Abstract
We have identified potent monocyte/macrophage activating bacterial lipoproteins within commonly used immune enhancing botanicals such as Echinacea, American ginseng and alfalfa sprouts. These bacterial lipoproteins, along with lipopolysaccharides, were substantially more potent than other bacterially derived components when tested in in vitro monocyte/macrophage activation systems. In experiments using RAW 264.7 and mouse peritoneal macrophages the majority (85–98%) of the activity within extracts from eight immune enhancing botanicals was eradicated by treatment with agents (lipoprotein lipase and polymyxin B) known to target these two bacterial components. Alfalfa sprouts exhibited the highest activity of those botanicals tested but the appearance of this activity during the germination of surface sterilized seeds was abolished by the presence of antibiotics. These studies indicate that the majority of the in vitro macrophage activating properties in extracts from these botanicals can be attributed to the presence of lipoproteins and lipopolysaccharides derived from bacteria and that bacterial endophytes may be a significant source of these components.
Keywords: Echinacea, ginseng, macrophage activation, lipoprotein, lipopolysaccharide, melanin
1. Introduction
High molecular weight polysaccharides represent the most frequently identified plant component that enhance monocyte/macrophage activation in vitro and these molecules are thought to contribute to immune enhancing properties of many botanicals [1]. An additional high molecular weight component within immune enhancing botanicals was identified in our laboratory that was a potent activator of monocytes/macrophages in vitro. This material was characterized as melanin [2]. Treatment of THP-1 human monocytes with this melanin fraction resulted in the Toll-like receptor (TLR) 2 dependent-activation of NF-kappa B and the production of IL-1 beta. Ingestion by mice of American ginseng or alfalfa sprout melanin for four days increased the production ex vivo of interferon-gamma by spleen cells and IgA and interleukin-6 by Peyer’s patch cells. These results suggested that this fraction was a previously unrecognized botanical component that contributed to their immune enhancing properties.
During the isolation of polysaccharide and melanin fractions chemical purity is not sufficient to assess whether these molecules are responsible for the activity they exhibit or if the activity is due to a similar type of high molecular weight component present at trace amounts. Because of this, isolated polysaccharide preparations are routinely tested by investigators for the presence of contaminating bacterial LPS using various types of the Limulus Amebocyte Lysate (LAL) test and/or by determining if the in vitro activity is blocked by the specific antibiotic polymyxin B. A significant contribution by LPS to the activity of the melanin fraction was ruled out in our earlier studies since THP-1 monocyte activation was not influenced by polymyxin B and that TLR2 mediated activation by melanin [2] rather than TLR4 which mediates the action of most characterized forms of LPS.
In addition to the gram-negative bacterial component LPS, bacterial lipoproteins present in both gram-positive and gram-negative bacteria have recently come to light as potent innate immune cell activators [3–5]. In fact, contamination of various LPS preparations with bacterial lipoproteins was responsible for the initial view that LPS required TLR2 in addition to TLR4 for the activation of macrophages [5,6]. Similarly, possible contamination by trace amounts of LPS, lipoproteins and other bacterial components has been a major conundrum in research aimed at identifying possible endogenous TLR ligands such as pancreatic elastase and C-reactive protein [7,8]. In the present study we show that bacterial lipoproteins present at trace amounts account for a substantial portion of the activity exhibited by the melanin fraction derived from botanicals traditionally used as immune enhancers. In addition, these experiments suggest that the majority of the in vitro macrophage activating properties in extracts from eight immune enhancing botanicals is due to the presence of lipoproteins and LPS derived from bacteria and that bacterial endophytes may be a significant source of these components.
2. Materials and Methods
2.1. Materials
Ultra pure Salmonella minnesota LPS was from List Biological Laboratories, Inc. Ultra pure E. coli LPS (0111:B4 strain), purified LTA from Staphylococcus aureus, synthetic bacterial lipoprotein Pam3CSK4, and soluble sonicated peptidoglycan from E. coli K12 were obtained from InvivoGen. Zymosan A from Saccharomyces cerevisiae, insoluble peptidoglycan from Staphylococcus aureus, lipoprotein lipase from Pseudomonas sp., proteinase K from Tritirachium album, and polymyxin B (7870 units/mg solid) were obtained from Sigma. SDS-out reagent was purchased from Pierce.
The following botanicals were obtained from commercial sources: Juglans nigra hulls (Nature’s Sunshine Products, Inc., Spanish Fork, UT); Echinacea purpurea root (Frontier Natural Products, Norway, IA and Mountain Rose Herbs, Eugene, OR); Echinacea purpurea herb (Frontier Natural Products, Norway, IA); Medicago sativa sprouts (Wal-Mart, Oxford, MS); and, Panax quinquefolius root (American Mercantile Corporation, Memphis, TN). The remaining botanicals (Glycyrrhiza glabra root, Astragalus membranaceus root, Panax ginseng root, and Tinospora cordifolia stem) were identified by a trained plant taxonomist (V. Joshi) and authenticated vouchers deposited in the NCNPR, University of Mississippi.
Bacterial endotoxin levels were determined using a LAL assay (Pyrochrome Chromogenic Test Kit with diazo coupling) from Associates of Cape Cod Inc.
2.2. Extraction of Botanicals
Botanical melanin fractions were prepared as previously described [2]. In brief, plant material was extracted with 90% aqueous phenol at 70°C and crude melanin fraction precipitated by addition of two volumes of ethyl acetate. Precipitate was then re-dissolved in 90% aqueous phenol and repeatedly partitioned against an equal volume of water until the water layer was clear. Melanin fraction was precipitated from the phenol layer by addition of 2 volumes of ethyl acetate and 4 volumes of ether:acetone (1:6), washed extensively with ethyl acetate and isopropanol and dried.
Crude extracts were prepared for each botanical using three different extraction methods: hot water at 98°C for 30 min, 50% ethanol at 27°C for 2 hours, and 4% SDS at 98°C for 1 hour. For each extraction method, 0.5 g of plant material was extracted with 5 mls of solvent. Extracted material was precipitated by addition of 4 volumes of 95% ethanol to the hot water and 4% SDS extracts, and 2 volumes of 95% ethanol to the 50% ethanol extracts. Before precipitation, SDS was removed from the SDS extract using SDS-out reagent. All precipitates were washed twice with 95% ethanol, air dried at 50–55°C and then dissolved in 2% octylglucoside for analysis.
2.3. Monocyte and macrophage activation assays
The THP-1 human monocyte cell line was transfected with a luciferase reporter gene construct containing two copies of NF-kappa B motif from HIV/IgK as described previously [9]. RAW 264.7 cells were cultured in RPMI 1640 medium supplemented with FBS (10% v/v) and amikacin (60 mg/L) at 37°C, under 5% CO2. Actively growing cells were transiently transfected with the same reporter plasmid as used for THP-1 cells using electroporation (at 150V and one 70 ms pulse) based on a published method [10]. Following electroporation, cells were resuspended in culture medium at 0.5 million cells/ml and plated at a density of 1 x 105 cells/well in 96-well plates. After 24 hours, test samples were added. After a six hour incubation, the medium was aspirated and cells lysed by addition of 40 μl of a 1:1 mixture of luciferase assay reagent (Promega) and PBS containing 1 mM calcium and magnesium. Light output was measured using a Packard microplate scintillation counter in single photon mode.
2.4. Bacterial lipoprotein characterization and identification
For lipoprotein lipase treatment of the melanin fractions (prepared using the 90% aqueous phenol procedure) samples were dissolved in 4% SDS at 100°C for 30 minutes. SDS insoluble material (inactive in monocyte activation assay, data not shown) was removed by centrifugation and discarded. SDS was removed from each sample using SDS-out reagent in the presence of 0.88% octylglucoside. To determine sensitivity to lipoprotein lipase, samples were incubated at 37°C for 16 hours with lipoprotein lipase (1 mg/ml), 10 μM AEBSF protease inhibitor cocktail solution (Sigma), 0.44% octylglucoside and 0.2% BSA. Control samples (without lipoprotein lipase) were run under identical conditions. This same procedure was also used to treat the botanical crude extracts, except that these extracts were dissolved in 2% octylglucoside and lipoprotein lipase digestion was carried out in 1% octylglucoside.
For proteinase K treatment and SDS polyacrylamide gel analysis of melanin fractions (prepared using the 90% aqueous phenol procedure) melanin was dissolved in 4% SDS at 100°C for 30 minutes. SDS insoluble material (inactive in monocyte assay, data not shown) was removed by centrifugation and discarded. Samples were diluted to a final concentration of 1% SDS and incubated with 0.1 mg/ml (3.6 units/ml) proteinase K in 50mM TRIS (pH 7.5), 5 mM ß-mercaptoethanol, and 5 mM CaCl2 for 2 hours at 50°C. Digests were then heated at 98°C for 10 minutes. Control samples (without proteinase K) were run under identical conditions. For SDS polyacrylamide gel analysis 20 μl (125–150 μg) of each sample (proteinase K treated and untreated) was mixed with 1 volume of Tris-Tricine sample buffer (Bio-Rad) and loaded in nonadjacent lanes of a 16.5% Tris-Tricine precast gel (Ready Gel, Bio-Rad). Wide molecular weight range (Sigma) or Blue range prestained protein markers (Pierce) were run in each gel. Individual gel lanes were cut into 12 equal sections (0.5 cm/section), each section was crushed and then extracted with 150 μl of 1.5% octylglucoside, 50 mM TRIS (pH 8.5), 5 mM CaCl2 at 95°C for 5 minutes. Supernatants of sample were collected and evaluated for activity in the THP-1 monocyte activation assay.
2,3-dihydroxypropyl cysteine isolation and detection was accomplished using a published method [11]. Echinacea purpurea root (14 g) was exhaustively extracted with chloroform/methanol (2:1) at room temperature to remove non-polar substances. Remaining plant material was dried and then further extracted with 150 mls of 4% SDS for 1 hour at 98°C. SDS was removed from the extract using SDS-out reagent. Crude extract material was precipitated by addition of 4 volumes of 95% ethanol and precipitate was washed twice with 95% ethanol and air dried at 55°C. Extracted material was then dissolved in 90% aqueous phenol and partitioned 4 times against an equal volume of water. Phenol layer material was precipitated by addition of 2 volumes of ether:acetone (1:5) mixture and 4 volumes of ethyl acetate. Precipitate was washed extensively with ethyl acetate and isopropanol and dried. Dried precipitate was completely dissolved in 4% SDS, 10 mM TRIS at a concentration of 20 mg/ml and incubated with 1.5 mM ß-mercaptoethanol at 98°C for 10 minutes. Sample was then diluted to a final concentration of 1% SDS and incubated with 0.2 mg/ml proteinase K in 50 mM TRIS (pH 7.5), and 5 mM CaCl2 for 2 hours at 50°C. SDS was removed from the sample using SDS-out reagent in the presence of 1% octylglucoside. Proteinase K digested sample was then applied to a 10 g C-18 column (Supelclean LC-18 SPE, Supelco). A 100% water to 100% isopropanol gradient was run and fractions were collected and evaluated using the THP-1 monocyte activation assay. Fractions in the region containing the highest activity (70% isopropanol to 90% isopropanol) were pooled and evaporated to dryness.
Dried samples were analyzed for 2,3-dihydroxypropyl cysteine by the Protein Chemistry Laboratory at Texas A&M University using the following protocol. Sample was hydrolyzed using 4N methanesulfonic acid for 18 hours at 102°C. Hydrolysate was analyzed using a Hewlett Packard AminoQuant System. In this system the hydrolyzed amino acids undergo pre-column derivitization with o-phthalaldehyde and are then separated by reverse phase HPLC and detected using fluorescence. Quantitation of 2,3-dihydroxypropyl cysteine was achieved by using Pam3CSK4 as a standard. Pam3CSK4 is a synthetic tripalmitoylated bacterial lipopeptide analogue that, after hydrolysis with methanesulfonic acid, contains a known amount 2,3-dihydroxypropyl cysteine.
2.5. Alfalfa sprout germination, treatments and extraction
Organically-grown alfalfa sprouting seeds (Johnny’s Selected Seeds lot # 25089, Winslow, ME) were washed with water followed by a 60 second wash in 70% ethanol and rinsed with sterile water. Seeds were surface sterilized by soaking for 12 minutes in a solution of 10% household bleach (final concentration 0.615% sodium hypochlorite) with 0.1% Tween-20 followed by four rinses with sterile water. Fifty seeds were aseptically transferred to 250 ml Erlenmeyer flasks containing five milliliters of water or water plus antibiotic solution with three replicates for each treatment. Antibiotic solutions were 30 mg/L ampicillin (Sigma, A9393), 150 mg/L cefotaxime (Agribio, 2000), or 100 mg/L vancomycin (Sigma, V1764). Seeds were germinated for four days on an orbital shaker at 100 rpm at ambient temperature under a 16-hour-day photoperiod provided by metal halide lighting.
At the end of the fourth day the seeds were rinsed and five ml of treatment solution was added to each flask. Treatment solutions were water alone or water plus 50 mg/ml chitin (Sigma, C9752), antibiotic or antibiotic with chitin. Chitin suspensions were prepared by homogenization in water followed by autoclaving. Twelve hours post-treatment, the seeds were rinsed and five ml of either water or antibiotic solution was added to the flasks. Seventy-two hours later the seedlings were gently blotted dry, frozen at −80°C for four hours, freeze dried overnight and then weighed. The dried material was then extracted with 90% aqueous phenol at 70°C. Crude melanin fractions were precipitated by addition of two volumes of ethyl acetate, washed extensively with ethyl acetate and isopropanol, and dried. For testing in cell culture the crude melanin fractions were resuspended in isopropanol.
2.6. Peritoneal macrophage isolation
The use of mice adhered to the University of Mississippi guide for the care and use of laboratory animals and were approved by the animal welfare committee. Male C57BL/6 mice (age 6 to 7 weeks) were purchased from Harlan Sprague/Dawley. Mice (20–30g) were sacrificed by CO2 asphyxiation and peritoneal cell suspensions were obtained by peritoneal cavity lavage with 5 ml cold PBS. The recovered peritoneal fluid was mixed with an equal volume of ice-cold RPMI media containing 10% FBS and amikacin (60 mg/L) and washed twice by centrifugation. The cells were plated in 100 mm tissue culture dish and incubated at 37°C/5% CO2 for 2 hours. Non-adherent cells were removed by gentle washing with sterile Ca/Mg free PBS (5ml, 2 times). The adherent macrophages were scraped into culture media, adjusted to the concentration of 0.5X106 cells/ml and plated in 96 well tissue-culture plate (0.1X106 cells/well). Test samples were added to cells and after 18 hours of incubation the cell culture supernatants were used for the estimation of TNF-α by ELISA (R&D systems) according to manufacturer’s instructions.
3. Results
3.1. Activation of NF-kappa B in THP-1 monocytes by botanical melanin fraction is due to trace amounts of bacterial lipoprotein
We reported previously [2] that when the melanin fraction of selected botanicals was dissolved by heating (98 C) in a buffer containing the chaotropic denaturant guanidine hydrochloride and fractionated in this buffer system by size exclusion chromatography the majority of the activity (NF-kappa B activation in THP-1 monocytes) eluted with melanin material that ranged in size from 100 to 1,000 kDa. If this same melanin fraction was dissolved in a buffer system containing 4% SDS (100 C for 30 min) and then fractionated by SDS-PAGE the majority of this activity eluted between 60 and 6 kDa (see white bars in Fig 1a–d). The activity of the SDS-dissolved melanin fraction also eluted around this size range during size exclusion chromatography in the presence of 1% SDS (data not shown). Since the majority of the UV absorbing melanin eluted at similar retention times in the presence or absence of SDS, it suggested that a minor component within the melanin fraction was responsible for activity and that in the presence of SDS this active component was dissociated from all size classes of melanin.
Fig. 1.
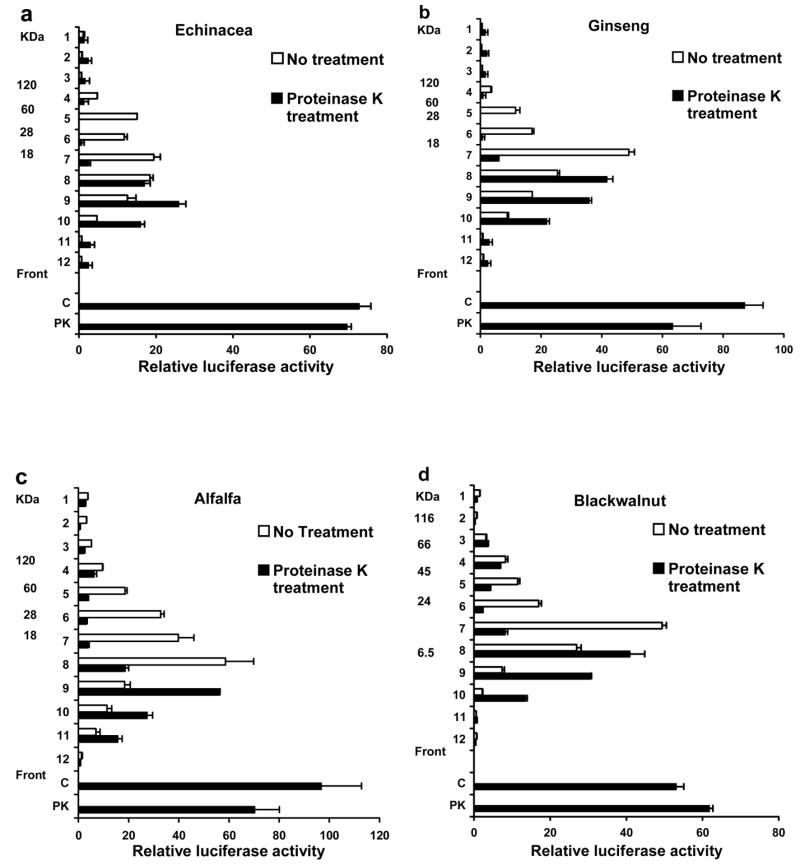
Treatment with proteinase K reduces the size of the active compounds in the melanin fraction of botanicals. The melanin fraction of the botanicals indicated was dissolved in 4% SDS and treated with proteinase K (black bars) or left untreated (white bars). Samples (125–150 μg) were heated at 98° C for five minutes in 1.5% SDS containing buffer and fractionated on a 16.5 % Tris-Tricine gel. Individual lanes were cut into 0.5 cm sections, extracted with octylglucoside and evaluated for activity in THP-1 human monocytes transfected with an NF-kappa B luciferase reporter plasmid. The black bars labeled C and PK represent the activity of the untreated and proteinase K treated samples respectively, before fractionation on the gel. Values are the average of duplicate determinations in a representative experiment that was repeated at least twice.
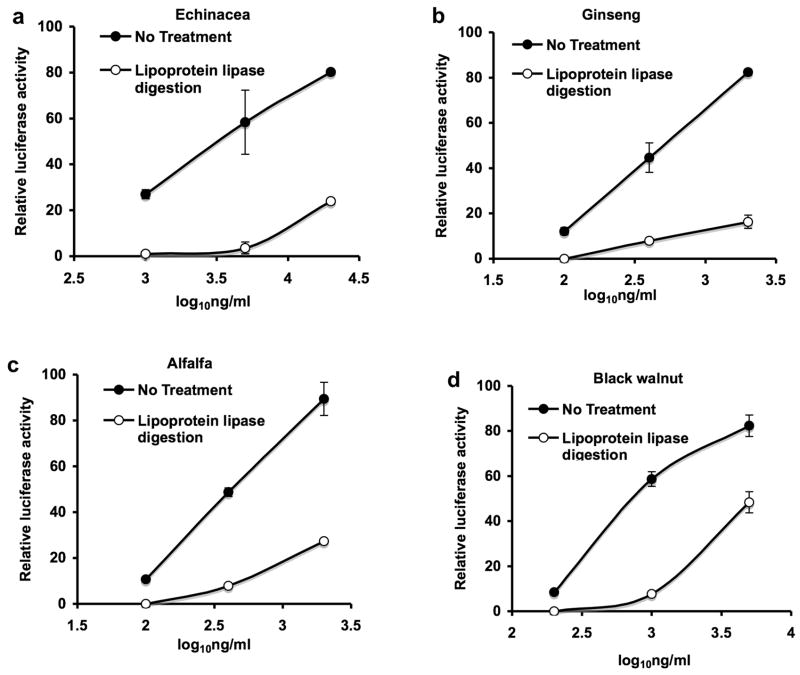
We had demonstrated previously that the melanin fraction from Echinacea, American Ginseng and alfalfa sprouts activated NF-kappa B in THP-1 monocytes through a TLR2-dependent process, was resistant to protease treatment, polymyxin B, and heat treatment (98 C for 2 hrs) but was irreversibly inactivated by treatment with strong base [2]. Since bacterial lipoproteins are of similar size, activate NF-kappa B through a TLR2-dependent process and also exhibit these other properties, we employed several established biochemical methods to characterize these bacterial components [3–5]. The results in Fig. 1a–d show that proteinase K treatment of the melanin fraction (black bars) decreased the over all size of the active component as compared to the untreated fraction (white bars) indicating that protein was part of the active molecule. The activity of the untreated (C) and proteinase K treated (PK) melanin fractions before gel electrophoresis were similar indicating that the protein component was not required for activity (Fig. 1a–d). The results presented in Figure 2a–d show that the activity present in the melanin fraction of the four botanicals tested is completely abrogated by removal of glycerol bound fatty acids by treatment with lipoprotein lipase (diacylglycerol lipase). This result together with the results presented in Figure 1 suggest that lipoproteins are responsible for the majority of the activity within the melanin fraction of these botanicals. A minor contribution to the activity of this fraction by bacterial lipoteichoic acid cannot be ruled out because under the conditions used in these studies lipoprotein lipase will completely inactivate both synthetic bacterial lipoprotein (Pam3CSK4) and bacterial lipoteichoic acid but not LPS (data not shown).
Fig. 2.
Complete inactivation of the melanin fraction by lipoprotein lipase treatment. The activity in the melanin fractions of the botanicals indicated was extracted with 4% SDS. The SDS was removed by SDS-out reagent in the presence of 0.88% octylglucoside and the samples were then incubated at 37° C for 16 hours with lipoprotein lipase. Control samples (without lipoprotein lipase) were run under identical conditions. Luciferase activity was determined in THP-1 human monocytes transfected with an NF-kappa B luciferase reporter plasmid. Values are the average of duplicate determinations in a representative experiment that was repeated at least twice.
Bacterial lipoproteins of the murein type are produced by both gram-negative and gram-positive bacteria and are thought to be unique to prokaryotes. These lipoproteins contain a diacylglycerol moiety that is linked by a thioester bond to the N-terminal cysteine of the peptide chain [12]. Evidence that the active lipoproteins detected in the Echinacea purpurea root melanin fraction were indeed of the murein type and of bacterial origin is indicated by the detection of the modified amino acid 2,3 dihydroxypropyl cysteine [11] using RP-HPLC (Peak A Figure 3). Peak A is not detectable in Echinacea purpurea root material exhibiting lipoprotein activity that is approximately 100 times less active than the material analyzed in Fig. 3. This provides further evidence that Peak A derives from the active lipoproteins.
Fig. 3.
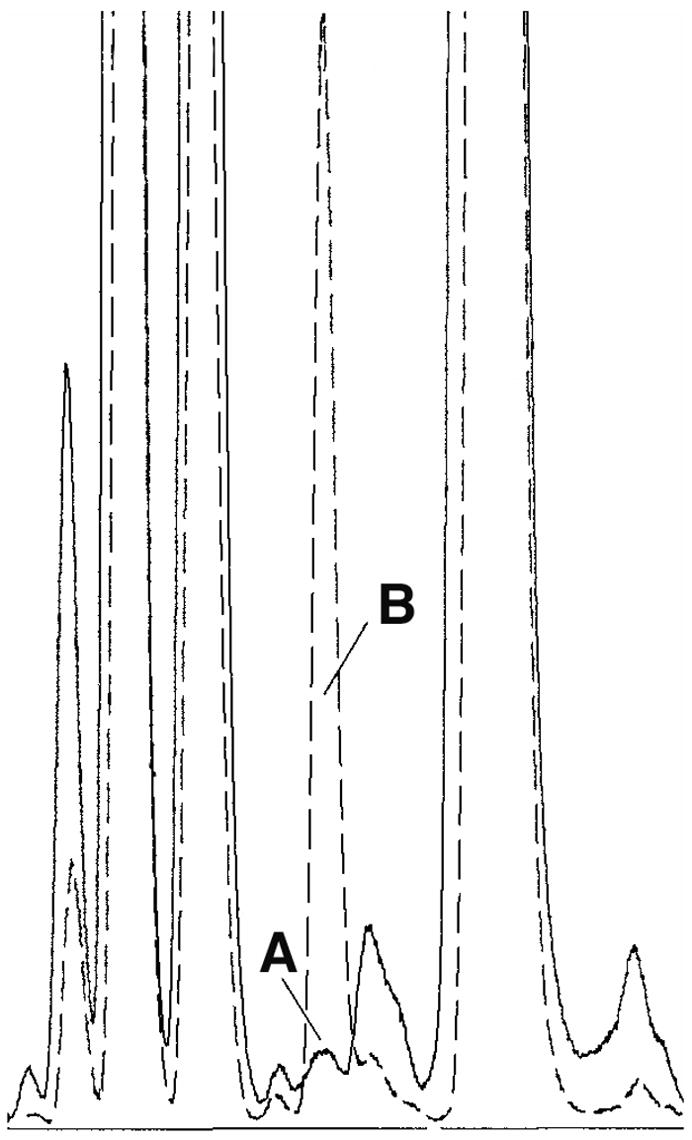
Identification within Echinacea purpurea root of 2,3-dihydroxypropyl cysteine, a structural component unique to prokaryotic lipoproteins. Lipoproteins were extracted using 4% SDS, were partitioned into phenol and then treated with proteinase K to remove the bulk of the protein component. Lipopeptide moieties were eluted from a C18 column using a water/isopropanol gradient and active fractions pooled. Active sample and the synthetic lipoprotein Pam3CSK4 (used as a standard) were hydrolyzed using methanesulfonic acid and hydrolysates analyzed using a Hewlet Packard AminoQuant System. Chromatogram section shown represents separation of active Echinacea sample (solid line) and active Echinacea sample spiked with standard (dashed line) using RP-HPLC with fluorescence detection after being derivitized with o-phthalaldehyde . Peak A eluted with a retention time (7.13 min) identical to the standard and identification was further supported by spiking of the active fraction with the standard (Peak B).
It would be expected that plant material containing high levels of bacterial lipoprotein would also contain higher amounts of other bacterial products such as LPS. This would of course be dependent on the relative abundance of gram-positive and gram-negative bacteria. LPS was extracted from plant material using the phenol:water procedure [13] and partitioned into the water phase. The melanin fraction is isolated from the phenol layer and contains 10–100 times less LPS than the water layer as determined by the LAL assay (data not shown). In general, with these four botanicals, the three (alfalfa sprouts, black walnut hulls and American ginseng) exhibiting the more potent lipoprotein activity of the melanin fraction (EC50s from 0.3–0.6 μg/ml) also contained the higher LPS content of the plant material (355–1,401 EU/mg dry plant material). Echinacea melanin fraction EC50 activity and LPS content was 3.0 μg/ml and 38 EU/mg, respectively.
3.2. The increase in Alfalfa sprout melanin fraction activity during germination and elicitation is prevented by antibiotics
In light of the data presented above a question arises as to whether the source of the bacterial products detected in these botanicals are derived from post-harvest contamination, from endophytic bacteria or a combination of both. Since alfalfa sprouts contained some of the highest levels of bacterial products and since germination and harvest are easily controllable, we used this model system to begin to address this question. We had previously shown that the melanin fraction from surface sterilized, aseptically germinated alfalfa seedlings that had been treated with the elicitor chitin exhibited 10–25 times greater activity than this fraction in untreated seedlings [2]. Figure 4 shows that the crude melanin fraction from surface sterilized alfalfa seeds contains minimal activity, but upon germination in vitro under aseptic conditions for seven days significant activity becomes evident (group A). Approximately 20 times more activity is present when the sprouts were treated with chitin from day three to the end of this seven day growing period (group C). Both untreated and chitin-treated sprouts cultured with ampicillin exhibited substantially reduced activity (groups B and D, respectively) suggesting that these activities were bacterially derived and that these bacteria were carried within the seeds as endophytes. Untreated and antibiotic-treated sprouts appeared identical and yielded similar biomasses (data not shown). Two other antibiotics commonly used to retard endophyte growth in vitro were also tested in this system. Cefotaxime at the concentration used (0.15/mg/ml) exhibits specificity for gram-negative bacteria and suppressed the activity to the same extent as ampicillin while vancomycin (0.1 mg/ml), an antibiotic specific for gram-positive bacteria, reduced the activity by half (data not shown).
Fig. 4.
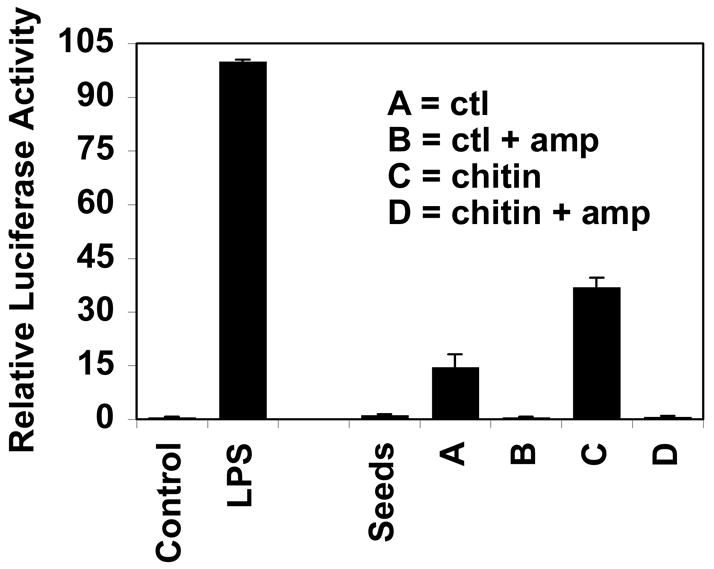
Antibiotics prevent the appearance of activity (NF-kappa B activation of THP-1 cells) during the aseptic germination of surface sterilized alfalfa seeds. Alfalfa seeds were surface sterilized (successively washed with 70% ethanol and 10% clorox/0.01% Tween -20) and germinated in aseptic conditions in the presence or absence of 30 μg/ml ampicillin (amp) for seven days. Sterile solutions of chitin (50 mg/ml) were present in some of the flasks for 12 hours on day four. Sprouts were weighed, freeze dried, and the crude melanin fraction (0.1 μg/ml) assessed for activity in THP-1 human monocytes transfected with an NF-kappa B luciferase reporter plasmid. THP-1 cells were left untreated or treated for 4 hours with crude E. coli LPS (10 μg/ml) or with the melanin fraction from alfalfa seeds or sprouts germinated under the conditions specified. The value for each condition is the average ± standard deviation of three flasks (~50 sprouts each) with extracts from each flask run in duplicate in the THP-1 assay.
3.3. Relative activity of bacterial and fungal components in monocyte/macrophage cell systems commonly used to assess immunostimulatory activity of botanicals
It was important to determine which bacterial components most influenced monocyte/macropage activation as the first step in assessing how much they contributed to the activity of botanicals or their extracts. Figure 5 a and b illustrates the relative efficacy of various bacterial and fungal components for NF-kappa B activation in the human monocyte cell line THP-1 and the mouse macrophage cell line RAW 264.7. Both cell lines were much more sensitive to LPS and the synthetic bacterial lipoprotein Pam3CSK4 than they were to the other components tested. THP-1 cells responded more robustly to Pam3CSK4 than to LPS while RAW 264.7 cells exhibited the opposite response. Resident macrophages from the peritoneal cavity of mice responded to LPS and lipoprotein with respect to TNF-α secretion in a fashion similar to that of NF-kappa B activation in RAW 264.7 cells (see Figure 5a insert).
Fig. 5.
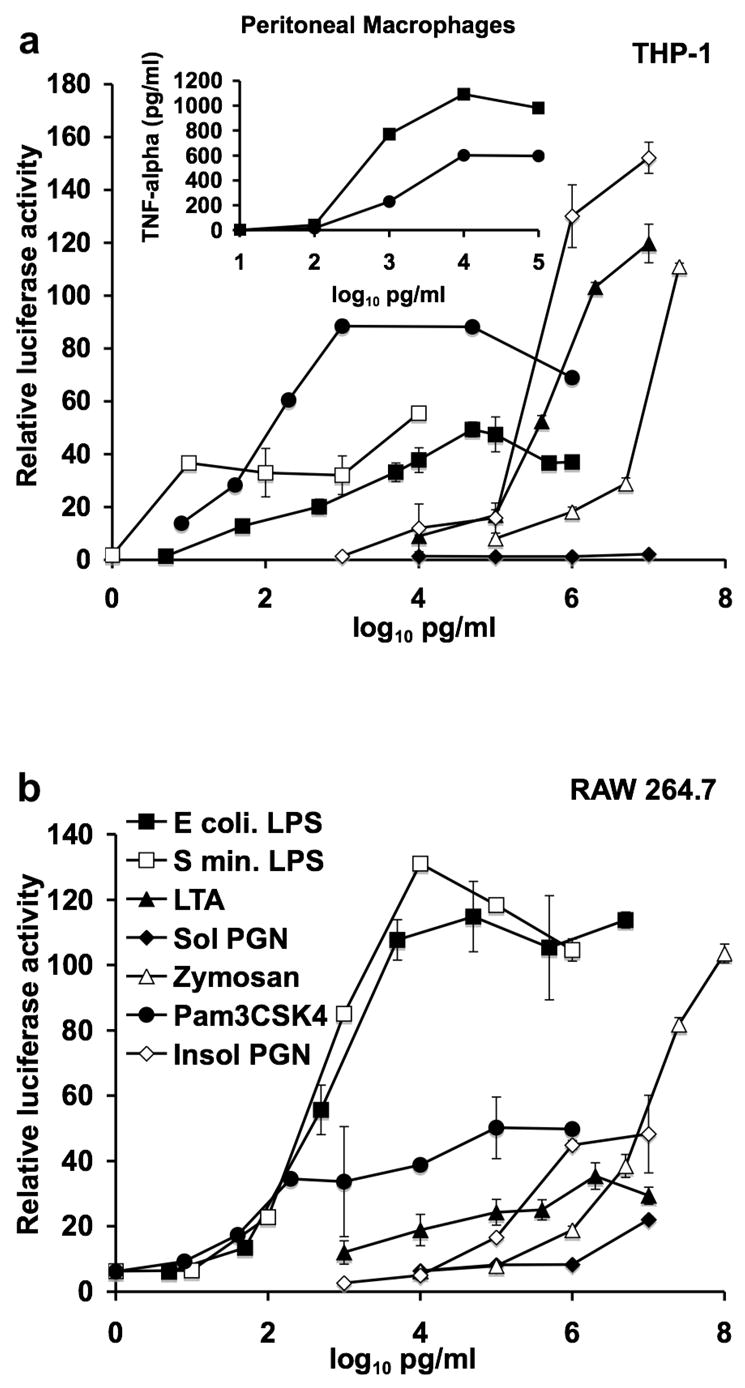
Sensitivity of three in vitro monocyte/macrophage systems to various bacterial and fungal components. (a) The human monocyte cell line THP-1, (a insert) resident peritoneal mouse macrophages, (b) the mouse macrophage cell line RAW 264.7 were treated with the indicated bacterial and fungal components: ultra pure LPS from E. coli (E coli LPS), ultra pure LPS from S. minnesota (S min. LPS), lipoteichoic acid (LTA), soluble peptidoglycan (Sol PGN), Zymosan, synthetic lipoprotein (Pam3CSK4), or insoluble peptidoglycan (Insol PGN). The activation of NF-kappa B was assessed in THP-1 and RAW 264.7 cells by the expression of the luciferase reporter gene. TNF-alpha production by mouse peritoneal macrophages was assessed by ELISA 18 hours after addition of LPS or Pam3CSK4. Data shown is from the average of duplicate samples from a representative experiment that was repeated at least twice.
3.4. A major portion of the in vitro macrophage activating property in extracts of immune enhancing botanicals is contributed by bacterially-derived lipoprotein and LPS
Since the results presented in Fig. 5 indicated that macrophage activation (as determined by NF-kappa B activation and TNF-α production) was most sensitive to bacterial LPS and lipoprotein, we proceeded to quantify the contribution of these two bacterial components (see Figure 6) to the total activity of extracts derived from several immune enhancing botanicals. Three types of extracts were tested; two that are common practice in the supplement industry to obtain polysaccharide-rich extracts (hot water extract 98° C for 30 min, bar 1) and to produce tinctures (50% ethanol extract, bar 2) and a hot water extract containing 4% SDS (conditions optimal for both LPS and lipoprotein extraction, bar 3). Following extraction, ethanol was added to each extract to a concentration of 85% and then cooled to precipitate extracted bacterial (LPS and lipoprotein) and plant components such as polysaccharides. The precipitate was then washed twice with 95% ethanol. The precipitation and washing steps are required to remove plant-derived anti-inflammatory compounds that substantially inhibit macrophage activation by plant- and bacterially-derived components (data not shown). Figure 6a shows that the total activity extracted from these botanicals can vary substantially and that while hot water 4% SDS conditions extracted more activity for some botanicals (e.g. licorice, astragalus and American ginseng) for other botanicals the other conditions were equally effective. What was most surprising about these results was the substantial amount of activity extracted from these botanicals. The white and black bars represent the total activity extracted from 120 μg and 2.5 μg, respectively of dried ground plant material and in some cases (black walnut hulls and alfalfa sprouts) the amount of activity extracted from 2.5 μg of plant material equaled 50% of the activity exhibited by maximally activating concentrations (10 μg/ml) of crude E. coli LPS. The average extract concentrations in the macrophage cultures for the hot water, 50% ethanol and hot water 4% SDS extracts were 82, 27 and 136 μg/ml, respectively for the 120 μg group and 1.7, 0.6 and 2.8 μg/ml, respectively for the 2.5 μg group. Figure 6b shows that the majority of the extracted activity (white bars) is lost when the three types of extracts were treated (black bars) with lipoprotein lipase (to inactivate lipoprotein) and the LPS inhibitor polymyxin B. Likewise, TNF-α production (Fig. 6c, white bars) by mouse peritoneal macrophages was substantially reduced after lipase and polymyxin B treatment (black bars). The two most active extracts (black walnut and alfalfa sprouts) contained substantially higher LAL positive material (numbers above each bar) further supporting the bacterial origin of this activity. The base sensitive nature of LPS has been reported [14] and we have shown that the activity of the melanin fraction (i.e. lipoproteins) is very sensitive to base treatment [2]. Treatment of the 4% SDS extracts with 0.5M NaOH at 50° C for 2 hours resulted in the reduction of NF-kappa B activation by 75 to 99%, almost identical to that seen with polymyxin B and lipase in Fig. 6b (85 to 98%). This is consistent with the inactivation of LPS and lipoproteins by the base-dependent removal of ester-and amide-linked fatty acids.
Fig. 6.
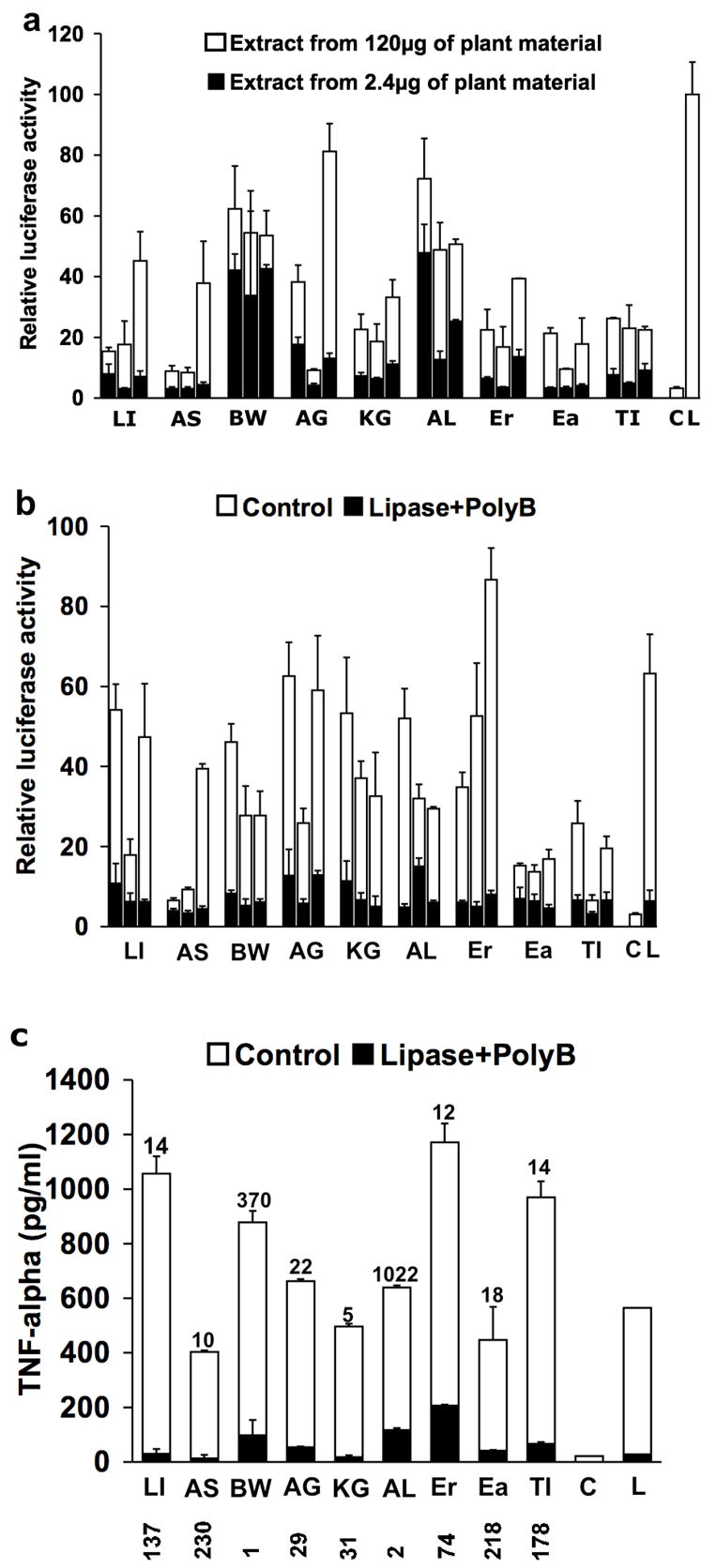
The majority of the in vitro macrophage stimulatory activity of various botanical extracts is abrogated by treatment with polymyxin B and lipoprotein lipase. (a and b) The indicated botanicals were extracted with hot water (first bar in each group), 50% ethanol (second bar in each group) or hot water containing 4% SDS (third bar in each group and extracts used in Fig. 6c), followed by precipitation and washes with ethanol. The activation of NF-kappa B was assessed in RAW 264.7 cells by the expression of the luciferase reporter gene (a and b) and production of TNF-alpha by resident peritoneal mouse macrophages by ELISA (c). The numbers above each bar in c indicate the amount of LPS in each sample (colorimetric LAL assay) and is expressed as EU/mg of extracted plant material and the numbers below the x axis are the medium concentrations of each extract in μg/ml. The botanicals studies were: Glycyrrhiza glabra-licorice (LI), Astragalus membranaceus (AS), Juglans nigra-black walnut hulls (BW), Panax quinquefolius-American ginseng (AG), Panax ginseng-Korean ginseng (KG), Medicago sativa-alfalfa sprouts (AL), Echinacea purpurea root (Er) and aerial (Ea), Tinospora cordifolia (TI). Bars labeled with a (C) or (L) indicate untreated cells and cells treated with 100 ng/ml ultra pure LPS from E. coli, respectively and the black bar within L indicates treatment with polymyxin B. Control values for lipase + polymyxin B for b were 3.4 ± 0.9 and for c were 11.4 ± 4 pg/ml.
4. Discussion
This study strongly suggests that bacterially-derived lipoprotein components within the melanin fraction substantially contribute to the in vitro macrophage activation properties of this fraction for the immune enhancing botanicals studied. These studies also point out that in addition to LPS, it is important to rule out the contribution of bacterial lipoproteins to the activity of a “purified” botanical component such as a polysaccharide or other large molecular weight plant component. Although these lipoproteins are optimally extracted with hot 90% phenol in water, we have detected substantial activity due to this potent bacterial component contaminating column purified polysaccharides and in commercially available products such as polysaccharide preparations and in hot water and aqueous ethanol extracts of certain botanicals (data not shown). The synthetic lipoprotein Pam3CSK4 (MW = 1.5 kDa) exhibited EC50s of 0.1 to 1.0 ng/ml in three monocyte/macrophage cell systems (Figure 5). Since the average molecular weight exhibited by these lipoproteins in SDS gels was approximately 10 kDa (Figure 1), quantities of less than 10 ng/ml of these lipoproteins in these in vitro systems would cause substantial activation. If polysaccharide preparations or botanical extracts are tested in vitro at concentrations of from 10 to 100 μg/ml, contaminating bacterial lipoproteins present at 10 ng/ml (0.10% to 0.01% contaminants) would contribute substantially to the assessed activity.
Earlier studies reporting the in vivo immune enhancing activity of black tea melanin used an isolation procedure involving hydrolysis in 7N HCl at 100° C for 2 hrs [15]. This would have completely inactivated both LPS and lipoprotein and indicates that the melanin itself was most likely responsible for the observed activity. Melanin from Nigella sativa seeds also stimulates macrophage cytokine production in vitro [16,17]. We have also detected immune enhancing activity associated with base and acid treated melanin and this will be the subject of another report.
Our research also suggests that the majority of the activity detected in extracts from the eight immune enhancing botanicals tested was due to the presence of bacterial LPS and lipoproteins (Figure 6). Many of these botanicals have been reported to contain immune enhancing polysaccharides as assessed by activation of macrophages in vitro [18–23]. The activity of these polysaccharides however has not been assessed relative to that of the original plant material or extract. Only then can one begin to assess the contribution of a particular polysaccharide to the overall therapeutic potential of a botanical or botanical extract. Most of the polysaccharides isolated from these eight botanicals were tested in in vitro macrophage activation assays at concentrations from 50–500 μg/ml [18–23]. Even at these high concentrations their activity is usually quite lower than that exhibited by maximally stimulatory concentrations of LPS. In addition, these polysaccharides usually represent less than 1% (wt/wt) of the dry weight of starting plant material. The crude extracts tested in the present research constituted an average yield of from 5 to 25% (wt/wt) and at concentrations of from 0.5 to 230 μg/ml induced TNF-alpha production by mouse peritoneal macrophages to levels equal to or more than levels observed with maximal concentrations (10 ng/ml) of purified E. coli LPS (Figure 6c). Similarly, the melanin fraction (containing trace lipoprotein contaminants) from the studied botanicals make up from 0.9 to 9% (avg. 3.3%) of their dry weight and when tested at 0.1 to 9.0 μg/ml activate NF-kappa B to levels 50% of that observed with maximal concentrations of crude E. coli LPS [2]. Since the majority of the activity present in these extracts is due to bacterial LPS and lipoproteins these bacterial components are at least as relevant as the activity reported for purified polysaccharides isolated from some of these botanicals.
The high levels of in vitro macrophage activation generated by these crude botanical extracts in the present study derives from their method of preparation. To fully detect this type of activity in crude extracts from most botanicals it is imperative to separate plant and bacterial components that activate macrophages from the anti-inflammatory plant components (i.e. inhibitors of macrophage activation). We used a precipitation and subsequent ethanol wash steps to separate most of the macrophage activating compounds (present in the precipitate) from compounds that inhibit macrophage activation that are mostly present in the supernatant and washes. Extracts made from most of the eight botanicals tested do not exhibit activity or exhibit substantially reduced activity in these types of macrophage activation assays if the precipitation and washing steps are omitted.
Although bacteria contaminating the surface of these plants may be a significant source of LPS and lipoproteins detected in these botanicals, a significant contribution by endophytic bacteria is also probable. The dramatic suppression by ampicillin of the appearance of activity (NF-kappa B activation of THP-1 cells) during the aseptic germination of surface sterilized alfalfa seeds indicates that endophytes can be a substantial source for this activity (Figure 4). The activity appearing during germination is significant in that 100 ng/ml concentrations of the extract from chitin elicited sprouts activated NF-kappa B to levels 40% of those achieved by maximal levels (10 μg/ml) of crude E. coli LPS. This is comparable to the activity detected in extracts from the most potent of the eight botanicals (black walnut hulls and alfalfa sprouts) presented in Figure 6a. The enhanced activity in sprouts after treatment with chitin was unexpected since elicitation of plant defense mechanisms usually reduces the number of endophytes [24]. We do not know at this time if the enhanced activity was due to an increase in the number of endophytes or increased levels within these bacteria of active lipoproteins or other bacterial components. We reported previously that the activity of the melanin fraction of 18 commonly used vegetables was several orders of magnitude less than the melanin fraction from botanicals traditionally used to enhance immune function [2]. Since the majority of the activity of this fraction is due to bacterial lipoproteins it may indicate that these immune enhancing botanicals contain higher levels of bacterial endophytes than the tested vegetables. This possibility is presently being investigated in our laboratory.
Recent research indicates that consumption of foods containing certain bacteria can have a beneficial effect on the immune system. This has been most extensively studied with respect to the ingestion of the probiotic lactic acid bacteria Lactobacillus and Bifidobacterium strains found in yoghurt and similar foods [reviewed in 25]. For example, a double blind, three-stage before-and-after intervention trial showed that consumption of Bifidobacterium lactis HN019 for three weeks by healthy volunteers significantly enhanced polymorphonuclear cell phagocytosis and natural killer cell tumor cell killing [26]. Many of these immune enhancing effects do not require viable bacterial cells since they can be mimicked by consumption of heat killed organisms. Consumption of heat killed L. plantarum L-137 by healthy subjects enhanced con A-induced proliferation and increased the percentage of INF-gamma and IL-4 producing CD4+ T cells [27]. In a mouse study, immune parameters that changed in response to probiotic administration to wild type mice were not seen in mice deficient in TLR2 [28]. These studies therefore suggest that recognition of bacterial components by Toll-like and other receptors on immune cells within the gut may mediate some of the immune effects of orally consumed bacteria. Likewise, the consumption of botanicals which contain compounds that interact with these receptors would mimic bacterial components and have similar effects. Recent research suggests that the immune enhancing polysaccharides isolated from astragalus [20], Korean ginseng [21], Siberian ginseng [22], Platycodon grandiflorum [28] activate macrophages through TLR4 while a (1,4)-alpha-d-glucan from Tinospora cordifolia requires TLR2/6 [23]. Since the present research suggests that the bacterial components LPS and lipoproteins are responsible for the majority of the in vitro macrophage stimulatory activity of the tested botanical extracts, and since these components act through TLR4 and TLR2, respectively, their role in contributing to the immune enhancing properties of these botanicals should not be ignored.
Acknowledgments
This research was partially funded by grants from the National Institutes of Health RO1 AT002360 (NCAAM) to DSP and by the USDA, Agricultural Research Service Specific Cooperative Agreement No. 58-6408-7-012.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schepetkin IA, Quinn MT. Botanical polysaccharides: macrophage immunomodulation and therapeutic potential. Int Immunopharmacol. 2006;6:317–333. doi: 10.1016/j.intimp.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Pugh ND, Balachandran P, Lata H, Dayan FE, Joshi V, et al. Melanin: dietary mucosal immune modulator from Echinacea and other botanical supplements. Int Immunopharmacol. 2005;5:637–647. doi: 10.1016/j.intimp.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 3.Aliprantis AO, Weiss DS, Radolf JD, Zychlinsky A. Release of Toll-like receptor-2-activating bacterial lipoproteins in Shigella flexneri culture supernatants. Infect Immun. 2001;69:6248–6255. doi: 10.1128/IAI.69.10.6248-6255.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hashimoto M, Tawaratsumida K, Kariya H, Aoyama K, Tamura T, Suda Y. Lipoprotein is a predominant Toll-like receptor 2 ligand in Staphylococcus aureus cell wall components. Int Immunol. 2006;18:355–362. doi: 10.1093/intimm/dxh374. [DOI] [PubMed] [Google Scholar]
- 5.Hashimoto M, Asai Y, Ogawa T. Separation and structural analysis of lipoprotein in a lipopolysaccharide preparation from Porphyromonas gingivalis. Int Immunol. 2004;16:1431–1437. doi: 10.1093/intimm/dxh146. [DOI] [PubMed] [Google Scholar]
- 6.Hirschfeld M, Ma Y, Weis JH, Vogel SN, Weis JJ. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine toll-like receptor 2. J Immunol. 2000;165:618–22. doi: 10.4049/jimmunol.165.2.618. [DOI] [PubMed] [Google Scholar]
- 7.Tsan MF, Gao B. Endogenous ligands of Toll-like receptors. J Leukoc Biol. 2004;76:514–519. doi: 10.1189/jlb.0304127. [DOI] [PubMed] [Google Scholar]
- 8.Tsan MF, Gao B. Pathogen-associated molecular pattern contamination as putative endogenous ligands of Toll-like receptors. J Endotoxin Res. 2007;13:6–14. doi: 10.1177/0968051907078604. [DOI] [PubMed] [Google Scholar]
- 9.Pugh N, Ross SA, ElSohly MA, Pasco DS. Characterization of Aloeride, a new high-molecular-weight polysaccharide from Aloe vera with potent immunostimulatory activity. J Agric Food Chem. 2001;49:1030–1034. doi: 10.1021/jf001036d. [DOI] [PubMed] [Google Scholar]
- 10.Subbaramaiah K, Bulic P, Lin Y, Dannenberg AJ, Pasco DS. Development and use of a gene promoter-based screen to identify novel inhibitors of cyclooxygenase-2 transcription. J Biomol Screening. 2001;6:101–110. doi: 10.1177/108705710100600206. [DOI] [PubMed] [Google Scholar]
- 11.Muhlradt PF, Meyer H, Jansen R. Identification of S-(2,3-Dihydroxypropyl) cysteine in a macrophage-activating lipopeptide from Mycoplasma fermentans. Biochemistry. 1996;35:7781–7786. doi: 10.1021/bi9602831. [DOI] [PubMed] [Google Scholar]
- 12.Wu H. Biosynthesis of lipoproteins. In: Neidhardt FC, et al., editors. Echerichia coli and Salmonella: cellular and molecular biology. 2. ASM Press; Washington, D.C: 1966. pp. 1005–1014. [Google Scholar]
- 13.Westphal O, Jann K. Bacterial lipopolysaccharides: Extraction with phenol-water and further applications of the procedure. In: Whistler RL, editor. Methods in Carbohydrate Chemistry: Volume V, General Polysaccharides. Academic Press; New York, NY: 1965. pp. 83–91. [Google Scholar]
- 14.Barthel CR, Levin LG, Reisner HM, Trope M. TNF-alpha release in monocytes after exposure to calcium hydroxide treated Escherichia coli LPS. Int Endod J. 1997;30:155–159. doi: 10.1046/j.1365-2591.1997.00066.x. [DOI] [PubMed] [Google Scholar]
- 15.Sava VM, Galkin BN, Hong M-Y, Yang P-C, Huang GS. A novel melanin-like pigment derived from black tea leaves with immunostimulating activity. Food Res International. 2001;34:337–343. [Google Scholar]
- 16.El-Obeid A, Hassib A, Ponten F, Westermark B. Effect of herbal melanin on IL-8: a possible role of Toll-like receptor 4 (TLR4) Biochem Biophys Res Commun. 2006;344:1200–1206. doi: 10.1016/j.bbrc.2006.04.035. [DOI] [PubMed] [Google Scholar]
- 17.El-Obeid A, Al-Harbi S, Al-Jomah N, Hassib A. Herbal melanin modulates tumor necrosis factor alpha (TNF-alpha), interleukin 6 (IL-6) and vascular endothelial growth factor (VEGF) production. Phytomedicine. 2006;13:324–333. doi: 10.1016/j.phymed.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Stimpel M, Proksch A, Wagner H, Lohmann-Matthes ML. Macrophage activation and induction of macrophage cytotoxicity by purified polysaccharide fractions from the plant Echinacea purpurea. Infect Immun. 1984;46:845–849. doi: 10.1128/iai.46.3.845-849.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nose M, Terawaki K, Oguri K, Ogihara Y, Yoshimatsu K, Shimomura K. Activation of macrophages by crude polysaccharide fractions obtained from shoots of Glycyrrhiza glabra and hairy roots of Glycyrrhiza uralensis in vitro. Biol Pharm Bull. 1998;21:1110–1112. doi: 10.1248/bpb.21.1110. [DOI] [PubMed] [Google Scholar]
- 20.Shao BM, Xu W, Dai H, Tu P, Li Z, Gao XM. A study on the immune receptors for polysaccharides from the roots of Astragalus membranaceus, a Chinese medicinal herb. Biochem Biophys Res Commun. 2004;320:1103–1111. doi: 10.1016/j.bbrc.2004.06.065. [DOI] [PubMed] [Google Scholar]
- 21.Nakaya TA, Kita M, Kuriyama H, Iwakura Y, Imanishi J. Panax ginseng induces production of proinflammatory cytokines via toll-like receptor. J Interferon Cytokine Res. 2004;24:93–100. doi: 10.1089/107999004322813336. [DOI] [PubMed] [Google Scholar]
- 22.Han SB, Yoon YD, Ahn HJ, Lee HS, Lee CW, Yoon WK, Park SK, Kim HM. Toll-like receptor-mediated activation of B cells and macrophages by polysaccharide isolated from cell culture of Acanthopanax senticosus. Int Immunopharmacol. 2003;3:1301–1312. doi: 10.1016/S1567-5769(03)00118-8. [DOI] [PubMed] [Google Scholar]
- 23.Nair PK, Melnick SJ, Ramachandran R, Escalon E, Ramachandran C. Mechanism of macrophage activation by (1,4)-alpha-D-glucan isolated from Tinospora cordifolia. Int Immunopharmacol. 2006;6:1815–1824. doi: 10.1016/j.intimp.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 24.Iniguez AL, Dong Y, Carter HD, Ahmer BMM, Stone JM, Triplett EW. Regulation of enteric endophytic bacterial colonization by plant defenses. Mol Plant Microbe Interact. 2005;18:169–178. doi: 10.1094/MPMI-18-0169. [DOI] [PubMed] [Google Scholar]
- 25.Ezendam J, van Loveren H. Probiotics: Immunomodulation and evaluation of safety and efficacy. Nutr Rev. 2006;64:1–14. doi: 10.1111/j.1753-4887.2006.tb00168.x. [DOI] [PubMed] [Google Scholar]
- 26.Chiang BL, Sheih YH, Wang LH, Liao CK, Gill HS. Enhancing immunity by dietary consumption of a probiotic lactic acid bacterium (Bifidobacterium lactis HN019): optimization and definition of cellular immune responses. Eur J Clin Nutr. 2000;54:849–855. doi: 10.1038/sj.ejcn.1601093. [DOI] [PubMed] [Google Scholar]
- 27.Hirose Y, Murosaki S, Yamamoto Y, Yoshikai Y, Tsuru T. Daily intake of heat-killed Lactobacillus plantarum L-137 augments acquired immunity in healthy adults. J Nutr. 2006;136:3069–3073. doi: 10.1093/jn/136.12.3069. [DOI] [PubMed] [Google Scholar]
- 28.Yoon YD, Han SB, Kang JS, Lee CW, Park SK, Lee HS, Kang JS, Kim HM. Toll-like receptor 4-dependent activation of macrophages by polysaccharide isolated from the radix of Platycodon grandiflorum. Int Immunopharmacol. 2003;3:1873–1882. doi: 10.1016/j.intimp.2003.09.005. [DOI] [PubMed] [Google Scholar]