.16. [DOI] [PubMed] [Google Scholar]
- Hollenberg SM, Tamburro P, Johnson MR, Burns DE, Spokas D, Costanzo MR, Parrillo JE, Klein LW. Simultaneous intracoronary ultrasound and Doppler flow studies distinguish flow-mediated from receptor-mediated endothelial responses. Catheter Cardiovasc Interv. 1999;46(3):282–288. doi: 10.1002/(SICI)1522-726X(199903)46:3<282::AID-CCD5>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Pepe A, Lombardi M, Takacs I, Positano V, Panzarella G, Picano E. Nitrate-induced coronary vasodilation by stress-magnetic resonance imaging: a novel noninvasive test of coronary vasomotion. J Magn Reson Imaging. 2004;20(3):390–394. doi: 10.1002/jmri.20136. [DOI] [PubMed] [Google Scholar]
- Terashima M, Meyer CH, Keeffe BG, Putz EJ, de la Pena-Almaguer E, Yang PC, Hu BS, Nishimura DG, McConnell MV. Noninvasive assessment of coronary vasodilation using magnetic resonance angiography. J Am Coll Cardiol. 2005;45(1):104–110. doi: 10.1016/j.jacc.2004.09.057. [DOI] [PubMed] [Google Scholar]
- Yang PC, Meyer CH, Terashima M, Kaji S, McConnell MV, Macovski A, Pauly JM, Nishimura DG, Hu BS. Spiral magnetic resonance coronary angiography with rapid real-time localization. J Am Coll Cardiol. 2003;41(7):1134–1141. doi: 10.1016/S0735-1097(03)00079-2. [DOI] [PubMed] [Google Scholar]
- Kerr AB, Pauly JM, Hu BS, Li KC, Hardy CJ, Meyer CH, Macovski A, Nishimura DG. Real-time interactive MRI on a conventional scanner. Magn Reson Med. 1997;38(3):355–367. doi: 10.1002/mrm.1910380303. [DOI] [PubMed] [Google Scholar]
- Davignon J, Ganz P. Role of endothelial dysfunction in atherosclerosis. Circulation. 2004;109(23 Suppl 1):III27–32. doi: 10.1161/01.CIR.0000131515.03336.f8. [DOI] [PubMed] [Google Scholar]
- Nishimura RA, Lerman A, Chesebro JH, Ilstrup DM, Hodge DO, Higano ST, Holmes DR, Jr., Tajik AJ. Epicardial vasomotor responses to acetylcholine are not predicted by coronary atherosclerosis as assessed by intracoronary ultrasound. J Am Coll Cardiol. 1995;26(1):41–49. doi: 10.1016/0735-1097(95)00142-M. [DOI] [PubMed] [Google Scholar]
- Nitenberg A, Valensi P, Sachs R, Cosson E, Attali JR, Antony I. Prognostic value of epicardial coronary artery constriction to the cold pressor test in type 2 diabetic patients with angiographically normal coronary arteries and no other major coronary risk factors. Diabetes Care. 2004;27(1):208–215. doi: 10.2337/diacare.27.1.208. [DOI] [PubMed] [Google Scholar]
- London GM, Pannier B, Agharazii M, Guerin AP, Verbeke FH, Marchais SJ. Forearm reactive hyperemia and mortality in end-stage renal disease. Kidney Int. 2004;65(2):700–704. doi: 10.1111/j.1523-1755.2004.00434.x. [DOI] [PubMed] [Google Scholar]
- Lambert J, Aarsen M, Donker AJ, Stehouwer CD. Endothelium-dependent and -independent vasodilation of large arteries in normoalbuminuric insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996;16(5):705–711. doi: 10.1161/01.atv.16.5.705. [DOI] [PubMed] [Google Scholar]
- Calver A, Collier J, Vallance P. Inhibition and stimulation of nitric oxide synthesis in the human forearm arterial bed of patients with insulin-dependent diabetes. J Clin Invest. 1992;90(6):2548–2554. doi: 10.1172/JCI116149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogikyan RV, Galecki AT, Pitt B, Halter JB, Greene DA, Supiano MA. Specific impairment of endothelium-dependent vasodilation in subjects with type 2 diabetes independent of obesity. J Clin Endocrinol Metab. 1998;83(6):1946–1952. doi: 10.1210/jc.83.6.1946. [DOI] [PubMed] [Google Scholar]
- Johnstone MT, Creager SJ, Scales KM, Cusco JA, Lee BK, Creager MA. Impaired endothelium-dependent vasodilation in patients with insulin-dependent diabetes mellitus. Circulation. 1993;88(6):2510–2516. doi: 10.1161/01.cir.88.6.2510. [DOI] [PubMed] [Google Scholar]
- McVeigh GE, Brennan GM, Johnston GD, McDermott BJ, McGrath LT, Henry WR, Andrews JW, Hayes JR. Impaired endothelium-dependent and independent vasodilation in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1992;35(8):771–776. doi: 10.1007/BF00429099. [DOI] [PubMed] [Google Scholar]
- van Etten RW, de Koning EJ, Verhaar MC, Gaillard CA, Rabelink TJ. Impaired NO-dependent vasodilation in patients with Type II (non-insulin-dependent) diabetes mellitus is restored by acute administration of folate. Diabetologia. 2002;45(7):1004–1010. doi: 10.1007/s00125-002-0862-1. [DOI] [PubMed] [Google Scholar]
- Balletshofer BM, Rittig K, Enderle MD, Volk A, Maerker E, Jacob S, Matthaei S, Rett K, Haring HU. Endothelial dysfunction is detectable in young normotensive first-degree relatives of subjects with type 2 diabetes in association with insulin resistance. Circulation. 2000;101(15):1780–1784. doi: 10.1161/01.cir.101.15.1780. [DOI] [PubMed] [Google Scholar]
- Adams MR, Robinson J, McCredie R, Seale JP, Sorensen KE, Deanfield JE, Celermajer DS. Smooth muscle dysfunction occurs independently of impaired endothelium-dependent dilation in adults at risk of atherosclerosis. J Am Coll Cardiol. 1998;32(1):123–127. doi: 10.1016/S0735-1097(98)00206-X. [DOI] [PubMed] [Google Scholar]
- Nakanishi T, Ishigami Y, Otaki Y, Izumi M, Hiraoka K, Inoue T, Takamitsu Y. Impairment of vascular responses to reactive hyperemia and nitric oxide in chronic renal failure. Nephron. 2002;92(3):529–535. doi: 10.1159/000064078. [DOI] [PubMed] [Google Scholar]
- Oflaz H, Pusuroglu H, Genchallac H, Demirel S, Bugra Z, Sever MS, Yildiz A. Endothelial function is more impaired in hemodialysis patients than renal transplant recipients. Clin Transplant. 2003;17(6):528–533. doi: 10.1046/j.1399-0012.2003.00100.x. [DOI] [PubMed] [Google Scholar]
- Sakuma H, Blake LM, Amidon TM, O'Sullivan M, Szolar DH, Furber AP, Bernstein MA, Foo TK, Higgins CB. Coronary flow reserve: noninvasive measurement in humans with breath-hold velocity-encoded cine MR imaging. Radiology. 1996;198(3):745–750. doi: 10.1148/radiology.198.3.8628864. [DOI] [PubMed] [Google Scholar]
- Wang L, Jerosch-Herold M, Jacobs DR, Jr., Shahar E, Folsom AR. Coronary risk factors and myocardial perfusion in asymptomatic adults: the Multi-Ethnic Study of Atherosclerosis (MESA) J Am Coll Cardiol. 2006;47(3):565–572. doi: 10.1016/j.jacc.2005.09.036. [DOI] [PubMed] [Google Scholar]
- Schroeder S, Kopp AF, Baumbach A, Meisner C, Kuettner A, Georg C, Ohnesorge B, Herdeg C, Claussen CD, Karsch KR. Noninvasive detection and evaluation of atherosclerotic coronary plaques with multislice computed tomography. J Am Coll Cardiol. 2001;37(5):1430–1435. doi: 10.1016/S0735-1097(01)01115-9. [DOI] [PubMed] [Google Scholar]
- Hoffmann MH, Shi H, Schmitz BL, Schmid FT, Lieberknecht M, Schulze R, Ludwig B, Kroschel U, Jahnke N, Haerer W, Brambs HJ, Aschoff AJ. Noninvasive coronary angiography with multislice computed tomography. Jama. 2005;293(20):2471–2478. doi: 10.1001/jama.293.20.2471. [DOI] [PubMed] [Google Scholar]
- Gullu H, Erdogan D, Caliskan M, Tok D, Yildirim E, Ulus T, Turan Sezgin A, Muderrisoglu H. Interrelationship between noninvasive predictors of atherosclerosis: transthoracic coronary flow reserve, flow-mediated dilation, carotid intima-media thickness, aortic stiffness, aortic distensibility, elastic modulus, and brachial artery diameter. Echocardiography. 2006;23(10):835–842. doi: 10.1111/j.1540-8175.2006.00342.x. [DOI] [PubMed] [Google Scholar]
- Miyazaki C, Takeuchi M, Yoshitani H, Otani S, Sakamoto K, Yoshikawa J. Optimum hypoglycemic therapy can improve coronary flow velocity reserve in diabetic patients: demonstration by transthoracic doppler echocardiography. Circ J. 2003;67(11):945–950. doi: 10.1253/circj.67.945. [DOI] [PubMed] [Google Scholar]
- Otsuka R, Watanabe H, Hirata K, Tokai K, Muro T, Yoshiyama M, Takeuchi K, Yoshikawa J. Acute effects of passive smoking on the coronary circulation in healthy young adults. Jama. 2001;286(4):436–441. doi: 10.1001/jama.286.4.436. [DOI] [PubMed] [Google Scholar]
- Dayanikli F, Grambow D, Muzik O, Mosca L, Rubenfire M, Schwaiger M. Early detection of abnormal coronary flow reserve in asymptomatic men at high risk for coronary artery disease using positron emission tomography. Circulation. 1994;90(2):808–817. doi: 10.1161/01.cir.90.2.808. [DOI] [PubMed] [Google Scholar]
- Sundell J, Janatuinen T, Ronnemaa T, Raitakari OT, Toikka J, Nuutila P, Knuuti J. Diabetic background retinopathy is associated with impaired coronary vasoreactivity in people with Type 1 diabetes. Diabetologia. 2004;47(4):725–731. doi: 10.1007/s00125-004-1340-8. [DOI] [PubMed] [Google Scholar]
- Kim WY, Astrup AS, Stuber M, Tarnow L, Falk E, Botnar RM, Simonsen C, Pietraszek L, Hansen PR, Manning WJ, Andersen NT, Parving HH. Subclinical coronary and aortic atherosclerosis detected by magnetic resonance imaging in type 1 diabetes with and without diabetic nephropathy. Circulation. 2007;115(2):228–235. doi: 10.1161/CIRCULATIONAHA.106.633339. [DOI] [PubMed] [Google Scholar]
- Fayad ZA, Fuster V, Fallon JT, Jayasundera T, Worthley SG, Helft G, Aguinaldo JG, Badimon JJ, Sharma SK. Noninvasive in vivo human coronary artery lumen and wall imaging using black-blood magnetic resonance imaging. Circulation. 2000;102(5):506–510. doi: 10.1161/01.cir.102.5.506. [DOI] [PubMed] [Google Scholar]
- Dewey M, Hoffmann H, Hamm B. Multislice CT coronary angiography: effect of sublingual nitroglycerine on the diameter of coronary arteries. Rofo. 2006;178(6):600–604. doi: 10.1055/s-2006-926755. [DOI] [PubMed] [Google Scholar]
- Kiviniemi TO, Toikka JO, Koskenvuo JW, Saraste A, Saraste M, Parkka JP, Raitakari OT, Hartiala JJ. Vasodilation of epicardial coronary artery can be measured with transthoracic echocardiography. Ultrasound Med Biol. 2007;33(3):362–370. doi: 10.1016/j.ultrasmedbio.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Jerosch-Herold M, Hudsmith LE, Robson MD, Francis JM, Doll HA, Selvanayagam JB, Neubauer S, Watkins H. Evidence for microvascular dysfunction in hypertrophic cardiomyopathy: new insights from multiparametric magnetic resonance imaging. Circulation. 2007;115(18):2418–2425. doi: 10.1161/CIRCULATIONAHA.106.657023. [DOI] [PubMed] [Google Scholar]
- Jain H SS., Iwuchukwu C, Wang Y, Schapiro W, Abela J, Reichek N . American Heart Association Scientific Sessions. Chicago, Illinois ; 2006. Coronary vasomotion in normal subjects with risk factors for atherosclerosis: Noninvasive assessment . [Google Scholar]
- Yilmaz A, Mahrholdt H, Athanasiadis A, Sechtem U. Non-invasive evaluation of coronary vasospasm using a combined hyperventilation and cold-pressure-test perfusion CMR protocol. J Cardiovasc Magn Reson. 2007;9(5):759–764. doi: 10.1080/10976640701544662. [DOI] [PubMed] [Google Scholar]