Abstract
Background
Cyclin A1 is a cell cycle regulator that has been implicated in the progression of prostate cancer. Its role in invasion and metastasis of this disease has not been characterized.
Methods
Immunohistochemistry and cDNA microarray analyses were used to assess protein and mRNA expression of cyclin A1 and proteins with roles in metastasis, including vascular endothelial growth factor (VEGF), metalloproteinase 2 (MMP2), and MMP9, in human prostate cancer. Transient transfection and infection with viral vectors expressing cyclin A1 and short hairpin RNA (shRNA) targeting cyclin A1 were used to study the effects of altered cyclin A1 expression in PC3 prostate cancer cells. The BrdU assay, annexin V staining, and invasion chambers were used to examine cyclin A1 effects on proliferation, apoptosis, and invasion, respectively. The role of cyclin A1 and androgen receptor (AR) in transcription of VEGF and MMP2 was assessed by promoter mutation and chromatin immunoprecipitation. The effect of cyclin A1 expression on tumor growth and metastasis was analyzed in a mouse model of metastasis. All statistical tests were two-sided.
Results
Cyclin A1 protein and mRNA expression were statistically significantly higher in prostate cancers than in adjacent benign tissues. A statistically significant correlation between expression of cyclin A1 and of MMP2, MMP9, and VEGF was observed in prostate tumors from 482 patients (P values from Spearman rank correlation tests < .001). PC3 cells that overexpressed cyclin A1 showed increased invasiveness, and inhibition of cyclin A1 expression via shRNA expression reduced invasiveness of these cells. Eight of 10 mice (80%) bearing PC3 cells overexpressing cyclin A1 had infiltration of tumor cells in lymph node, liver, and lung, but all 10 mice bearing tumors expressing control vector were free of liver and lung metastases and only one mouse from this group had lymph node metastasis (P values from Fisher exact tests < .001). Cyclin A1, in concert with AR, bound to and increased expression from the VEGF and MMP2 promoters.
Conclusions
Cyclin A1 contributes to prostate cancer invasion by modulating the expression of MMPs and VEGF and by interacting with AR.
CONTEXT AND CAVEATS
Prior knowledge
Cyclin A1 is a cell cycle regulatory factor that is highly expressed in human prostate cancers.
Study design
Cyclin A1 expression was altered in cultured prostate cancer cells by introducing expression vectors or RNA interference. The effect of alterations in cyclin A1 expression was examined in terms of cellular processes relevant to the progression of prostate cancer, including invasion of prostate cancer cells in vitro and in mouse models of tumor growth and metastasis, and regulation at the promoter level of genes that play a role in metastasis.
Contribution
This work demonstrated, and characterized in some molecular detail, the role of cyclin A1 in promoting invasion and metastasis of prostate cancer cells.
Implications
A cell cycle regulatory factor may contribute to prostate cancer invasion and metastasis.
Limitations
The manipulations of cyclin A1 expression were confined to cell lines and animal models that may not recapitulate the process of prostate cancer invasion and metastasis in humans.
From the Editors
Once prostate cancer becomes hormone refractory, cancer cells may rapidly gain the ability to invade and to metastasize to lymph nodes and distant organs. Approximately one-third of patients treated for hormone-refractory prostate cancer will relapse, and there is no curative treatment for metastatic disease.
The progression through hormone-dependent to hormone-refractory and metastatic prostate cancer is poorly understood, but some proteins with critical roles in the process have been partially characterized. Dissemination of tumor cells requires expression of metastasis-promoting genes involved in basement membrane degradation—metalloproteinases (MMPs), urokinase-type plasminogen activator (uPA), adhesion molecules, cell surface receptors, and many other proteins (1). MMPs are endopeptidases that regulate cell growth, migration, and extracellular matrix remodeling and are expressed in primary prostate cancer cells, osteoblasts, and osteoclasts (2). MMP1 and MMP2 are highly expressed in metastatic cells, suggesting that they may have causal roles in tumor metastasis (3–5).
Tumor cells invade either the blood or lymphatic vessels to access the general circulation and establish themselves in other tissues. Vascular endothelial growth factor (VEGF) and its receptors play an important role in vessel formation, and high levels of VEGF are frequently observed in prostate cancer (6,7). VEGF stimulation can enhance the motility of targeted tumor cells in cooperation with MMPs and urokinase plasminogen activator (uPA)-mediated pathways (8). Androgen receptor (AR), a member of the superfamily of ligand-activated nuclear receptors, plays a central role in the pathogenesis of primary and metastatic prostate cancer (9,10). Somatic mutations in the gene encoding this receptor and its amplification contribute to progression and metastasis of prostate cancer (11–13).
Cyclin A1, a cell cycle regulatory factor, has been demonstrated to be required for the G2/M phase transition in meiotic division of male germ cells by gene targeting (14), and it contributes to the G1/S cell cycle progression in leukemic cell lines (15). Its role in pathogenesis of leukemia has been demonstrated in a transgenic mouse model in which targeted overexpression of cyclin A1 in early myeloid cells initiated acute myeloid leukemia in mice (16). This study suggested that cyclin A1 has a cell cycle–independent function in carcinogenesis. Elevated levels of cyclin A1 expression have been observed in various types of solid tumors, including testicular, ovarian, and breast tumors (17–19). The homeo-protein Six1 is functionally linked to cyclin A1 in breast cancer cell lines, and interactions between Six1 and cyclin A1 are needed to promote tumorigenic activity in breast cancer cells (20). Recently, we have shown that cyclin A1 are highly expressed in advanced prostate cancer and that its expression is associated with tumor histology and VEGF expression (21). However, the precise role of cyclin A1 in the pathogenesis of primary and metastatic prostate cancer is largely unknown.
In this study, we investigated whether cyclin A1 contributes to prostate cancer invasion and metastasis and explored the molecular mechanisms by which cyclin A1 contributes to tumor metastasis.
Methods
Tissue Specimens and Tissue Microarrays
Paraffin-embedded sections of prostate samples from 482 patients with prostate cancer were obtained as archival specimens from the Department of Pathology at the University Hospital in Malmö. Hematoxylin–eosin (H&E)–stained slides of prostate biopsies were examined by a national board–certified pathologist (L. Helczynski). Selected areas of prostate cancer and corresponding benign samples were applied in duplicate to tissue microarray constructs of 1.0 mm in diameter. The study was approved by the Ethics Committee, Lund University, and the Helsinki Declaration of Human Rights was strictly observed.
Immunohistochemistry and Quantification of Angiogenesis
Immunohistochemistry on tumor tissue arrays was performed as previously described (21). The slides were incubated with the following primary antibodies: anti–cyclin A1, 1:50 (Pharmingen, San Diego, CA); anti-VEGF, 1:100 (Santa Cruz Biotechnology, Santa Cruz, CA); anti-MMP9, 1:100 (Chemicon, Temecula, CA); anti-MMP2, 1:100 (Lab Vision, Fremont, CA); and anti-AR, 1:200 (Dako, Glostrup, Denmark A/S). Anti-rabbit or anti-mouse peroxidase–conjugated secondary antibodies were applied. Diaminobenzidine colorimetric reagent solution from Dako was used. Slides were counterstained with hematoxylin (Sigma, St Louis, MO). The specimens were viewed with an Olympus BX51 microscope at magnification of ×20 or ×40. The staining intensity was scored as 0 (negative), 1 (weakly positive or positive), 2 (moderate positive), 3 (strongly positive), or 4 (very strong positive) using an arbitrary semiquantitative scale as previously described (21). The scores are representative for at least 60%–70% of the area of tissue analyzed, and the highest score for both cytoplasmic and nuclear compartments was considered in the statistical analyses. For analysis of tumor angiogenesis, tumor sections were stained with an antibody against CD31 (BD Pharmingen, San Diego, CA, and Dako). Regions of high vascular density within the tumors were examined. The number of CD31-positive pixels per microscopic field was recorded. At least two sections per tumor and three views per section were determined.
Cell Culture and Androgen R1881 Treatment
Human prostatic cell lines LNCaP and PC3 were purchased from the American Type Culture Collection (Manassas, VA), and treatment with the synthetic androgen R1881 (Dupont/NEN, Boston, MA) was performed as previously described (22). Time-resolved analyses of cyclin A1–GFP fusion protein in living cells in response to R1881 treatment were performed using a Fluorescence Zeiss Apoptome Axiovert microscope at ×40 magnification.
Retroviral- and Adenoviral-Mediated Transduction
Micro-RNA–adapted short hairpin RNA (shRNAmir) against human cyclin A1 in pSM2 vector was obtained from Open Biosystems (Huntsville, AL). The shRNAmirCyclin A1 sequence was subcloned to MSCV-LTRmiR30-PIG (LMP) vector (Open Biosystems) with XhoI and EcoRI restriction enzymes. Clones were verified by restriction site analysis and sequencing. For production of retrovirus, HEK 293 packaging cells were transiently transfected with shRNAmir-cyclinA1-1α-LMP, VSVG, and Gag-Pol plasmids by use of Lipofectamine 2000 (Invitrogen, Inc, Carlsbad, CA). Media containing viruses were collected every 12 hours, and supernatants were added to the culture medium containing PC3 cells followed by further incubation for 14 hours. PC3 cells were then selected with 5 μg/mL of puromycin (Sigma) for 1 week, and the knockdown of cyclin A1 was tested by immunoblotting and reverse transcription–polymerase chain reaction (RT-PCR). Adenoviruses containing cyclin A1 were prepared by standard protocols as previously described (23). Adenovirus-cyclin A1 vector was also generated by cloning full-length cyclin A1 cDNA into the XbaI and SalI sites of adenoviral vector pShuttle-PGK-EGFP vector kindlly provided by Dr. Xiaolong Fan (Department of Molecular Medicine and Gene Therapy, Lund University). This vector contains Ad5-PGK1-IRES-EGFP-B-globin intron-poly A and pShuttle backbone. Cyclin A1 cDNA was cloned between PGK1 and IRES. This adenoviral vector carrying cyclin A1 cDNA was designated as Ad5-Cyclin A1. The control vector was the empty adenoviral vector expressing EGFP and was designated as Ad5-Control. These vectors were used for the induction of long-term expression of cyclin A1 in prostate cancer cell lines.
Mouse Models of Tumor Growth and Metastasis
Three sets of mouse experiments were performed. All animal experiments met the requirements of Lund University Animal Care Facility and the National Institutes of Health guidelines. To analyze of the effects of cyclin A1 overexpression in subcutaneous tumor xenografts, 40 athymic nude mice aged 8–12 weeks (Harlan Nederland, Horst, The Netherlands) were used. For measurement of tumor growth, 10 of these mice were injected subcutaneously with PC3 cells infected with adenoviruses expressing cyclin A1–EGFP and 10 mice were injected with PC3 cells infected with adenoviruses expressing control-EGFP (4 × 106 cells per mouse). Tumor diameters were measured twice weekly using calipers, and volumes were calculated using the equation a(b2/2), where a and b represent the larger and smaller diameters, respectively. Mice were killed 6 weeks after inoculation of tumor cells. Tumor invasion and metastasis was examined in the remaining 20 mice (10 injected with PC3 cells expressing cyclin A1–EGFP and 10 with PC3 cells expressing control-EGFP) that were killed 12–14 weeks after injection. For assessment of cyclin A1 knockdown in subcutaneous tumor xenografts, 20 mice were injected with PC3 cells infected with retroviruses containing cyclin A1 shRNA-EGFP or control shRNA-EGFP (4 × 106 per mouse, 10 mice per group). For assessment of cyclin A1–overexpressing tumors in orthotopic tumor xenografts, eight mice were injected in their prostates with PC3 cells (1 × 106 cells per mouse) infected with adenovirus expressing cyclin A1–EGFP or control-EGFP (four mice for each tumor type). Mice were killed by CO2 asphyxia or by cervical dislocation. Prostates, lymph node, liver, lung, brain, spleen, and femurs were removed from each mouse. (These tissues have been taken from all the mice used in this study.) Half of the tissues were used for histology and immunohistochemical analysis. For histology analysis, tissues were fixed in 4% paraformaldehyde and embedded in paraffin. The sections were stained with H&E and were subjected to analysis under microscopy. The other half of the tissues were snap frozen in liquid nitrogen for use in protein analyses.
Immunohistochemistry
For immunohistochemistry of mouse organs, tissues were fixed for 24 hours in 4% paraformaldehyde and embedded in paraffin. The following antibodies were used: anti–cyclin A1, 1:50 (US Biological) and anti–cyclin A1, 1:100 (Santa Cruz Biotechnology); anti–cytokeratin-5, 1:200 (CK5) and anti-CD31, 1:200 (BD Pharmingen and Dako); and anti-VEGF, 1:100 (Santa Cruz Biotechnology).
Detection of VEGF Expression by Enzyme-Linked Immunosorbent Assay
The enzyme-linked immunosorbent assay (ELISA) for the detection of human VEGF in tissue lysates was performed as previously described (21). Briefly, tissue homogenate was diluted to the final concentration of 1 mg/mL and 100 μL of the lysate was used for measuring human VEGF using the VEGF ELISA kit (R&D Systems, Minneapolis, MN).
Cell Synchronization and Cell Cycle Analysis
To obtain cells in G0 cell cycle phase, LNCaP cells were starved in RPMI-1640 containing 0.1% fetal bovine serum for 48 hours as previously described (24). Cells were then fed with medium containing 10% FBS for 6 hours (G1 phase), followed by growth in medium containing 200 μM mimosine dissolved in ethanol (Sigma) for additional 24 hours (late G1 phase). To obtain G1/S cells, 2 μg/mL aphidicolin in dimethyl sulfoxide (Sigma) was added to the cells; after 24 hours, cells were washed and cultured in medium containing 10% FBS for additional 6 hours (S phase). Nocodazole (0.1 μg/mL) in DMSO (Sigma) was then added into the medium, and cells were grown for 30 hours (G2/M phase). Cells were harvested at different time points. A total of 1 × 106 cells were fixed and permeabilized. Cells were then washed twice with phosphate-buffered saline (PBS) containing 10% fetal calf serum and resuspended and incubated in PBS containing 5 μg/mL propidium iodide (Sigma), 100 mM sodium citrate, pH 7.3, and 0.05 mg RNase A (Sigma) for 20 minutes at 4°C. The cell fluorescence was measured in a FACSCalibur cytofluorometer (Becton Dickinson, Franklin Lakes, NJ) and analyzed using CELL Quest software (Becton Dickinson).
BrdU Proliferation Assay
The effect of cyclin A1 expression on cell proliferation was determined using the nonradioactive BrdU–based cell proliferation assay (Roche, Basel, Switzerland) according to the manufacturer’s protocol. Twenty-four hours after transfection, PC3 cells (3 × 103 cells per well) were incubated for 24 hours in a 96-well plastic plate that contained complete growth medium (triplicate cultures). BrdU was then added, and cells were cultured for another 8 hours. BrdU incorporation into the DNA was determined by measuring the absorbance at both 450 and 690 nm on an ELISA plate reader.
Invasion Assay
The invasion of PC3 cells was measured using Boyden transwell chambers (Chemicon) according to the manufacturer's protocol. Briefly, the cells were seeded onto the membrane of the upper chamber at a concentration of 3 × 104 in 400 μL medium. RPMI-1640 medium containing 10% FBS was then added to the lower chamber. Cells that passed through the Matrigel-coated membrane to the lower chamber were stained with crystal violet and were then dissolved in 10% acetic acid. The absorbance of the stained cells was measured on an ELISA plate reader.
uPA Activity Assay and Zymography
uPA activities in conditioned medium of cells transfected with small interfering RNA (siRNA) targeting uPA and control siRNA was measured with a colorimetric uPA activity assay kit (Millipore, Billerica, MA) following the manufacturer's protocol. Zymography was performed using Novex Zymogram Gels (Invitrogen) following the manufacturer's protocol. Briefly, PC3 cells were transfected with pCMS-EGFP-Cyclin A1 or control pCMS-EGFP vector (see below), or cells were stably transduced with shRNA against cyclin A1; 24 hours after transfection or seeding the stably transduced cells, culture supernatants were collected, and equal amount of volumes were subjected to gelatin zymography experiment (Invitrogen).
Preparation of Nuclear and Cytoplasmic Extracts
Nuclear and cytoplasmic fractions from control LNCaP cells or LNCaP cells treated with R1881 were prepared using the subcellular fractionation kit from Pierce (Rockford, IL) following the manufacturer's protocol.
Plasmids, Transfection, siRNA, and Luciferase Assay
The pCMS-EGFP-Cyclin A1 and pCMS-EGFP plasmids have been described previously (21). Human cyclin A1 cDNA (1.8 kb) was cloned into pCMS-EGFP plasmid at the XhoI and SalI restriction sites. These vectors were used in experiments assessing the effects of induction of transient expression of cyclin A1 in prostate cancer cell lines. AR-pcDNA3.1 was kindly provided by Dr Steven Balk (Beth Israel Deaconess Medical Center, Harvard Medical School). pcDNA3.1 was purchased from Invitrogen. PSA-luc and control constructs were kindly provided by Dr Hans Lilja (Memorial Sloan-Kettering Cancer Center, New York, NY). VEGF promoter constructs were kindly provided by Dr Dieter Marme (Tumor Biology Center, Freiburg, Germany) (25). The fragment designated as 2068 contains the full-length promoter; fragment 1340 consists of two activator protein-1 (Ap-1) sites; fragment 840 contains only one Ap-1 site; and fragments 102, 105, 316, and 415 lack the Ap-1 motifs. Transient transfection of pCMS-EGFP-Cyclin A1, pCMS-EGFP, AR-pcDNA3.1, or luc vectors containing different fragments into prostate cancer cell lines was performed using the electroporation system with Nucleofection kit (AmaxaBiosystems, Gaithersburg, MD) according to the manufacturer's instructions. In luciferase assay, 100–200 ng of luc plasmids were used. Luciferase activity was measured using the Luciferase Assay kit (Promega, Madison, WI) following the manufacturer's protocol. siRNAs against MMP2, uPA, and VEGF and scrambled siRNA were purchased from Ambion, Austin, TX. siRNA (50 nM) in 3.5 μL of nuclease-free water was transfected into 1 × 104 PC3 cells in a total volume 250 μL of Optimem medium using 7 μL of Lipofectamine 2000 (Invitrogen). Cells were used 24 hours later for invasion assays.
Immunoblotting and Immunoprecipitation Assays
Immunoblot analysis was performed as previously described (21). Co-immunoprecipitation of cyclin A1 and AR was performed as previously described (23). Protein extracts were separated on 12% sodium dodecyl sulfate (SDS)–polyacrylamide gels and were subsequently transferred to nitrocellulose membrane Hybond ECL (Amersham Pharmacia Biotech, Buckinghamshire, UK). The membranes were probed with appropriate primary antibodies, including: anti–cyclin A1, 1:200 (Pharmingen and US Biological, Swampscott, MA) and anti–cyclin A1, 1:500 (Santa Cruz Biotechnology); anti-AR, 1:1000 (Dako); anti–β-actin, 1:1000 and anti–lamin B, 1:1000 (Santa Cruz Biotechnology); anti-uPA, 1:500 (American Diagnostics, Stamford, CT); and anti–prostate-specific antigen (PSA), 1:1000 (code 2E9) (26). Membranes were then incubated with horseradish peroxidase–conjugated secondary antibodies (Amersham Life Sciences, Alesbury, UK) at 1:5000 dilution and visualized using the Enhanced ChemiLuminescence detection system (ECL) and ECL films (Amersham Pharmacia Biotech).
RNA Preparation, RT-PCR Analysis, and cDNA Microarray Analysis
RNA was isolated from PC3 cells as previously described (21). cDNA was synthesized from 2 μg of RNA (Invitrogen) and was used for semiquantitative RT-PCR or real-time RT-PCR. β-Actin was used as an internal control. Primers for human VEGF were previously described (27). The other primers were as follows: cyclin A1: 5′-CTCCTCTCCCAGTCTGAAGA-3′ and 5′-CAGGAAGTTGACAGCCAGAT-3′; MMP2: 5′-CGGCCGCAGTGACGGAAA-3′ and 5′-CATCCTGGGACAGACGGAAG-3′; MMP9: 5′-GACGCAGACATCGTCATCCAGTTT-3′ and 5′-GCCGCGCCATCTGCGTTT-3′; MMP11: 5′-CGATGTGACGCCACTCACCTTT-3′ and 5′-GGCCAGGGCTGGCCATATA-3′; MT1MMP: 5′-CCAGGGTCTCAAATGGCAACATAATGAAA-3′ and 5′-CCATGGAAGCCCTCGGCAAA-3′; GAPDH: 5′-AACAGCGACACCCACTCCTC-3′ and 5′-GGAGGGGAGATTCAGTGTGGT-3′; and β-actin: 5′-CGCGAGAAGATGACCCAGATC-3′, 5′-TCACCGGAGTCCATCACGA-3′. For cDNA microarray analysis, the experimental procedures have been described in Varambally et al. (28). We used expression profiling data that were available in the Gene Expression Omnibus (GEO) under accession number GSE3325 (available at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE3325). We used the data from benign prostate tissues (n = 6, N1–N4, NX1, NX2, GEO Profiles numbers: GSM74875–GSM74880), clinically localized primary prostate cancer (n = 7, P1–P5, PX1-2-tissue pools, GEO Profiles numbers: GSM74881–GSM74887), and metastatic prostate cancer (n = 6, W1–W4, WX1-2-tissue pools, GEO Profiles numbers: GSM74888–GSM74893).
Chromatin Immunoprecipitation
LNCaP cells were incubated with 0.1 nM R1881 for 1 hour and then rinsed with PBS. Protein–DNA complexes were then cross-linked with 5% formaldehyde in serum-free medium for 5 minutes. The samples were precleared with 40 μL of protein A/G beads, and the supernatant was collected. Immunoprecipitation was performed on 0.5–1 mg protein with antibody against cyclin A1 or IgG (negative control) by rocking samples with 40 μL G beads and 200 μg/mL salmon sperm DNA at 4°C in total volume of 500 μL for 3 hours. After incubation, beads were extensively washed with low-salt buffer (RIPA) and DNA–cyclin A1 complexes were eluted with 200 μL elution buffer (1% SDS, 1× Tris-Acetate Buffer) by rocking the beads at room temperature for 15 minutes. To dissociate histone–DNA complexes, 20 μL of 5 M NaCl was added and incubated for 4 hours at 65°C. To release DNA fragments, proteinase K was added in incubation buffer (10-μL 0.5 M EDTA, 20 μL 1 M Tris–HCl, pH 6.5) and incubated for 1 hour at 45°C. DNA was purified by phenol–chloroform extraction. For PCR the following primers were used: human VEGF promoter: 5′-CTGGCCTGCAGCAGACATCAAAGTGAG-3′ and 5′-CTTCCCGTTCTCAGCTCCACAAAC-3′ (amplicon: −913 to −783 bp in the promoter); and human MMP2 promoter: 5′-TGTTCCCTAAAACATTCCCC-3′ and 5′- GTCTCTGAGGAATGTCTTCT-3′ (amplicon: −1340 to −1120 bp in the promoter).
Sequence-Based Structure Prediction
The secondary structure of cyclin A1 was predicted by using the SABLE server (29).
Statistical Analysis
Possible pairwise correlations between the groups were analyzed using the Spearman rank correlation test. The statistical significance of differences between groups was evaluated by paired Wilcoxon rank sum test and Student t test or Mann–Whitney tests (comparison of means), and the Fisher exact test was used for comparison of incidence, as indicated. For measurement of tumor growth, two-way repeated analysis of variance (ANOVA) was performed using ANOVA test. All outcome variables are presented as the mean values with 95% confidence intervals (CIs). All data presented are representative of at least three independent experiments. All statistical tests were two-sided, and P values less than .05 were considered to be statistically significant.
Results
Expression of Cyclin A1, VEGF, and MMPs in Tumor Specimens From Prostate Cancer Patients
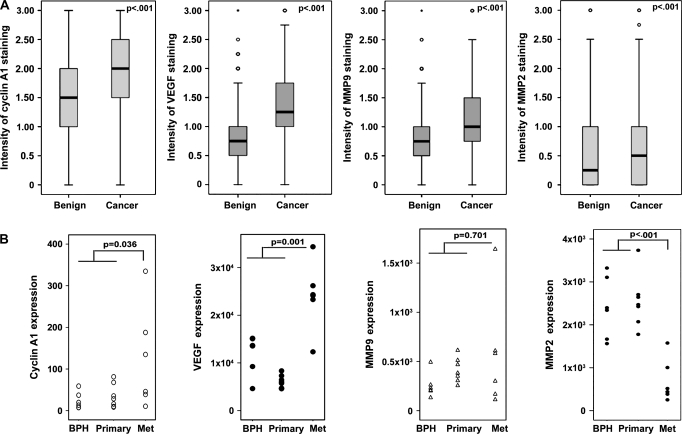
Because VEGF and MMPs play important roles in tumor metastasis, we examined whether their expression was increased in prostate cancer and whether their expression was correlated with that of cyclin A1 (6,7). Expression of cyclin A1, VEGF, MMP2, and MMP9 was examined by immunohistochemical analysis of tissue microarrays that contained primary malignant tumors and adjacent benign prostatic tissues from 482 patients with prostate cancers of various stages (Figure 1 and Supplementary Figure 1, A, available online). Prostate cancer specimens had statistically significantly higher levels of cyclin A1, VEGF, MMP2, and MMP9 expression than adjacent benign specimens (all P < .001, Wilcoxon signed rank test; Figure 1, A, and Supplementary Table 1, available online). There were statistically significant correlations between cyclin A1 and VEGF expression (r2 = 0.656, P < .001, Spearman rank correlation test), cyclin A1 and MMP2 expression (r2 = 0.123, P < .001), and cyclin A1 and MMP9 expression (r2 = 0.432, P < .001) (Supplementary Table 2, available online).
Figure 1.
Evaluation of the expression of cyclin A1, vascular endothelial growth factor (VEGF), metalloproteinase (MMP)9, and MMP2 in prostate cancer specimens. A) Tissue microarrays of sections from benign tissue and adjacent tumor tissue designated as Gleason grade 3 (81%) or Gleason grade 4–5 (18%) were immunostained with antibodies against cyclin A1, VEGF, MMP9, and MMP2. Differences in the expression of cyclin A1 (tumor n = 876, benign n = 856), VEGF (tumor n = 864, benign n = 787), MMP2 (tumor n = 806, benign n = 791), and MMP9 (tumor n = 863, benign n = 836) between groups were assessed using the paired Wilcoxon signed rank test (P < .001). The mean values of intensities of staining (horizontal lines) with error bars representing 95% confidence intervals for the mean are shown. The outliers are labeled by open circles. The boxes represent the distribution of the expression of each protein in the groups. B) Dot plots showing the expression of genes encoding cyclin A1, VEGF, MMP9, and MMP2 in tumor specimens from patients with benign prostatic hyperplasia (BPH, n = 6), primary prostate cancers (n = 7), and metastatic prostate cancers (Met, n = 6) analyzed by cDNA microarray. Differences between metastatic cancers (Met) and nonmetastatic disease (benign prostatic cancer and primary tumors in localized cancer) were assessed using the Mann–Whitney test. P values from two-sided tests are indicated.
Using the gene expression profiles obtained from the cDNA microarray analyses reported by Varambally et al. (28), we assessed the expression of genes encoding cyclin A1, VEGF, MMP2, and MMP9 in tumor specimens from benign prostatic hyperplasia (n = 6), primary prostate cancers (n = 7), and metastatic prostate cancers (n = 6). Expression of cyclin A1 and VEGF was statistically significantly higher in metastatic prostate cancer specimens than in benign prostatic hyperplasia tissue and primary tumors (Figure 1, B, P value from Mann–Whitney test for cyclin = .036, P value for VEGF < .001). There was no statistically significant difference in the levels of MMP9 in metastatic tumors compared with benign and primary tumors. In contrast to the immunohistochemistry result, MMP2 levels derived from cDNA microarrays were lower in metastatic tumors. The inconsistency between protein and mRNA may be due to the fact that a proportion of nontumor cells were present in samples used for the cDNA microarray assay because tissues containing metastatic tumor cells were grossly dissected.
Effect of Cyclin A1 Overexpression or Depletion on Invasion and Growth of PC3 Cells In Vitro
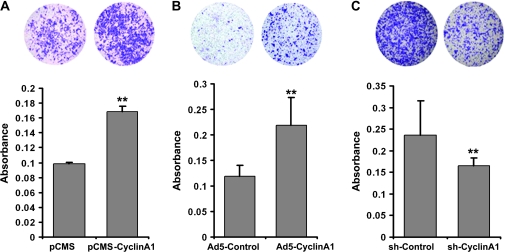
Based on the positive correlation between cyclin A1 expression and metastatic prostate cancer, we examined the role of cyclin A1 in tumor cell invasion. We achieved temporary cyclin A1 overexpression by transiently transfecting PC3 cells with cyclin A1–EGFP expressing vectors. Long-term overexpression of cyclin A1 in PC3 cells was achieved by infecting PC3 cells with adenoviruses expressing cyclin A1–EGFP. The effect of cyclin A1 overexpression on the invasiveness of PC3 cells was assessed using Matrigel coated Boyden chamber invasion assays. A higher proportion of PC3 cells overexpressing cyclin A1 passed through a Matrigel-coated membrane than of cells that did not overexpress cyclin A1 (Figure 2). Mean absorbances of cells containing pCMS-EGFP (designated as pCMS) and pCMS-EGFP-cyclin A1 (pCMS-Cyclin A1) vectors were 0.098 and 0.170, respectively (difference = 0.072, 95% CI = 0.059 to 0.083; P < .001); mean absorbance of cells infected with Adeno-5-PGK-EGFP (Ad5-Control) and Adeno-5-PGK-EGFP-Cyclin A1 (Ad5-Cyclin A1) were 0.118 and 0.218, respectively (difference = 0.100, 95% CI = 0.064 to 0.136; P < .001). Thus, cells overexpressing cyclin A1 were more invasive.
Figure 2.
Evaluation of the role of cyclin A1 in invasion of PC3 cancer cells in vitro. Invasiveness of PC3 cells transfected with pCMS-EGFP (pCMS) or pCMS-EGFP-A1 (pCMS–cyclin A1) vectors (A), with adenoviruses expressing EGFP (Ad5-Control) or cyclin A1–EGFP (Ad5-Cyclin A1) (B), or with retroviruses expressing cyclin A1 shRNA-EGFP or control shRNA-EGFP (C). Cells that passed through the Matrigel-coated membrane into the lower chamber stained with crystal violet and then dissolved in 10% acetic acid. The absorbance of the stained cells was measured on an enzyme-linked immunosorbent assay plate reader. Photomicrographs (top) were taken from the lower invasion chambers at ×4 magnification. Data in graphs at bottom are means of three independent experiments, each performed in duplicate, with upper 95% confidence intervals (**P < .001).
To determine if expression of cyclin A1 was required for invasion, we depleted endogenous cyclin A1 by infecting cells with retrovirus expressing cyclin A1 shRNA-EGFP. PC3 cells expressing cyclin A1 shRNA-EGFP displayed statistically significantly reduced invasiveness compared with control cells infected with shRNA-EGFP (Figure 2, C; mean invasion for control shRNA = 0.236, mean absorbance of invaded cells containing cyclin A1 shRNA = 0.166, difference = 0.070, 95% CI = 0.044 to 0.096; P < .001). We further examined the effect of cyclin A1 overexpression or depletion on proliferation and the cell cycle. There was no statistically significant effect of overexpression or inhibition of cyclin A1 on the proliferation of PC3 cells as assessed by the nonradioactive BrdU assay (Supplementary Figure 2, A and B, available online). Furthermore, overexpression or depletion of cyclin A1 did not influence the progression through the cell cycle in PC3 cells (Supplementary Figure 2, C and D, available online).
Effect of Cyclin A1 Overexpression on Growth and Vascularization of Tumors In Vivo
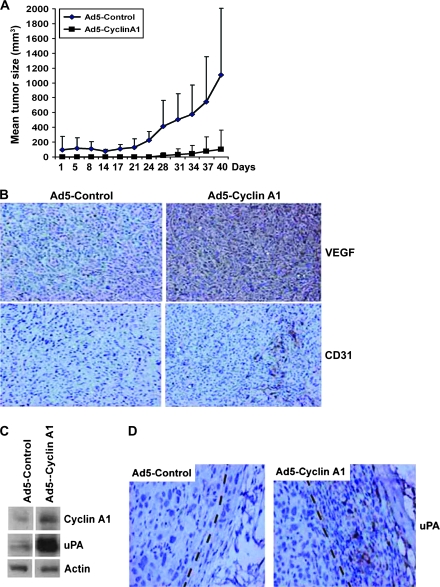
We examined the effect of cyclin A1 expression on tumor growth and invasion in vivo using mouse tumor xenografts. Equal numbers of PC3 cells infected with adenoviruses containing cyclin A1–EGFP or control EGFP were subcutaneously injected into nude mice, and tumor volume was assessed for 42 days beginning 14 days postinjection. The growth of tumors expressing cyclin A1–EGFP was delayed compared with that of tumors expressing control-EGFP (Figure 3, A, mean volume on day 40 of control-EGFP = 1110 mm3, Cyclin A1–EGFP = 102 mm3, difference = 1010 mm3, 95% CI = 434 to 1580 mm3; P = .005, two-sided t test; cyclin A1 overexpression effect: P = .003, sampling time effect: P = .014, cyclin A1 × sampling time effect: P = .03 [two-way repeated-measures ANOVA test for tumor growth]).
Figure 3.
The effect of cyclin A1 on the invasive phenotype of tumor xenografts in vivo. Subcutaneous xenografts were established with PC3 cells that contained adenovirus expressing cyclin A1–EGFP (cyclin A1 xenografts) or EGFP (control xenografts). A) Growth of cyclin A1 and control xenografts (n = 10 mice per group). Mean tumor volumes and upper 95% confidence intervals are shown. B) Immunohistochemical analysis of tumors overexpressing cyclin A1 and control tumors for the expression of vascular endothelial growth factor and CD31 on day 42. C) Immunoblot showing the expression of cyclin A1 and urokinase-type plasminogen activator (uPA) in control tumors and tumors overexpressing cyclin A1. Actin was probed with a specific antibody to control for differences in protein amounts as loading control. D) Immunohistochemical analysis of uPA expression in control and cyclin A1 tumors. uPA expression is seen in human tumor PC3 cells in cyclin A1–overexpressing tumor (Ad5–Cyclin A1), and the uPA-positive tumor cells are located in the fibroblast capsule of the invasive front of the tumor area (as marked with the dashed line).
We also characterized tumor histology and measured the expression of VEGF, uPA, CD31, and Ki-67, proteins that are important for invasion and metastasis. We observed statistically significantly higher expression of VEGF, as measured by ELISA, in tumors expressing cyclin A1–EGFP compared with tumors expressing control-EGFP: mean VEGF expression in tumors formed by cells that were infected with control adenoviruses and adenoviruses expressing cyclin A1 was 2.03 ng/mL per mg protein and 2.64 ng/mL per mg protein, respectively (difference = 0.61 ng/mL, 95% CI = 0.14 to 1.09 ng/mL; P = .016). These results were confirmed immmunohistochemically (Figure 3, B). We determined the extent of tumor vascularization by quantifying the expression of CD31 and formation of blood vessels in the tumor areas. There was a statistically significant increase in CD31 expression in tumors overexpressing cyclin A1 (mean numbers of CD31-expressing vessels in tumors formed from cells expressing control-EGFP and cyclin A1–EGFP were 4.2 and 46, respectively, difference = 42, 95% CI = 28 to 55; P < .001; Figure 3, B). An infiltrative growth pattern in the fibroblast capsule of the tumor along with an increased level of uPA was also observed in tumors that overexpressed cyclin A1 (Figure 3, C and D). Although growth of tumors overexpressing cyclin A1 was delayed, there was no statistically significant difference in the fraction of cells expressing Ki-67, suggesting that cyclin A1 overexpression did not inhibit tumor cell proliferation (Supplementary Figure 3, A [available online]; mean positive Ki-67 cells among control and cyclin A1–overexpressing cells was 45 and 42, respectively, difference = 3, 95% CI = 0 to 13; P = .45). Although cyclin A1–overexpressing tumors proliferated at similar rates compared with control tumors, the mean size of cyclin A1–overexpressing tumors was smaller.
Effect of Cyclin A1 shRNA on Tumor Invasion In Vivo
To further verify the effect of cyclin A1 on tumor invasion, we established tumor xenografts by subcutaneously injecting PC3 cells expressing shRNA-EGFP (control shRNA) or cells expressing cyclin A1 shRNA-EGFP (cyclin A1 shRNA) into nude mice (10 mice per group). Tumor incidence and volume were assessed 40 days later. Tumors appeared in all mice from the two groups. There was no statistically significantly difference in tumor size. We analyzed the histology of the H&E-stained sections of lymph nodes, livers, lungs, and bones from the two groups of mice and found that all the organs were free of metastasis. To examine the invasiveness of tumors with reduced cyclin A1 expression, we isolated fresh tumor cells from the mice (four mice per group) and tested these cells in Matrigel-coated Boyden chamber invasion assays. Invasion of tumor cells expressing cyclin A1 shRNA-EGFP was statistically significantly less than that of tumor cells expressing control shRNA-EGFP (mean absorbances were 0.151 and 0.180, respectively, difference = 0.029, 95% CI = 0.024 to 0.034; P < .001, Supplementary Figure 3, B, available online).
Effect of Cyclin A1 Overexpression on Tumor Metastasis
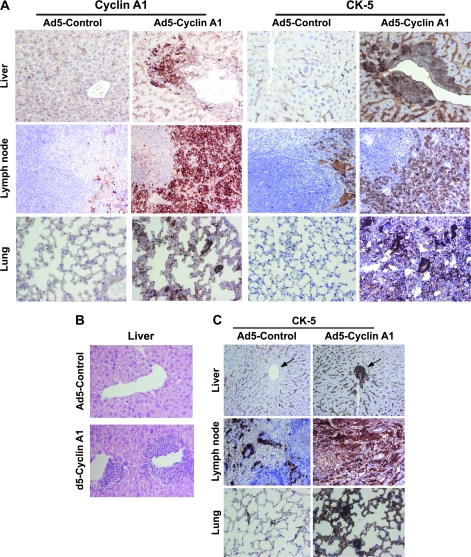
To investigate whether cyclin A1 overexpression promoted invasion of PC3 tumor cells to secondary sites, we isolated lymph nodes, livers, lungs, and bones from the mice 12–14 weeks after subcutaneous implantation. In eight of 10 mice bearing PC3 cells overexpressing cyclin A1, there was infiltration of tumor cells into lymph node, liver, and lung. None of the 10 mice bearing PC3 cells containing control vectors had liver and lung metastases. One control mouse had lymph node metastasis. The incidence of tumor metastasis to lymph nodes was 10% in control (Ad5-Control) mice and 80% in cyclin A1–overexpressing mice (Ad5–Cyclin A1) (P < .001, Fisher exact test). Cyclin A1 overexpression in livers, lymph nodes, and lungs was verified by immunostaining of all the tissues using antibody against cyclin A1 (Figure 4, A). Because CK5 is expressed in PC3 prostate cancer cells but is absent in lymph node, lung, and liver in mouse tissues (30), expression of CK5 was examined and quantified in livers, lungs, and lymph nodes from mice bearing cyclin A1–overexpressing tumors and control mice. The mean percentage of CK5-positive cells in livers of control mice and mice whose tumors overexpressed cyclin A1 was 0 and 21.6, respectively (difference = 21.6, 95% CI = 0.0 to 43.5; P = .05). In lungs the corresponding percentages were 0 and 37, respectively (difference = 37, 95% CI = 0.0 to 86; P = .12). The corresponding percentages in lymph nodes were 15.0 and 50.0, respectively (difference = 35.0, 95% CI = 0 to 109; P = .263) (Figure 4, A). Histological examination of liver specimens showed that tumors cells overexpressing cyclin A1 infiltrated into the livers in 70% of the mice, but none of the control mice showed signs of liver infiltration by tumor cells (Figure 4, B); the number of occluded vessels in the livers of control and cyclin A1–overexpressing mice was 0 and 8.7 (difference = 8.7, 95% CI = 0 to 19; P = .09).
Figure 4.
Effect of cyclin A1 on metastasis of prostate cancer cells in vivo. A) Immunohistochemical analysis of cyclin A1 and cytokeratin-5 (CK5) in livers, lungs, and lymph nodes of mice bearing control and cyclin A1–overexpressing tumors. About 5–10 sections were prepared from each tissue examined. B) Histology of the metastasis in the liver in mice bearing tumors with overexpression of cyclin A1 compared with noncancerous livers of mice whose tumors did not overexpress cyclin A1. C) Dependence of CK5 expression on cyclin A1 activity in an orthotopic mouse model of metastasis. PC3 cells overexpressing cyclin A1, or control PC3 cells expressing vector were orthotopically injected into mouse prostates, and the resulting metastasis was assessed 30 days later for organ histology and for CK5 expression. Sections of lymph nodes, lungs, and livers stained with antibody against CK5 are shown. The arrows indicate the portal vein areas in the liver sections. The infiltrated human PC3 tumor cells in the portal vein areas in the cyclin A1–overexpressing liver are positively stained with CK5.
To further examine the effect of cyclin A1 overexpression on tumor metastasis, we established an orthotopic mouse model by inoculating PC3 cells that were infected with adenoviruses containing cyclin A1-EGFP or control-EGFP into the prostates of nude mice (n = 4 mice per group). Metastasis was measured 30 days later. We observed liver and lung metastases in three of four mice bearing tumors overexpressing cyclin A1 when we examined H&E-stained sections prepared from livers and lungs of all the mice in the two groups. Only one mouse from the control group had metastases in the lung and liver. This result was also confirmed by the assessment of CK5 staining in lungs and livers from the mice overexpressing cyclin A1 and control mice (four mice per group) (Figure 4, C, mean percentage of CK5-positive cells in lungs of controls and mice overexpressing cyclin A1 was 2.5 and 20, respectively, difference = 18, 95% CI = 0.0 to 43; P = .13). All (16/16) lymph nodes analyzed from cyclin A1–overexpressing tumors had micrometastases compared with 45% (5/11) of lymph nodes in the control group, and this difference was statistically significant (P = .017). One of four cyclin A1–overexpressing mice had enlarged axillary, para-aortic lymph nodes, and spleen (Supplementary Figure 4, available online), as well as large colonies of tumor cells positive for CK5 (Figure 4, C), suggesting that metastatic growth was responsible for the enlargement. Furthermore, vascularization was determined by the quantification of CD31-positive vessels in tumor areas. There were higher numbers of CD31-positive vessels in cyclin A1–overexpressing tumors than controls (mean CD31-positive vessels in control and cyclin A1–overexpressing mice was 7 and 18, respectively, difference = 11, 95% CI = 1 to 22; P = .032).
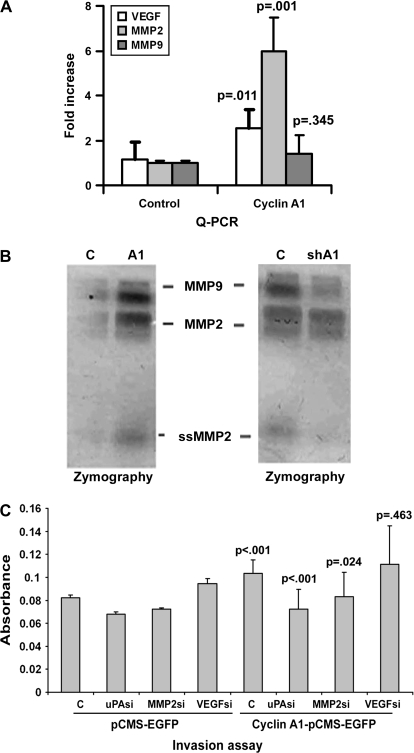
Expression and Activity of MMP2 and MMP9 in Cyclin A1–Overexpressing Cells
MMP2 and VEGF mRNA expression levels were statistically significantly higher in PC3 cells transiently transfected with pCMS-EGFP-Cyclin A1 than in PC3 cells transfected with empty pCMS-EGFP vector, as measured by semiquantitative RT-PCR (Supplementary Figure 5, available online) and real-time quantitative RT-PCR (Figure 5, A). Gelatin zymography assays were performed to examine the effect of overexpression or inhibition of cyclin A1 on the activity of MMPs. PC3 cells expressing cyclin A1–EGFP displayed increased activity of MMP2 and MMP9 proteins, compared with control PC3 cells (Figure 5, B). In contrast, the gelatinase activities of these proteins were reduced in PC3 cells expressing cyclin A1 shRNA (Figure 5, B). Because we observed strong effects of cyclin A1 overexpression on uPA and MMP2 levels in vivo, we attempted to determine which of them is involved in cyclin A1–mediated invasion. We used siRNAs corresponding to the mRNAs encoding these proteins to inhibit the levels of uPA, MMP2, or VEGF in PC3 cells overexpressing cyclin A1 (Supplementary Figure 5, B and C, available online). We overexpressed cyclin A1 by transiently transfecting PC3 cells with pCMS-EGFP-Cyclin A1 or empty pCMS-EGFP vectors. Reduced expression of MMP2 or uPA inhibited the cyclin A1–mediated invasion in vitro. Increased invasion of PC3 cells due to overexpression of cyclin A1 (mean absorbance of invaded control cells = 0.0823, mean absorbance value of invaded cyclin A1–overexpressing cells = 0.1033, difference = 0.021, 95% CI = 0.011 to 0.031; P = .004) was partially abrogated when uPA was depleted in the cells by introduction of corresponding siRNA (mean absorbance of invaded control cells expressing control siRNA = 0.1033, mean absorbance of invaded cells expressing siRNA targeting uPA = 0.072, difference = 0.031, 95% CI = 0.018 to 0.044; P < .001) or when MMP2 was depleted (means absorbance of invaded control cells expressing control siRNA = 0.103 invaded cells expressing siRNA targeting MMP2 = 0.083, difference = 0.021, 95% CI = 0.003 to 0.037; P = .024). However, inhibition of VEGF did not prevent the increase in invasion mediated by cyclin A1 overexpression (P = .46). These results suggest that uPA and MMP2 are important for cyclin A1–mediated invasion (Figure 5, D). Thus, our findings suggest that cyclin A1 may promote tumor cell invasion in part through uPA- and MMP2-mediated pathways.
Figure 5.
Activation of metalloproteinases (MMPs) in PC3 cells overexpressing cyclin A1. A) Quantitative real-time polymerase chain reaction (Q-PCR) for vascular endothelial growth factor (VEGF), MMP2, and MMP9 was performed using RNA isolated from PC3 cells infected with adenoviruses expressing EGFP (C) or Cyclin A1–EGFP (A1). B) Assessment of MMP2 and MMP9 activity using gelatin zymography. Culture media of PC3 cells transfected with pCMS-EGFP (C) or pCMS-EGFP-Cyclin A1 (A1) or culture media of PC3 cells infected with cyclin A1 shRNA or control shRNA (C) were subject to electrophoresis, and MMP9, MMP2, and small size MMP2 activities were distinguished based on electrophoretic mobility. C) Invasiveness of PC3 cells overexpressing cyclin A1 and/or expressing siRNA constructs targeting urokinase-type plasminogen activator, MMP2, or VEGF. Data are means of three independent experiments performed in duplicate with upper 95% confidence intervals.
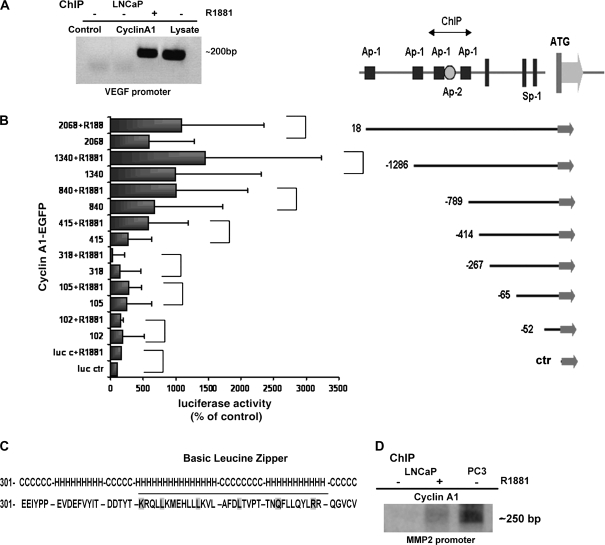
Interaction of Cyclin A1 With AP-1 Sites Within the Promoters of VEGF and MMP2
Next, we investigated whether cyclin A1 contributes to tumor cell invasion by regulating the expression and activity of genes encoding VEGF and MMP2. We performed chromatin immunoprecipitation (ChIP)–binding assays in androgen-dependent LNCaP cells. In LNCaP cells treated with R1881, a synthetic androgen, cyclin A1 precipitated with the VEGF promoter region (Figure 6, A). To define the sites on the VEGF promoter to which cyclin A1 binds, seven VEGF sequences spanning different transcription factor–binding sites in the promoter were constructed and cloned so that they controlled luciferase expression: fragment 2068 contained the full-length promoter; fragment 1340 contained two Ap-1 sites; fragment 840 contained only one Ap-1 site; and fragments 102, 105, 318, 415 lacked Ap-1 motifs (Figure 6, B). The constructs were cotransfected with cyclin A1–EGFP or control vectors into LNCaP cells for 24 hours followed by treatment with 0.1 nM R1881 (Figure 6, B). Cyclin A1 overexpression did not induce luciferase activity from promoter sequences lacking Ap-1 sites (102–415). In the presence of cyclin A1 expression we observed higher expression of luciferase under the control of promoter fragment 840 with only one Ap-1 site (mean value of luciferase activities from the control promoter and the promoter containing one Ap-1 site [840] in the presence of cyclin A1 expression was 0.011 and 0.074, respectively, difference = 0.063, 95% CI = 0 to 0.115; P = .078). Cyclin A1 greatly increased luciferase expression from a promoter sequence that contained two closely spaced putative Ap-1 sites (1340) (Figure 6, B) (mean value = 0.1093, difference = 0.098, 95% CI = 0.0516 to 0.145; P = .026). The effect of cyclin A1 on this region was even greater when cells were treated with 0.1 nM R1881 for 24 hours (means value of luciferase expression from control and the promoter containing the putative sites in the presence of R1881 was 0.018 and 0.159, respectively, difference = 0.141, 95% CI = 0.0852 to 0.197; P = .019). Analysis of the cyclin A1 amino acid sequence revealed that it contains a leucine zipper–like motif with helix-loop-helix structure similar to that of the jun transcription factor (Figure 6, C). To examine whether cyclin A1 occupied the Ap-1 sites located in the promoter regions of MMPs, we performed additional ChIP assays. Similar to what was observed for VEGF, cyclin A1 was bound to the MMP2 promoter in LNCaP cells treated with R1881 and in PC3 cells (Figure 6, D).
Figure 6.
Evaluation of the role of cyclin A1 in mediating vascular endothelial growth factor (VEGF) transcriptional activity. A) Chromatin immunoprecipitation (ChiP) in LNCaP cells or in LNCaP cells treated with 0.1 nM R1881, a synthetic androgen. Polymerase chain reaction (PCR) was performed on immunocomplexes obtained by precipitation with antibody to cyclin A1 or to IgG (Control); Lysate = total lysate. B) The schematic organization of the VEGF promoter (25). Consensus sites for Ap-1 (filled black squares) and Ap-2 (open circle) along with translation start site and Sp-1 sites are indicated. Luciferase assay was performed in LNCaP cells that were cotransfected with pCMS-EGFP-A1 and various VEGF promoter fragments and treated with R1881 or vehicle for 24 hours. (The P values for each comparison to examine the effect of cyclin A1 overexpression on VEGF promoters were: promoter fragment 102: P = .395; 105: P = .354; 318: P = .780; 415: P = .132; 840: P = .078; 1340: P = .026, full-length promoter: P = .016; two-sided t test.) (The P values for each comparison to examine the effect of cyclin A1 overexpression in combination with R1881 were: promoter fragments 102: P = .495; 105: P = .028; 318: P = .005; 415: P = .076; 840: P = .01; 1340: P = .019; full-length promoter: P = .016; two-sided t test.) C) Secondary structure of cyclin A1 that forms the leucine-like zipper fragment. The helix-coiled-helix loop is underlined (H = helix; C = coil). D) ChiP assay in LNCaP cells or in LNCaP cells treated with 0.1 nM R1881 (+) and in PC3 cells. PCR was performed with cyclin A1 immunocomplexes as template; primers spanning the Ap-1 site as described in “Materials and Methods” in the metalloproteinase 2 promoter were used to amplify precipitated sequences.
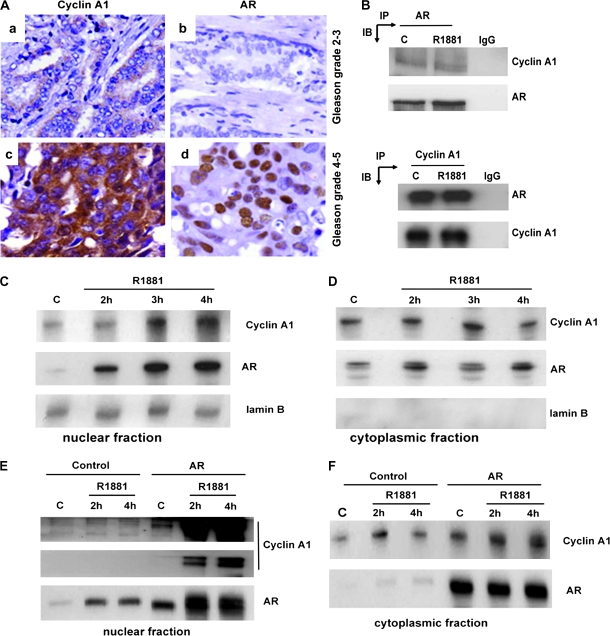
Interaction of Cyclin A1 With AR in Prostate Cancer Cells
Because cyclin A1 binds to the VEGF and MMP2 promoters in LNCaP cells in an androgen-dependent manner, we investigated how cyclin A1 associates with AR. We examined the expression of cyclin A1 and AR in the tissue microarrays containing 99 prostate cancers that were chosen from a total of 482 because they were scored with higher Gleason grades and were therefore defined as advanced prostate cancers. There was a statistically significant correlation between cyclin A1 and AR expression in the 99 specimens from advanced cancers (r2 = 0.214, P = .036) (Figure 7, A, Supplementary Table 3, available online). We have previously shown that the regulation of VEGF promoter by cyclin A1 is partially dependent on AR in prostate cancer cell lines (21). Therefore, we determined if AR interacted physically with cyclin A1. A complex between cyclin A1 and AR was detected in both control and R1881-treated LNCaP cells using antibodies to cyclin A1 to precipitate the complex and antibody to AR for detection on immunoblots or antibody to AR for immunoprecipitation and antibody to cyclin A1 for detection on immunoblots (Figure 7, B).
Figure 7.
Evaluation of the associations between androgen receptor (AR) and cyclin A1 in patients and in LNCaP cells. A) Immunohistochemical analysis of cyclin A1 and AR in prostate cancer tissue samples. Cyclin A1 staining is shown in panels a and b; AR staining is indicated in c and d. B) Immunoprecipitation of cyclin 1 complexed to AR in LNCaP cells. Antibodies to AR and cyclin A1 were used for immunoprecipitation in the upper and lower panels, respectively, and for detection on immunoblots in the lower and upper panels, respectively. C = untreated control cells, R1881 = synthetic androgen–treated cells, IgG = negative control. C and D) Nuclear and cytoplasmic fractions from LNCaP control cells and LNCaP cells treated with R1881 for the indicated times, electrophoresed, transferred to nitrocellulose membranes, and probed with antibodies to cyclin A1 and AR. E and F) Expression of cyclin A1 and AR was examined in the subcellular fractions from LNCaP cells transfected with control vector or AR vector in the presence or absence of R1881 treatment (2–4 hours). Chemiluminescent reactions were allowed to proceed for the indicated times.
The Effect of AR Stimulation on Cyclin A1 Localization in Prostate Cancer Cells
To determine whether localization of cyclin A1 was affected by androgen stimulation, we assessed expression of cyclin A1 and AR in cytosolic and nuclear fractions of LNCaP cells that had been treated or not treated with R1881. An increase in the levels of both proteins was observed in the nuclear fraction after treatment with 0.1 nM R1881 over a period of 2–4 hours (Figure 7, C and D). This time-dependent increase suggests that R1881 either promotes nuclear translocation or mediates cyclin A1 stabilization in the nucleus. To test whether androgen effects on the subcellular distribution of cyclin A1 are mediated via AR, LNCaP cells were transfected with the AR-pcDNA3.1 expression vector or a control pcDNA3.1 vector and treated with R1881 for 2 or 4 hours. AR overexpression in combination with R1881 treatment resulted in an increase in the overall expression of cyclin A1 relative to levels in cells transfected with vector alone with a much more pronounced increase in nuclear cyclin A1 expression (Figure 7, E and F), suggesting that AR is involved in cyclin A1 translocation to the nucleus. In PC3 cells that lacked a functional endogenous AR and were treated with R1881, overexpression of AR induced a maximal increase in cyclin A1 expression only in the nucleus (Supplementary Figure 6, available online). To confirm our observation that androgen-triggered activation of AR is involved in cyclin A1 translocation to the nucleus, we performed time-resolved studies on live cells in which a construct encoding cyclin A1–GFP fusion protein was transfected into LNCaP cells. LNCaP cells that had been transiently transfected with vectors that expressed cyclin A1–GFP or GFP alone were treated with R1881, and fluorescence images were taken every 3 minutes for 30 minutes. Although the subcellular location of control GFP did not change after R1881 treatment, we observed rapid translocation of cyclin A1–GFP to the nucleus (Supplementary Figures 7, A–C, and 8, A and B, available online).
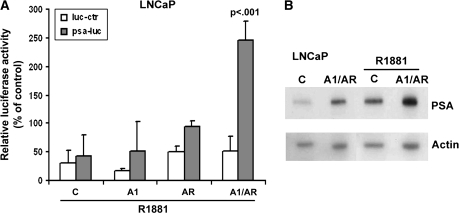
Cyclin A1 Transactivation of AR on the PSA Promoter
Finally, we asked whether a change in the levels of cyclin A1 is associated with changes in AR-mediated gene expression. PSA promoter activity was determined in LNCaP cells overexpressing cyclin A1, AR, or both proteins followed by treatment with R1881. Although cyclin A1 by itself did not increase the activation of the PSA promoter to a statistically significant extent when cyclin A1 was cotransfected with AR into the LNCaP cells, the activity of the PSA promoter and PSA protein levels were greatly increased compared with cells transfected with AR alone (Figure 8, A and B). Similar to what was observed in LNCaP cells, the greatest increase in PSA expression was observed upon coexpression of cyclin A1 and AR in PC3 cells lacking a functional AR, followed by the treatment with 0.1 nM R1881 (Supplementary Figure 8, C, available online). These results suggest that cyclin A1 acts as a coactivator of AR.
Figure 8.
Cyclin A1 regulates androgen receptor (AR) activity. A) LNCaP cells were transfected with prostate-specific antigen (PSA) promoter reporter constructs plus either control vector (C) or cyclin A1–expressing vector (A1) or AR-expressing vector (AR) or cyclin A1 and AR vectors (A1/AR) followed by treatment with 0.1 nM R1881. Results in A are presented as mean luciferase activity of three experiments with 95% confidence intervals. The P value is indicated. B) Expression of PSA was examined in cells transfected with control vectors or cyclin A1- and AR-expressing vectors by immunoblot analysis.
Discussion
In this study we found that cyclin A1 plays an important role in prostate cancer invasion and metastasis. We demonstrated that cyclin A1 expression was elevated in most of the primary and metastatic prostate cancer specimens examined compared with adjacent benign specimens. Furthermore, there was a statistically significant correlation between cyclin A1 and VEGF, MMP2, and MMP9 protein expression in tumor specimens from prostate cancer patients. Overexpression of cyclin A1 in PC3 cells by transient transfection or by adenoviral transduction promoted tumor cell invasion in vitro, and the cells became more invasive in vivo and metastatic. Silencing of cyclin A1 by shRNA inhibited migration and invasion in PC3 cells. We also demonstrated that inhibition of MMP2 or uPA by siRNA-mediated knockdown could partially inhibit the increased invasiveness of PC3 cells that overexpressed cyclin A1. Our data suggest that cyclin A1 may promote tumor cell invasion in part by increasing the expression and activity of MMPs, uPA, and VEGF. In addition, we showed that induced AR expression enhanced nuclear cyclin A1 expression. Finally, we observed that cyclin A1 interacted with AR and enhanced AR-mediated activation of AR target genes, suggesting that cyclin A1 and AR may act as cofactors, possibly to promote prostate tumor progression and invasion.
To mimic the gradual low-level dissemination of carcinoma cells in vivo, we used both subcutaneous and orthotopic xenograft models. We detected lymph node and lung metastases, regardless of primary tumor size, in mice bearing tumors overexpressing cyclin A1, but not in mice bearing tumors expressing control vectors. Despite their more aggressive and metastatic properties, cyclin A1–transduced cells proliferated at similar rates in vitro and the mean size of tumors overexpressing cyclin A1 was smaller than that of tumors expressing control vectors. One possible interpretation for these results is that PC3 cells overexpressing cyclin A1 may tend to spread rather than grow. Our findings are supported by results of several other studies. For example, breast tumors with alterations in the HER-2 gene could invade into the lung without forming larger tumors in mouse models, and the number of disseminated cancer cells was similar for small and large tumors in patients with HER-2 alterations (31). Vascular endothelial growth factor-C expression has also been shown to promote metastasis of lung tumors without promoting proliferation in tumor cells (32). Similarly, overexpression of p16 not only increased invasiveness in tumors in breast and colorectal cancers but also inhibited cell cycle (33).
The subcutaneous and orthotopic xenograft tumors overexpressing cyclin A1 also had higher levels of vascularization and angiogenesis than control tumors as reflected by higher CD31 and VEGF expression. The fact that cyclin A1–overexpressing tumors did not grow larger than control tumors agrees with recent findings that higher vascularization does not always correlate with tumor growth (34).
We demonstrated that cyclin A1 regulates VEGF and MMP2 expression by binding directly to their promoters. Overexpression of cyclin A1 also enhanced the activities of MMPs. When we introduced cyclin A1–expressing vector into PC3 cells together with constructs expressing siRNAs to MMP or uPA, we found that reduced expression of MMPs and uPA inhibited the cyclin A1–mediated invasion and migration in vitro. The finding that cyclin A1 regulates the expression of a growth factor signaling molecule (VEGF) and extracellular proteases (MMPs) and promotes tumor cell invasion and metastasis in part through MMPs and uPA suggests that cyclin A1 may be a key regulator of tumor invasion and metastasis.
The mechanisms underlying the transition from androgen dependent to androgen-independent metastatic cancer are unknown, but an important finding is the strong increase in expression of AR that is associated with the transition to latter condition (11). R1881-induced AR overexpression in LNCaP cells and PC3 cells increased levels of cyclin A1 in the nucleus, suggesting that AR could either promote trafficking of cyclin A1 from the cytoplasm to the nucleus or promote its stabilization in the nucleus. The nuclear localization of cyclin A1 may be linked to its binding, in concert with AR, to the promoter region of VEGF and MMP2.
Our study has several limitations. One is the lack of an appropriate in vivo model to study the cooperative effects of cyclin A1 and AR on tumor invasion and metastasis. We used PC3 cells to initiate the invasive tumors in mice. However, PC3 cells do not have functional AR. LNCaP cells contain a functional AR, but when they are subcutaneously injected into mice, the resultant tumors are unable to invade to the secondary tissues. Why cyclin A1–overexpressing tumors are smaller but more invasive compared with control tumors is unknown and an appropriate model to separate tumor growth from tumor metastasis is needed. In some experiments we were limited by the number mice used. For example, our results that cyclin A1 overexpression led to increased metastasis to lung and liver in an orthotopic tumor mouse model did not achieve statistical significance. Finally, the mechanisms by which cyclin A1 is stabilized in the nucleus by AR-mediated pathways remain to be elucidated.
In conclusion, understanding the mechanisms underlying the invasion and metastasis of prostate cancer cells may be essential to developing effective therapeutics for the hormone-refractory form of the disease. We have shown that overexpression of cyclin A1 increased migration and invasiveness of prostate cancer in vitro and promoted metastasis. The importance of cyclin A1 in tumor cell invasion and metastasis is suggested by its high level of nuclear expression in aggressive prostate cancer and its ability to mediate the activities of MMPs and VEGF in cultured cells.
Funding
The Swedish Cancer Society (4573-B02-02XBB to J.L.P., 4294 to A.B., and 4864-1306-04XBC to P.H.), The Swedish National Research Council (to J.L.P. and K2006-32X-20103-01-3 to P.H.), Malmö Hospital Cancer Foundation, Swedish Royal Physiographic Society in Lund, Crafoord Foundation and MAS Foundation (to J.L.P.), The Foundation for Urology Research in Malmö, The Thulefjord Foundation (to A.B.) The Gunnar Nilsson Cancer Foundation (to J.L.P.) and the Sigrid Jusélius Foundation (to P.H.).
Supplementary Material
Footnotes
The sponsors had no role in the study design, data collection and analysis, interpretation of results, the preparation of the manuscript, or the decision to submit the manuscript for publication.
We are grateful to Dr Steven Balk (Beth Israel Deaconess Medical Center, Harvard Medical School), Dr Hans Lilja (Memorial Sloan-Kettering Cancer Center, New York, NY), and Dr Dieter Marme (Tumor Biology Center, Freiburg, Germany) for providing construct plasmids. We thank Dr Sooryanarayana Varambally (University of Michigan Medical School, US) for providing cDNA microarray data (28). We thank Dr Martin Bilban (Vienna Medical University, Austria) for help with the silencing technology. Dr Sven Påhlman (Lund University, Sweden) is acknowledged for helpful discussions about the manuscript.
References
- 1.Wong SY, Hynes RO. Lymphatic or hematogenous dissemination: how does a metastatic tumor cell decide? Cell Cycle. 2006;5(8):812–817. doi: 10.4161/cc.5.8.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ortega N, Behonick DJ, Werb Z. Matrix remodeling during endochondral ossification. Trends Cell Biol. 2004;14(2):86–93. doi: 10.1016/j.tcb.2003.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carmeliet P, Moons L, Lijnen R, et al. Urokinase-generated plasmin activates matrix metalloproteinases during aneurysm formation. Nat Genet. 1997;17(4):439–444. doi: 10.1038/ng1297-439. [DOI] [PubMed] [Google Scholar]
- 4.He Y, Liu XD, Chen ZY, et al. Interaction between cancer cells and stromal fibroblasts is required for activation of the uPAR-uPA-MMP-2 cascade in pancreatic cancer metastasis. Clin Cancer Res. 2007;13(11):3115–3124. doi: 10.1158/1078-0432.CCR-06-2088. [DOI] [PubMed] [Google Scholar]
- 5.Gupta GP, Nguyen DX, Chiang AC, et al. Mediators of vascular remodelling co-opted for sequential steps in lung metastasis. Nature. 2007;446(7137):765–770. doi: 10.1038/nature05760. [DOI] [PubMed] [Google Scholar]
- 6.Qi L, Robinson WA, Brady BM, Glode LM. Migration and invasion of human prostate cancer cells is related to expression of VEGF and its receptors. Anticancer Res. 2003;23(5A):3917–3922. [PubMed] [Google Scholar]
- 7.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2(3):161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 8.McCawley LJ, Matrisian LM. Tumor progression: defining the soil round the tumor seed. Curr Biol. 2001;11(1):R25–R27. doi: 10.1016/s0960-9822(00)00038-5. [DOI] [PubMed] [Google Scholar]
- 9.Krishan A, Oppenheimer A, You W, Dubbin R, Sharma D, Lokeshwar BL. Flow cytometric analysis of androgen receptor expression in human prostate tumors and benign tissues. Clin Cancer Res. 2000;6(5):1922–1930. [PubMed] [Google Scholar]
- 10.Sharma M, Zarnegar M, Li X, Lim B, Sun Z. Androgen receptor interacts with a novel MYST protein, HBO1. J Biol Chem. 2000;275(45):35200–35208. doi: 10.1074/jbc.M004838200. [DOI] [PubMed] [Google Scholar]
- 11.Buchanan G, Irvine RA, Coetzee GA, Tilley WD. Contribution of the androgen receptor to prostate cancer predisposition and progression. Cancer Metastasis Rev. 2001;20(3–4):207–223. doi: 10.1023/a:1015531326689. [DOI] [PubMed] [Google Scholar]
- 12.Culig Z, Hobisch A, Cronauer MV, et al. Mutant androgen receptor detected in an advanced-stage prostatic carcinoma is activated by adrenal androgens and progesterone. Mol Endocrinol. 1993;7(12):1541–1550. doi: 10.1210/mend.7.12.8145761. [DOI] [PubMed] [Google Scholar]
- 13.Buchanan G, Yang M, Harris JM, et al. Mutations at the boundary of the hinge and ligand binding domain of the androgen receptor confer increased transactivation function. Mol Endocrinol. 2001;15(1):46–56. doi: 10.1210/mend.15.1.0581. [DOI] [PubMed] [Google Scholar]
- 14.Liu D, Matzuk MM, Sung WK, Guo Q, Wang P, Wolgemuth DJ. Cyclin A1 is required for meiosis in the male mouse. Nat Genet. 1998;20(4):377–380. doi: 10.1038/3855. [DOI] [PubMed] [Google Scholar]
- 15.Ji P, Agrawal S, Diederichs S, et al. Cyclin A1, the alternative A-type cyclin, contributes to G1/S cell cycle progression in somatic cells. Oncogene. 2005;24(16):2739–2744. doi: 10.1038/sj.onc.1208356. [DOI] [PubMed] [Google Scholar]
- 16.Liao C, Wang XY, Wei HQ, et al. Altered myelopoiesis and the development of acute myeloid leukemia in transgenic mice overexpressing cyclin A1. Proc Natl Acad Sci USA. 2001;98(12):6853–6858. doi: 10.1073/pnas.121540098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mèuller-Tidow C, Diederichs S, Schrader MG, et al. Cyclin A1 is highly expressed in aggressive testicular germ cell tumors. Cancer Lett. 2003;190(1):89–95. doi: 10.1016/s0304-3835(02)00582-7. [DOI] [PubMed] [Google Scholar]
- 18.Liao C, Li SQ, Wang X, Muhlrad S, Bjartell A, Wolgemuth DJ. Elevated levels and distinct patterns of expression of A-type cyclins and their associated cyclin-dependent kinases in male germ cell tumors. Int J Cancer. 2004;108(5):654–664. doi: 10.1002/ijc.11573. [DOI] [PubMed] [Google Scholar]
- 19.Rivera A, Mavila A, Bayless KJ, Davis GE, Maxwell SA. Cyclin A1 is a p53-induced gene that mediates apoptosis, G2/M arrest, and mitotic catastrophe in renal, ovarian, and lung carcinoma cells. Cell Mol Life Sci. 2006;63(12):1425–1439. doi: 10.1007/s00018-006-5521-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coletta RD, Christensen K, Reichenberger KJ, et al. The Six1 homeoprotein stimulates tumorigenesis by reactivation of cyclin A1. Proc Natl Acad Sci USA. 2004;101(17):6478–6483. doi: 10.1073/pnas.0401139101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wegiel B, Bjartell A, Ekberg J, Gadaleanu V, Brunhoff C, Persson JL. A role for cyclin A1 in mediating the autocrine expression of vascular endothelial growth factor in prostate cancer. Oncogene. 2005;24(42):6385–6393. doi: 10.1038/sj.onc.1208795. [DOI] [PubMed] [Google Scholar]
- 22.Taneja SS, Ha S, Garabedian MJ. Androgen stimulated cellular proliferation in the human prostate cancer cell line LNCaP is associated with reduced retinoblastoma protein expression. J Cell Biochem. 2001;84(1):188–199. doi: 10.1002/jcb.1278. [DOI] [PubMed] [Google Scholar]
- 23.Ekberg J, Landberg G, Holm C, Richter J, Wolgemuth DJ, Persson JL. Regulation of the cyclin A1 protein is associated with its differential subcellular localization in hematopoietic and leukemic cells. Oncogene. 2004;23(56):9082–9089. doi: 10.1038/sj.onc.1208090. [DOI] [PubMed] [Google Scholar]
- 24.Yang R, Muller C, Huynh V, Fung YK, Yee AS, Koeffler HP. Functions of cyclin A1 in the cell cycle and its interactions with transcription factor E2F-1 and the Rb family of proteins. Mol Cell Biol. 1999;19(3):2400–2407. doi: 10.1128/mcb.19.3.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finkenzeller G, Sparacio A, Technau A, Marme D, Siemeister G. Sp1 recognition sites in the proximal promoter of the human vascular endothelial growth factor gene are essential for platelet-derived growth factor-induced gene expression. Oncogene. 1997;15(6):669–676. doi: 10.1038/sj.onc.1201219. [DOI] [PubMed] [Google Scholar]
- 26.Steiner H, Berger AP, Godoy-Tundidor S, et al. An autocrine loop for vascular endothelial growth factor is established in prostate cancer cells generated after prolonged treatment with interleukin 6. Eur J Cancer. 2004;40(7):1066–1072. doi: 10.1016/j.ejca.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 27.Jozkowicz A, Nigisch A, Wegrzyn J, Weigel G, Huk I, Dulak J. Opposite effects of prostaglandin-J2 on VEGF in normoxia and hypoxia: role of HIF-1. Biochem Biophys Res Commun. 2004;314(1):31–38. doi: 10.1016/j.bbrc.2003.12.059. [DOI] [PubMed] [Google Scholar]
- 28.Varambally S, Yu J, Laxman B, et al. Integrative genomic and proteomic analysis of prostate cancer reveals signatures of metastatic progression. Cancer Cell. 2005;8(5):393–406. doi: 10.1016/j.ccr.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Adamczak R, Porollo A, Meller J. Accurate prediction of solvent accessibility using neural networks-based regression. Proteins. 2004;56(4):753–767. doi: 10.1002/prot.20176. [DOI] [PubMed] [Google Scholar]
- 30.Chu PG, Weiss LM. Expression of cytokeratin 5/6 in epithelial neoplasms: an immunohistochemical study of 509 cases. Mod Pathol. 2002;15(1):6–10. doi: 10.1038/modpathol.3880483. [DOI] [PubMed] [Google Scholar]
- 31.Husemann Y, Geigl JB, Schubert F, et al. Systemic spread is an early step in breast cancer. Cancer Cell. 2008;13(1):58–68. doi: 10.1016/j.ccr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Su JL, Yang PC, Shih JY, et al. The VEGF-C/Flt-4 axis promotes invasion and metastasis of cancer cells. Cancer Cell. 2006;9(3):209–223. doi: 10.1016/j.ccr.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 33.Palmqvist R, Rutegard JN, Bozoky B, Landberg G, Stenling R. Human colorectal cancers with an intact p16/cyclin D1/pRb pathway have up-regulated p16 expression and decreased proliferation in small invasive tumor clusters. Am J Pathol. 2000;157(6):1947–1953. doi: 10.1016/S0002-9440(10)64833-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Noguera-Troise I, Daly C, Papadopoulos NJ, et al. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006;444(7122):1032–1037. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.