Abstract
Recent studies reveal a critical role for copper in the development of the zebrafish notochord, suggesting that specific cuproenzymes are required for the structural integrity of the notochord sheath. We now demonstrate that β-aminopropionitrile, a known inhibitor of the copper-dependent lysyl oxidases, causes notochord distortion in the zebrafish embryo identical to that seen in copper deficiency. Characterization of the zebrafish lysyl oxidase genes reveals eight unique sequences, several of which are expressed in the developing notochord. Specific gene knockdown demonstrates that loss of loxl1 results in notochord distortion, and that loxl1 and loxl5b have overlapping roles in notochord formation. Interestingly, while notochord abnormalities are not observed following partial knockdown of loxl1 or loxl5b alone, in each case this markedly sensitizes developing embryos to notochord distortion if copper availability is diminished. Likewise, partial knockdown of the lysyl oxidase substrate col2a1 results in notochord distortion when combined with reduced copper availability or partial knockdown of loxl1 or loxl5b. These data reveal a complex interplay of gene expression and nutrient availability critical to notochord development. They also provide insight into specific genetic and nutritional factors that may play a role in the pathogenesis of structural birth defects of the axial skeleton.
Keywords: zebrafish, notochord, lysyl oxidase, copper, nutrition, disease model, col2a1
Introduction
Structural birth defects are a leading cause of morbidity and mortality in humans. Despite recent advances identifying the molecular genetic basis of several such disorders, the genetic and environmental determinants of most structural birth defects remain unknown (Epstein, 1995). One of the most important environmental factors influencing the outcome of fetal development is nutrition, and epidemiologic data, twin studies, and phenotype-genotype correlations all suggest that specific nutritional influences in combination with genetic susceptibility at multiple loci have profound long-term effects on pregnancy outcome (Miles et al., 2005). While in utero development severely limits experimental elucidation of the mechanisms and timing of critical events affected by nutrition during early embryonic development, recent studies suggest that the zebrafish may be an informative, genetically tractable model organism for such analysis (Jensen et al., 2006; Mendelsohn et al., 2006; Taylor et al., 2004).
The notochord is a useful structure for examining the complex interplay of genes and nutrition in early vertebrate development. This organ is readily visible throughout early zebrafish development, is the first to fully differentiate during embryogenesis, and is essential for the patterning of surrounding ectodermal, mesodermal, and endodermal tissues (Scott and Stemple, 2005). The notochord is also a critical midline structure required for locomotion in some chordates, and for axial skeletal formation in vertebrates (Scott and Stemple, 2005). Furthermore, the cell biological mechanisms underlying notochord maturation, involving cell vacuolation, matrix biosynthesis, and sheath formation, have begun to be elucidated through a series of elegant genetic experiments (Coutinho et al., 2004; Odenthal et al., 1996; Parsons et al., 2002; Stemple et al., 1996). Given the critical role of the notochord in chondrogenesis and fate determination of surrounding tissues including neural tube, heart, and skeleton (Stemple, 2005), as well as the large number of human structural birth defects arising from these tissues, elucidation of the nutritional and genetic mechanisms of notochord formation may be of direct relevance to our understanding of the etiology of congenital malformations.
During studies examining the role of copper in embryonic development, we discovered a critical role for this nutrient in late notochord formation (Mendelsohn et al., 2006). These findings suggested that specific cuproenzymes may be required for the structural integrity of the zebrafish notochord sheath, a concept supported by recent studies in Xenopus laevis (Geach and Dale, 2005). In this current study we have determined a role for two specific lysyl oxidase genes in notochord formation, and defined a complex biological pathway modulated by copper nutrition that underlies this process. Our data suggest that copper status during embryonic development should be interpreted in the context of environmental and genetic modulators of cuproenzyme activity. Indeed, the results raise the intriguing possibility that suboptimal copper availability, due either to nutritional or genetic variation during embryonic development, contributes to human birth defects of the axial skeleton.
Materials and methods
Zebrafish strains and maintenance
Zebrafish were reared under standard conditions at 28.5°C (Westerfield, 1993) and staged as described (Kimmel et al., 1995). Synchronous, in vitro fertilized embryos were obtained from AB or AB/WIK stocks for all experiments.
Pharmacologic treatment
Pharmacologic compounds were purchased from Sigma (St. Louis, MO). β-aminopropionitrile (A3134) and neocuproine (N1501) were prepared as 100 mM stocks in egg water and dimethyl sulfoxide, respectively, and diluted in egg water. Embryos were incubated in compound starting at 3 hpf and examined with an Olympus SZX12 zoom stereomicroscope at 24, 30, 48, and 72 hpf. Images of representative fish mounted in 2% methylcellulose were acquired with an Olympus DP70 camera.
Identification and annotation of zebrafish lysyl oxidase genes
Exons encoding conserved regions of the lysyl oxidase catalytic domain (Kagan and Li, 2003) were detected by TBLASTN search of the Ensembl zebrafish genome (Zv5). A clone of lox was commercially available (Open Biosystems #6971279); full-length cDNA sequences of all other family members were obtained using 5′ and 3′ rapid amplification of cDNA ends (RACE) and RT-PCR based on sequence homology to human, mouse, and frog lysyl oxidase sequences. Primers were as follows: loxl1 forward primer 5′-CGCCTTCTTTTATTAGTCTTCTGG-3′ and reverse primer 5′-ACAGCGACGTCAGGAATTCC-3′; loxl2a – forward primer 5′-ATGGCGGTGTCTTCTGCATTGTGC-3′ and reverse primer 5′-CTATCTGAGGTGGTTCAGCTGGTTGC-3′; loxl2b – forward primer 5′-GCCATACAATCTGAGCCTCTGTC-3′ and reverse primer 5′-CTTTACCTGTGGGTCACCTGG-3′; loxl3a – forward primer 5′-ATGGGACAGTTTGCTAACAGC-3′ and reverse primer 5′-TTATGAGATCTTGTTGTTGAGCTGCC-3′; loxl3b – forward primer 5′-GTGTTTGTGTCCTTTGATGC-3′ and reverse primer 5′-GCTGTACATGAAGAGTGATCT-3′; loxl5a – nested 5′RACE primers 5′-CACTCGAGCTGTACGCTGAACTGGAAAGGC-3′ (first reaction) and 5′-GCACAGGGCTTGAACGCACCACATGCCC-3′ (second reaction), and 3′ PCR amplification with forward primer 5′-CATTAAGATGTGCCGCAGAGG-3′and reverse primer 5′-CTCACCCGGTGATCCTACAGTTG-3′; loxl5b – forward primer 5′-CCCACATCCAGAGGAGCGAA-3′ and reverse primer 5′-TCCTCACCCAGTTATGATGCAG-3′. GenBank accession numbers are: lox – EF030479; loxl1 – EF030480; loxl2a – EF030481; loxl2b – EF030482; loxl3a – EF030483; loxl3b – EF030484; loxl5a – EF030485; loxl5b – EF030486. An alignment of the C-terminal portion of zebrafish and human lysyl oxidases was created in ClustalW with human CD163 as outgroup (Chenna et al., 2003), and phylogenetic analysis carried out according to the parsimony method (Felsenstein, 2005). Bootstrap values over 100 replicates are noted. Signal peptides and scavenger-receptor, cysteine rich domains were predicted using SignalP 3.0 and Motif scan (Bendtsen et al., 2004; Pagni et al., 2004). Copper binding domains, Bone Morphogenic Protein-1 (BMP-1) cleavage sites, and lysyl-tyrosyl quinone cofactor residues were identified based on homology to known lysyl oxidase sequences (Borel et al., 2001; Csiszar, 2001; Panchenko et al., 1996).
Whole mount in situ hybridization and frozen sections
Lysyl oxidase and col2a1 (Yan et al., 1995) probe constructs were generated as partial clones by RT-PCR and ligated into pCRII (Invitrogen). DIG-labeled antisense RNA probes were synthesized from these constructs using a DIG-labeling kit (Roche), and whole mount in situ hybridization performed as previously described (Mendelsohn et al., 2006; Thisse et al., 1993). Embryos for frozen sections were subsequently embedded in PBS containing 1.5% agarose/5% sucrose. Blocks were equilibrated in 30% sucrose at 4°C, mounted with O.C.T. (Tissue-Tek), and cut on a Leica Cryostat after freezing with liquid nitrogen. 14 μM sections were collected on Superfrost slides (Fisher) and mounted in 50% glycerol-PBS for visualization on an Olympus BX60 microscope.
Morpholino and mRNA injections
Morpholino oligonucleotides (Nasevicius and Ekker, 2000) targeting splice sites of exons encoding the lysyl oxidase copper binding domain (Csiszar, 2001) were resuspended in Danieau buffer, diluted to include 0.05% phenol red, and injected into one- to four-cell embryos. Morpholinos targeting the start sites of loxl1 and loxl5b, the 5′ splice acceptor site of an exon corresponding to exon 48 of the human col2a1 orthologue, and standard control morpholino (Gene Tools, LLC) were likewise prepared and injected. Morpholino sequences are as follows: loxl1 (splice) – 5′-GTGTAGATGTGGACTCACTGATGGC-3′; loxl1 (splice) – 5′-GTAATGCCTGATGGAGACAAGAGAC-3′ (Fig. 7 and Fig. 8); loxl1 (start) – 5′-AGTACATGCAGCATATTGAGAAGAC-3′; loxl2b – 5′-GATCTGGAGCAGCTAGAAAAAACAA-3′; loxl3b – 5′-CAGCTGCGGACATAAACAAACAAAT-3′; loxl5b (splice) – 5′-GCCTGTGGAATAAACACCAGCCTCA-3′; loxl5b (start) – 5′-TAAAGCTGTATGATTCGCTCCTCTG-3′; col2a1 – 5′-CCTGAAGGTCCCTATTATAAATAAC-3′. Capped, polyadenylated mRNA for rescue experiments was generated from full-length clones of loxl1, loxl5b, and control transposase (Kawakami et al., 2004) using the mMESSAGE mMACHINE kit (Ambion), with 300 pg of mRNA injected per embryo. Rescue was calculated by taking the difference between the percentages of embryos with distorted notochords injected with lysyl oxidase and control mRNA, and then dividing this number by the percentage of embryos with distorted notochords injected with control mRNA. Statistical analysis was conducted using one-way ANOVA for independent samples based on the percentages of embryos with the distorted notochord phenotype from at least three separate experiments. In all cases, abnormal splicing was detected by RT-PCR of RNA from 15 embryos injected with control or lysyl oxidase morpholino using primers to exons flanking the putative splice site. Nucleotide sequencing of putative splice products identified on agarose or polyacrylamide gels confirmed the presence of a premature stop codon in the splice variants. The intensity of wild-type PCR products was quantified using non-saturated gel pictures and ImageJ 1.37v software (Rasband, 1997–2006), and the percentage of wild-type splice form remaining after morpholino knockdown calculated.
Fig. 7.
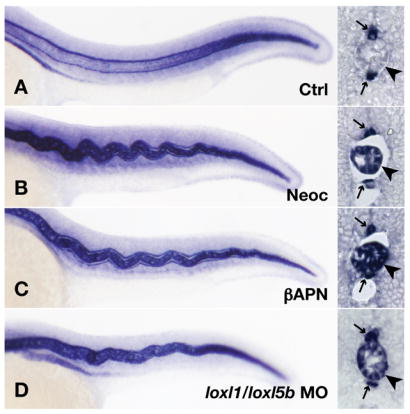
Expression of col2a1 mRNA by notochord vacuolar cells persists late following lysyl oxidase inhibition. Wild-type embryos were injected with 7.4 ng control morpholino (A), incubated in 10 μM neocuproine (B), incubated in 10 mM β-aminopropionitrile supplemented with 10 μM CuCl2 (C), or injected with 3.7 ng each of loxl1 and loxl5b morpholino (D). In situ hybridization was carried out at 24 hpf, and frozen sections confirmed persistent col2a1 expression following lysyl oxidase inhibition (B–D, arrowhead), as well as floorplate and hypochord staining (A–D, arrows).
Fig. 8.
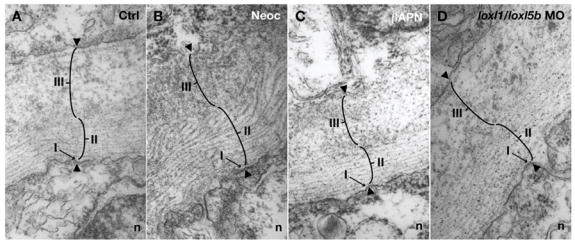
Electron micrographs of the notochord sheath from cross-sections of 30 hpf embryos: (A) 7.4 ng control morpholino; (B) 10 μM neocuproine; (C) 10 mM β-aminopropionitrile; (D) 3.7 ng each of loxl1 and loxl5b morpholino. Sheath components include a basal lamina (I), fibrillar layer (II), and granular layer (III). All three layers are preserved after lysyl oxidase inhibition.
Transmission Electron Microscopy
Dechorionated embryos were fixed in 2.5% glutaraldehyde/0.1 M sodium cacodylate and sequentially stained with osmium tetroxide and uranyl acetate. They were then dehydrated, embedded in PolyBed 812, and thin sectioned on a Reichert-Jung Ultra-Cut. Slices were post-stained in 4% uranyl acetate and lead citrate, and viewed on a Zeiss 902 electron microscope. Photographs were recorded with Kodak EM film.
Results
β-aminopropionitrile recapitulates the notochord phenotype of copper deficiency
Copper deficiency, induced by either chemical (neocuproine) or genetic (calamityvu69) means, results in a pleiotropic phenotype that includes a distorted, wavy notochord (Mendelsohn et al., 2006). Lysyl oxidases are copper-dependent enzymes that stabilize extracellular matrix by crosslinking elastin and collagens (Csiszar, 2001; Kagan and Li, 2003), a process thought to be important for maintaining notochord sheath integrity during notochord vacuolation. β-aminopropionitrile irreversibly inhibits lysyl oxidases by binding the active site of the catalytic domain (Molnar et al., 2003; Tang et al., 1983), and incubation of 3 hpf zebrafish embryos with β-aminopropionitrile resulted in striking notochord distortion and blunted somites, similar to what is observed with the copper chelator neocuproine (Fig. 1 and S. Fig. 1). Equivalent results were also obtained after incubation with semicarbazide, another known inhibitor of lysyl oxidases (data not shown), suggesting that these enzymes play a central role in notochord formation. β-aminopropionitrile treatment does not result in the pleiotropic phenotype of copper deficiency, including absence of melanin pigmentation, midbrain-hindbrain degeneration, and loss of red blood cells (Fig. 1B vs. 1C), reflecting the broader scope of cuproenzyme inhibition achieved with neocuproine. Furthermore, the effect of β-aminopropionitrile on notochord morphology was not due to copper chelation as this phenotype was not reversible with CuCl2 (data not shown). Although the notochord distortion observed with β-aminopropionitrile in Figure 1 is less than that with neocuproine, the mechanisms resulting in this process are likely the same because ten-fold higher doses of β-aminopropionitrile result in a degree of distortion identical to that seen with neocuproine (S. Fig. 1).
Fig. 1.
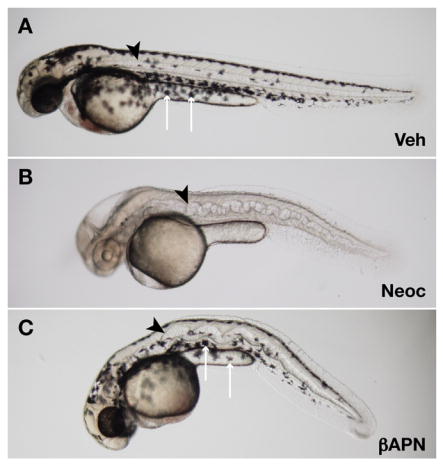
β-aminopropionitrile recapitulates the notochord phenotype of copper deficiency. Wild-type embryos were incubated in vehicle (A), 10 μM neocuproine (B), or 10 mM β-aminopropionitrile supplemented with 10 μM CuCl2 (C). At 48 hpf, the notochord (arrowheads) is distorted in embryos treated with neocuproine (B) and β-aminopropionitrile (C); however, melanocytes (arrows) are present after β-aminopropionitrile (C) but not neocuproine treatment (B), demonstrating that β-aminopropionitrile does not act through copper chelation.
The zebrafish genome encodes eight lysyl oxidases
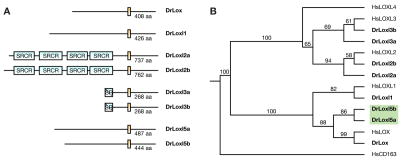
These data suggested a requirement for lysyl oxidase activity in zebrafish notochord development. The human genome encodes five lysyl oxidase family members characterized by the presence of a conserved copper binding domain and residues for a lysyl-tyrosyl quinone cofactor. Although experimental evidence suggests a role for two of these lysyl oxidases in elastin and collagen crosslinking (Hornstra et al., 2003; Liu et al., 2004; Maki et al., 2002; Maki et al., 2005), the precise role of each family member in extracellular matrix formation, cancer biology, and intracellular signaling remains undetermined. Using a bioinformatic approach, we identified and cloned eight unique zebrafish lysyl oxidase genes, revealing that each human lysyl oxidase has a zebrafish orthologue except LOXL4 (Fig. 2 and S. Fig. 2). While lysyl oxidase genes are well conserved between humans and zebrafish based on nucleotide sequence alignment, Loxl3a and Loxl3b lack a signal peptide and the full complement of scavenger receptor domains found in human LOXL3 (Fig. 2A and S. Fig. 2). Our analysis also revealed two novel zebrafish lysyl oxidases, Loxl5a and its paralogue Loxl5b, that are closely related to LOX and LOXL1 by phylogenetic analysis but have been not previously described in other species (Fig. 2B and S. Fig. 2A). The presence of paralogues for some zebrafish lysyl oxidases likely reflects a genome duplication event and subsequent partitioning of gene function (Lynch and Force, 2000; Woods et al., 2000).
Fig. 2.
Lysyl oxidase family members in zebrafish. (A) The zebrafish genome encodes eight distinct lysyl oxidases, all of which contain a conserved copper binding domain depicted in yellow. Scavenger receptor, cysteine-rich domains (SRCR) of unknown function are in blue and are truncated in Loxl3a and Loxl3b (SR with strikethrough). (B) Phylogenetic tree depicting the evolutionary relationship between human and zebrafish lysyl oxidases. HsCD163 is used as an outgroup, and bootstrap values over 100 replicates are noted. Loxl5a and Loxl5b (green) represent new additions to the lysyl oxidase family that closely resemble LOX and LOXL1 in structure. Zebrafish encode orthologues to all human lysyl oxidase genes except LOXL4.
Four lysyl oxidases are expressed throughout the developing notochord
To elucidate the role of specific lysyl oxidases in notochord formation, we assessed lysyl oxidase mRNA expression in zebrafish embryos by whole-mount in situ hybridization. Four lysyl oxidase family members – loxl1, loxl2b, loxl3b, and loxl5b – were readily detected throughout the notochord during early zebrafish development (Fig. 3), and were specifically expressed within the vacuolar cells, as seen in frozen sections of 15 somite embryos (Fig. 4). Lysyl oxidase expression was observed as early as the 5 somite stage (Fig. 3A,M), well before notochord vacuolation begins, and was extinguished after vacuolation is complete, between 24 hpf and 48 hpf (data not shown). While loxl1 and loxl5b were robustly expressed at the caudal tip (Fig. 3D,P, arrowheads), loxl2b and loxl3b were not (Fig. 3H,L), possibly reflecting a differential requirement for their activity during notochord elongation. Closer inspection revealed that loxl5b is expressed anterior to the notochord, in the prechordal plate region (Fig. 3O, asterix), and that loxl1 is expressed in the hypochord (Fig. 4A, arrows), providing a new marker for this structure and suggesting a role for loxl1 in crosslinking extracellular matrix near the developing aorta (Cleaver and Krieg, 1998; Eriksson and Lofberg, 2000). lox, loxl3a, and loxl5a were not expressed in or near the notochord between the 5 somite stage and 48 hpf, but could be detected by 5 dpf, while loxl2a was faintly and transiently expressed in a limited region of the anterior notochord before 24 hpf and was not studied further (data not shown).
Fig. 3.
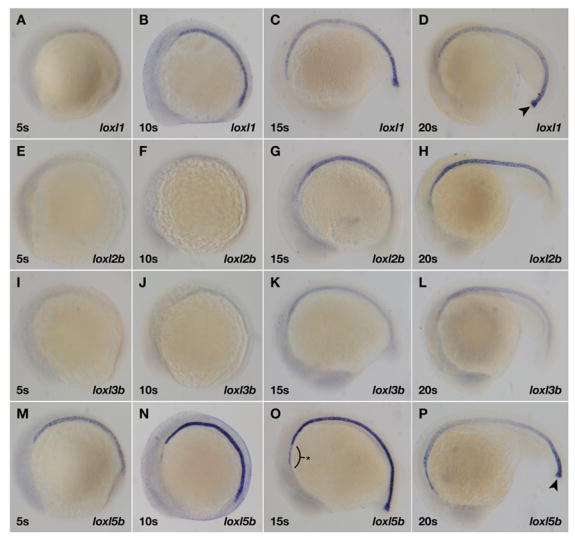
Four lysyl oxidases are expressed throughout the developing zebrafish notochord by in situ hybridization. (A–D) loxl1; (E–H) loxl2b; (I–L) loxl3b; and (M–P) loxl5b. Stages of development are 5 somites (A,E,I,M); 10 somites (B,F,J,N); 15 somites (C,G,K,O); and 20 somites (D,H,L,P). loxl1 and loxl5b are robustly expressed at the caudal notochord tip (arrowheads) while loxl5b is also expressed anterior to the notochord (asterix).
Fig. 4.
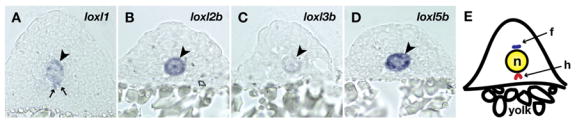
Lysyl oxidases are expressed by notochord vacuolar cells. (A–D) Frozen cross-sections of 15 somite embryos were obtained after in situ hybridization with the following probes: (A) loxl1; (B) loxl2b; (C) loxl3b; and (D) loxl5b. The notochord is indicated (arrowheads). Hypochord staining of loxl1 (arrows) suggests a role for this family member in extracellular matrix crosslinking near the developing aorta. (E) Cartoon demonstrating the location of the hypochord (h) and floorplate (f), which are closely apposed to the notochord (n). Lysyl oxidases are not expressed in the floorplate.
Morpholino knockdown of loxl1 or loxl1 and loxl5b together results in a distorted notochord
To determine the precise developmental functions of individual lysyl oxidases expressed throughout the notochord, we conducted knockdown experiments using morpholino antisense oligonucleotides (Fig. 5 and Table 1). Whereas control morpholino had no observable effect on developing zebrafish embryos (Fig. 5A and Table 1), a splice morpholino to loxl1 recapitulated the notochord distortion and blunted somites observed with β-aminopropionitrile and neocuproine (Fig. 5B and Table 1), revealing that this family member is necessary for late notochord formation in zebrafish. Morpholino knockdown of loxl2b, loxl3b, and loxl5b induced minor changes to notochord architecture, including occasional notochord kinks (Fig. 5C–E and Table 1), and the loxl5b morpholino also caused caudal vein edema (Fig. 5E, arrows). In all cases, a specific effect of these splice morpholinos on lysyl oxidase gene transcription was confirmed by demonstrating abnormal splicing and generation of a premature stop codon (S. Fig. 3 and data not shown). Since very few embryos injected with morpholinos to loxl2b, loxl3b, and loxl5b alone developed the distorted notochord phenotype at high morpholino doses (Fig. 5C–E and Table 1), we tested all possible combinations of lysyl oxidase morpholinos. Morpholino doses of loxl1 and loxl5b that did not result in notochord distortion when used alone, together recapitulated the notochord phenotype and blunted somites observed with β-aminopropionitrile and neocuproine (Fig. 5F and Table 1). This was the only morpholino combination resulting in a notochord phenotype (Table 1 and S. Table 1), and was similarly recapitulated with morpholinos targeting the start sites of loxl1 and loxl5b (Table 1 and S. Fig. 1). Taken together, these observations indicate that these two lysyl oxidases have specific overlapping functions in notochord development. The notochord distortion elicited by combined knockdown of loxl1 and loxl5b (Fig. 5F and Table 1) was specifically rescued with co-injection of either loxl1 or loxl5b mRNA (Fig. 5H,I and Table 2), but not control mRNA (Fig. 5G and Table 2). Importantly, each of the lysyl oxidase splice morpholinos also resulted in head necrosis (Fig. 5B–E) that was not rescued with specific mRNA injection (data not shown), suggesting that this phenotype results from a non-specific morpholino effect. Consistent with this concept, head necrosis is not observed following treatment with either β-aminopropionitrile or neocuproine (S. Fig. 1).
Fig. 5.
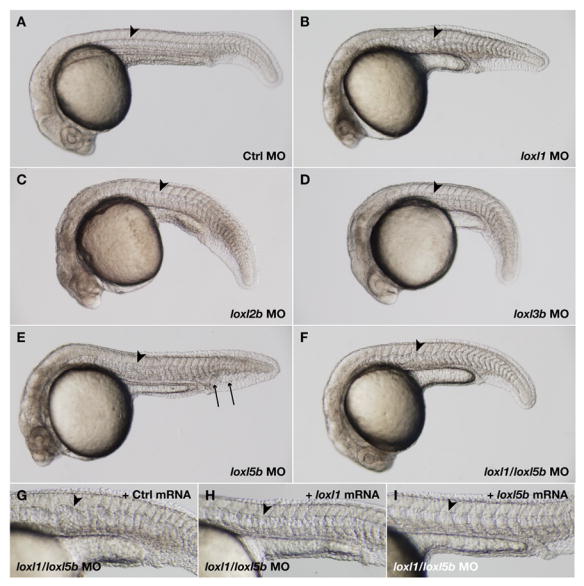
Morpholino knockdown of loxl1 or loxl1 and loxl5b together results in notochord distortion. Wild-type embryos were injected with morpholinos (MO) to notochord-expressed lysyl oxidases and photographed at 24 hpf. (A) 12 ng standard control; (B) 6 ng loxl1; (C) 3.7 ng loxl2b; (D) 12 ng loxl3b; (E) 7.4 ng loxl5b; (F) 2.4 ng loxl1 and 5 ng loxl5b. The notochord (arrowheads) is strikingly distorted after knockdown of loxl1 (B), and caudal vein edema develops with the loxl5b morpholino (E, arrows). Combining doses of loxl1 and loxl5b morpholino that do not cause notochord distortion alone also recapitulates the notochord phenotype seen with neocuproine and β-aminopropionitrile treatment (F). This distortion is specifically rescued by co-injection of mRNA encoding either loxl1 (H) or loxl5b (I), but not control sequence (G).
Table 1.
loxl1 is necessary for notochord formation in developing zebrafish embryos, and overlaps in function with loxl5b. Wild-type embryos were injected with morpholinos to notochord-expressed lysyl oxidases and examined at 24 hpf. The number and percentage of embryos with and without a distorted notochord phenotype is noted. Injection of 6 ng of loxl1 splice morpholino causes notochord distortion in 88% of embryos, an effect not seen with morpholinos to loxl2b, loxl3b, or loxl5b alone. However, injection of 2.4 ng of loxl1 morpholino and 5 ng of loxl5b morpholino together causes notochord distortion in 94% of embryos, demonstrating that loxl1 and loxl5b have overlapping roles in notochord formation. This finding was recapitulated in separate experiments with start site (ATG) morpholinos to loxl1 and loxl5b. The number of embryos examined includes dead embryos that could not be scored for notochord phenotype; the loxl2b morpholino causes significant embryo death at higher doses. Data shown are the pooled results of three independent experiments.
| Specific Morpholino | Dose of morpholino (ng) | # of embryos examined | Phenotype of notochord distortion | |
|---|---|---|---|---|
| − | + | |||
| Ctrl | 12 | 302 | 278 (100%) | 0 (0%) |
| loxl1 | 6 | 271 | 30 (12%) | 217 (88%*) |
| loxl2b | 2.4 | 212 | 122 (98%) | 3 (2%) |
| loxl3b | 12 | 190 | 128 (96%) | 6 (4%) |
| loxl5b | 5 | 263 | 237 (98%) | 6 (2%) |
| loxl1 | 2.4 | 297 | 272 (99%) | 2 (1%) |
| loxl1/loxl5b | 2.4/5 | 292 | 17 (6%) | 254 (94%*) |
| Ctrl | 12 | 173 | 138 (100%) | 0 (0%) |
| loxl1 ATG | 12 | 158 | 145 (100%) | 0 (0%) |
| loxl5b ATG | 12 | 159 | 140 (92%) | 12 (8%) |
| loxl1 ATG/loxl5b ATG | 6/6 | 188 | 33 (19%) | 145 (81%*) |
p < 0.01 versus controls by ANOVA
Table 2.
The distorted notochord phenotype resulting from combined loxl1 and loxl5b knockdown is specific and can be rescued by co-injection of mRNA encoding either loxl1 or loxl5b. Wild-type embryos injected with control or loxl1 and loxl5b morpholinos together were co-injected with control or lysyl oxidase mRNA, as indicated. The number and percentage of embryos with and without a distorted notochord phenotype at 24 hpf is noted, as well as the percentage rescue attributable to each mRNA. Exogenously-supplied loxl1 or loxl5b mRNA produces a marked improvement in notochord morphology, substantially decreasing the proportion of embryos with notochord distortion. Injection of lysyl oxidase mRNA alone did not result in any overexpression phenotype. The number of embryos examined includes dead embryos that could not be scored for notochord phenotype. Data shown are the pooled results of three independent experiments.
| Specific Morpholino | Dose of morpholino (ng) | Specific mRNA | # of embryos examined | Phenotype of notochord distortion | Rescue of notochord phenotype | |
|---|---|---|---|---|---|---|
| − | + | |||||
| Ctrl | 7.4 | Ctrl | 240 | 226 (100%) | 0 (0%) | NA |
| loxl1/loxl5b | 2.4/5 | Ctrl | 223 | 8 (4%) | 195 (96%) | NA |
| loxl1/loxl5b | 2.4/5 | loxl1 | 238 | 137 (63%) | 80 (37%*) | 62% |
| loxl1/loxl5b | 2.4/5 | loxl5b | 251 | 197 (86%) | 33 (14%*) | 85% |
p < 0.01 versus loxl1/loxl5b MO with Ctrl mRNA by ANOVA
Nutrient, gene interactions in notochord distortion
Since the notochord phenotype observed with combined loxl1 and loxl5b knockdown is identical to that seen with β-aminopropionitrile and neocuproine, we next examined the interaction of copper availability and lysyl oxidase gene expression in notochord formation (Fig. 6). For these experiments, doses of loxl5b morpholino and neocuproine were determined such that they did not cause notochord distortion when used alone (Fig. 6B,C and Table 3). In striking contrast, embryos subjected to combined treatment with these same doses of loxl5b morpholino and neocuproine developed distorted notochords (Fig. 6D and Table 3). Experiments using loxl1 morpholino and neocuproine yielded similar results (Table 3), supporting the model that specific inhibition of two lysyl oxidases accounts for the notochord abnormalities observed with β-aminopropionitrile and neocuproine. Most significantly, these data also reveal that copper availability influences the phenotypic outcome of notochord development according to the genetic content of the embryo.
Fig. 6.
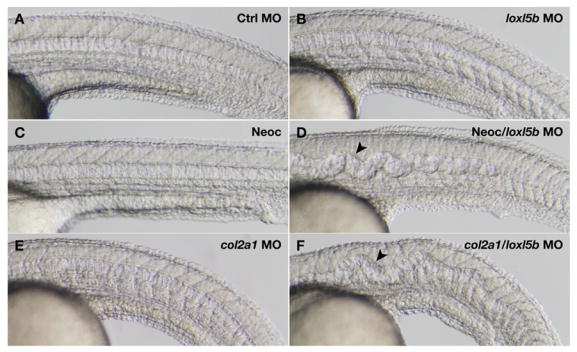
Partial knockdown of loxl5b sensitizes embryos to notochord distortion in the presence of suboptimal copper nutrition (A–D) or disruption of col2a1 (E,F). Wild-type embryos were injected with 5 ng of control morpholino (A,C) or loxl5b morpholino (B,D), and incubated with (C,D) or without (A,B) 2 μM neocuproine starting at 3 hpf. Embryos injected with loxl5b morpholino develop notochord distortion (arrowhead) in the context of diminished copper availability (D). Embryos injected with 7.4 ng of col2a1 morpholino (E) are sensitized to develop notochord distortion (arrowhead) upon co-injection of 5 ng of loxl5b morpholino (F), demonstrating a genetic interaction between col2a1 and loxl5b. Photographs were obtained at 30 hpf (A–D) and 24 hpf (E,F).
Table 3.
Partial knockdown of loxl1 or loxl5b sensitizes embryos to notochord distortion in the presence of suboptimal copper nutrition. Wild-type embryos were injected with control or lysyl oxidase-specific morpholino, as indicated, and incubated with or without neocuproine at doses that were determined not to cause notochord distortion alone. Embryos injected with lysyl oxidase-specific morpholino and incubated in 2 μM neocuproine were sensitized to develop notochord distortion. The number of embryos examined includes dead embryos that could not be scored for notochord phenotype. Each result is pooled from three independent experiments, which were scored at 24 hpf.
| Specific Morpholino | Dose of morpholino (ng) | Pharmacologic treatment | # of embryos examined | Phenotype of notochord distortion | |
|---|---|---|---|---|---|
| − | + | ||||
| Ctrl | 2.4 | None | 104 | 104 (100%) | 0 (0%) |
| Ctrl | 2.4 | 2 μM neocuproine | 113 | 111 (98%) | 2 (2%) |
| loxl1 | 2.4 | None | 143 | 127 (100%) | 0 (0%) |
| loxl1 | 2.4 | 2 μM neocuproine | 132 | 49 (39%) | 77 (61%*) |
| Ctrl | 5 | None | 139 | 136 (100%) | 0 (0%) |
| Ctrl | 5 | 2 μM neocuproine | 138 | 132 (99%) | 2 (1%) |
| loxl5b | 5 | None | 147 | 126 (95%) | 7 (5%) |
| loxl5b | 5 | 2 μM neocuproine | 147 | 1 (1%) | 140 (99%*) |
p < 0.01 versus controls by ANOVA
In this context, we also examined the role of collagen II, a structural component of the notochord sheath, in notochord formation. Collagen II is a lysyl oxidase substrate whose spatio-temporal pattern of expression corresponds with that of the notochord-expressed lysyl oxidases during early zebrafish development (Yan et al., 1995). We determined the highest dose of col2a1 morpholino that could be injected without phenotypic abnormalities (Fig. 6E), and incubated these injected embryos in a dose of neocuproine (2 μM) that was also without effect. This combined treatment resulted in notochord distortion (data not shown), revealing the importance of copper nutrition in the context of collagen II disruption and predicting a genetic interaction between collagen II and the notochord-expressed lysyl oxidases. Consistent with this prediction, about half of the embryos injected with loxl5b and col2a1 morpholinos together revealed a conspicuously distorted notochord (Fig. 6F and Table 4), demonstrating that decreased expression of loxl5b sensitizes embryos to col2a1 disruption. A comparable result was also obtained using the loxl1 and col2a1 morpholinos (Table 4). The specificity of this effect was confirmed by detection of a splicing defect resulting from the col2a1 morpholino that causes a premature stop codon (S. Fig. 3 and data not shown). The observation that in each case only about 50% of the embryos displayed the abnormal notochord phenotype may be due either to additional lysyl oxidase substrates that play a role in notochord formation or to submaximal disruption of col2a1 gene expression at this morpholino dose (S. Fig. 3). Taken together, these data illustrate the complex interplay of multigenic expression and nutrition required for notochord formation in developing zebrafish embryos.
Table 4.
Genetic interaction of loxl1 and loxl5b with col2a1. Wild-type embryos were injected with the indicated morpholino combinations and scored for notochord distortion at 24 hpf. Partial knockdown of loxl1 and col2a1 together, but not alone, causes the distorted notochord phenotype. An identical result is obtained with morpholinos to loxl5b and col2a1, suggesting that diminished activity of these lysyl oxidases sensitizes embryos to notochord distortion in the presence of col2a1 disruption. Importantly, collagen II is a lysyl oxidase substrate produced by the notochord vacuolar cells and present in the notochord sheath. The number of embryos examined includes dead embryos that could not be scored for notochord phenotype. Each result is pooled from three independent experiments.
| Specific Morpholino | Dose of morpholino (ng) | # of embryos examined | Phenotype of notochord distortion | |
|---|---|---|---|---|
| − | + | |||
| loxl1/ctrl | 2.4/7.4 | 202 | 146 (99%) | 1 (1%) |
| col2a1/ctrl | 7.4/2.4 | 206 | 161 (98%) | 4 (2%) |
| col2a1/loxl1 | 7.4/2.4 | 258 | 118 (58%) | 84 (42%*) |
| loxl5b/ctrl | 5/7.4 | 151 | 137 (93%) | 10 (7%) |
| col2a1/ctrl | 7.4/5 | 122 | 116 (99%) | 1 (1%) |
| col2a1/loxl5b | 7.4/5 | 217 | 111 (54%) | 96 (46%*) |
p < 0.01 versus controls by ANOVA
col2a1 expression persists in notochord vacuolar cells after lysyl oxidase inhibition
The foregoing experiments suggest that collagen II is a substrate for both Loxl1 and Loxl5b in zebrafish. As recently demonstrated (Tilton et al., 2006), col2a1 expression persists in the vacuolar cells of the notochord at 24 hpf when embryos are treated with a dose of neocuproine that causes notochord distortion (Fig. 7B). One possible explanation for this phenomenon is impairment of a feedback mechanism that normally downregulates production of col2a1 upon collagen crosslinking in the notochord sheath. Consistent with this idea, embryos treated with β-aminopropionitrile (Fig. 7C) or injected with morpholinos to loxl1 and loxl5b (Fig. 7D) continued to express col2a1 in the vacuolar cells of the notochord at 24 hpf, whereas control embryos did not (Fig. 7A). The persistent expression of col2a1 was not due to developmental delay, as embryos added somites at the same rate under all experimental conditions (data not shown).
We next considered whether the persistent col2a1 expression was specific to a reduction in crosslinking or was a generalized response to mechanical distortion of the notochord. To explore this idea, we incubated embryos in a dose of neocuproine (2 μM) that did not cause notochord distortion but that partially disrupted crosslinking, as revealed by the combined neocuproine and loxl5b studies. Persistent col2a1 expression was observed within the notochord vacuolar cells at 24 hpf under these conditions (S. Fig. 4), similar to what is observed in neocuproine-treated embryos incubated with anesthetic to prevent notochord distortion (Tilton et al., 2006). These data are consistent with a feedback mechanism originating from the products of the crosslinking process.
The col2a1 expression data suggest a model where lysyl oxidase inhibition affects the final stages of notochord development at a time when the extracellular matrix components, including collagen II, are already in place in the notochord sheath. The notochord sheath in zebrafish is composed of three distinct layers: an internal basal lamina, a middle fibrillar layer, and an external granular layer (Fig. 8A) (Cerda et al., 2002; Stemple, 2005). Inspection of the notochord sheath by electron microscopy revealed that inhibition of lysyl oxidase activity by neocuproine, β-aminopropionitrile, or combined loxl1 and loxl5b knockdown was without effect on these layers (Fig. 8B–D). This observation is consistent with the idea that lysyl oxidase inhibition alters the final stages of notochord sheath formation.
Discussion
These studies reveal an essential role for two specific lysyl oxidase genes, loxl1 and loxl5b, in notochord formation, and demonstrate a complex interplay of gene expression and nutrient availability that is relevant to the pathogenesis of human structural birth defects (Fig. 9A,B). A role for lysyl oxidases in notochord formation was hypothesized from previous work using small molecules (Geach and Dale, 2005; Mendelsohn et al., 2006), and our current data establish a genetic basis for this pharmacologic notochord distortion. Various lysyl oxidases have been implicated in tumor metastasis, wound healing, cardiac fibrosis and elastin fiber homeostasis (Erler et al., 2006; Lau et al., 2006; Liu et al., 2004; Yu et al., 2006). However, the precise function of individual lysyl oxidase family members in development has been difficult to discern given the lack of inhibitors unique to any given enzyme. This study is the first to demonstrate that genetic deficiency of specific lysyl oxidases results in notochord distortion, and elucidates a specific and novel interaction between gene function (lysyl oxidases) and nutrition (copper) during development. While it is logical that copper-dependent enzymes such as lysyl oxidases would be inactive in the complete absence of copper, it is more difficult to predict the effect of mild copper deficiency on enzyme activity, and more importantly, to determine what components of the developing notochord would become essential in the setting of impaired – but not absent – lysyl oxidase function. The specific role for loxl1 and loxl5b in notochord formation is supported by sensitization experiments using neocuproine (Fig. 6 and Table 3), and these genes may be candidates for late notochord mutants identified in previous forward genetic screens (Odenthal et al., 1996; Stemple et al., 1996) or for human disorders of the axial skeleton. Though we cannot completely rule out the possibility that the low penetrance of a distorted notochord phenotype using splice morpholinos to loxl2b, loxl3b, or loxl5b alone (Table 1) results from incomplete disruption of gene expression (S. Fig. 3), combinations involving these morpholinos did not convincingly demonstrate a role for loxl2b or loxl3b in notochord formation (Table 1 and S. Table 1).
Fig. 9.
Model of pathways involved in notochord formation. (A) The cuproenzymes Loxl1 and Loxl5b crosslink collagen II in the notochord sheath, which suppresses vacuolar cell expression of col2a1 as the notochord differentiates. (B) Polymorphisms at multiple genetic loci interact with environmental factors to cause disease. As a result, what is normally considered adequate nutrition may in fact be suboptimal during a specific developmental window. The gene atp7a encodes a transporter required for copper uptake (Mendelsohn et al., 2006).
Our data also demonstrate a genetic interaction between two lysyl oxidases (loxl1 and loxl5b) and the collagen substrate col2a1 (Fig. 6). This could reflect either direct crosslinking of this collagen substrate or crosslinking of additional extracellular matrix proteins that interact with collagen II for proper notochord formation. The latter possibility has been proposed in recent morphological studies on the notochord sheath of teleosts (Grotmol et al., 2006) and could account for observations in coatomer I protein complex mutants (Coutinho et al., 2004), where defects in the secretion of lysyl oxidases or such additional extracellular matrix components would give rise to the notochord sheath abnormalities. In all cases, reduced crosslinking of collagen fibrils from impaired lysyl oxidase activity would prevent the feedback inhibition of col2a1 produced by notochord vacuolar cells, resulting in prolonged col2a1 expression (Fig. 7). Alternatively, persistent col2a1 expression could result indirectly from a general loss of notochord sheath integrity.
The observed lysyl oxidase expression patterns and phenotypes (Fig. 3, Fig. 4, Fig. 5 and data not shown) suggest that the zebrafish will be a useful model organism for elucidating the complex and specific roles of lysyl oxidases in development. In addition, the phylogenetic analysis (Fig. 2B) may prove helpful. The lack of a zebrafish orthologue to LOXL4 is consistent with a BLAST search revealing such orthologues only in mammals (data not shown), and the markedly different expression patterns of this gene in human and mouse (Asuncion et al., 2001; Ito et al., 2001; Maki et al., 2001) may indicate that it has divergent, specialized roles in these organisms. Moreover, it is now apparent that scavenger receptor, cysteine-rich domains are a more variable feature of lysyl oxidase structure than previously anticipated. LOXL2, LOXL3, and LOXL4 are considered related because they all possess four scavenger receptor, cysteine-rich domains, which are completely absent in LOX and LOXL1. However, zebrafish loxl3a and loxl3b encode only a single scavenger receptor, cysteine-rich domain that is truncated (Fig. 2A), as does a Xenopus clone of loxl3 previously assumed to be partial (S. Fig. 2C) (Geach and Dale, 2005). While the function of these domains remains unknown, a splice variant of LOXL3 that excludes some scavenger-receptor, cysteine-rich domains has recently been described, and appears to have altered substrate specificity (Lee and Kim, 2006).
Definitive demonstration of a role for specific lysyl oxidases in notochord formation is of direct relevance to embryonic environmental exposures to nitrile compounds in plasticizers and carbamates in pesticides. Recent toxicological studies demonstrate that such pesticides induce notochord distortion in developing zebrafish embryos (Teraoka et al., 2006; Tilton et al., 2006) as well as persistent expression of col2a1 by vacuolar cells of the notochord (Tilton et al., 2006). These findings are identical to our observations with loxl1, loxl5b, and col2a1 morpholino knockdown combined with neocuproine treatment (Fig. 5B,F, Fig. 6 and Fig. 7), suggesting that the pesticides act through direct or indirect inhibition of these lysyl oxidase family members. Importantly, these data raise the distinct possibility that embryonic exposure to such toxins in specific genetic contexts may predispose to the later development of structural birth defects (Fig. 9B), where the inheritance of this genetic variation would not be reflected in Mendelian ratios of the observable phenotypes.
The notochord is a critical structure required for vertebral column patterning in vertebrates (Fleming et al., 2004) and its distortion in zebrafish phenotypically mimics a number of axial skeletal defects in humans including idiopathic scoliosis. Of note, while β-aminopropionitrile causes notochord distortion in zebrafish (Fig. 1B and S. Fig. 1), it is also well known to cause scoliosis in rats and other mammals (Barrow et al., 1974). Interestingly, disruptions in extracellular matrix components involved in notochord formation have been identified in clinical syndromes that include scoliosis. Two different mutations in exon 48 of the COL2A1 gene cause spondyloepiphyseal dysplasia with scoliosis in humans (Tiller et al., 1995; Tiller et al., 1990). Furthermore, the kyphoscoliosis type of Ehler-Danlos syndrome (EDS VI) (Yeowell and Walker, 2000) results from loss of function mutations in the gene encoding lysyl hydroxylase type 1, which catalyzes the conversion of lysine to hydroxylysine in collagen prior to oxidization by lysyl oxidases. Taken together with the nutritional (copper) and toxicology findings noted above, these data raise the intriguing possibility that polymorphisms at multiple extracellular matrix component loci, including the lysyl oxidase genes identified here, may predispose to axial skeletal malformations in situations where nutrition or environment might otherwise be considered adequate (Fig. 9B). This concept is supported by our previous findings revealing a hierarchy of nutrient distribution to the developing embryo under limiting conditions (Mendelsohn et al., 2006) and as such is of broad relevance to our understanding of the pathogenesis of structural birth defects. The data reported here provide a testable model for these ideas with regards to common defects of the axial skeleton and reveal that zebrafish permit unique insights into the complex interplay of genes, environment and nutrition in development and disease.
Supplementary Material
S. Fig. 1. Comparison of the notochord distortion obtained in zebrafish using three distinct methods of lysyl oxidase inhibition at 24 hpf. Wild-type embryos were incubated in 10 μM neocuproine (A), 10 mM β-aminopropionitrile supplemented with 10 μM CuCl2 (B), 100 mM β-aminopropionitrile supplemented with 10 μM CuCl2 (C), or injected with 6 ng each of loxl1 and loxl5b start (ATG) morpholino (D). 10 μM neocuproine (A) causes greater notochord distortion than 10 mM β-aminopropionitrile (B), but an identical phenotype is obtained with 100 mM β-aminopropionitrile (A vs. C). Melanin pigmentation has not yet developed in the embryos pictured, but is not affected by β-aminopropionitrile treatment or by the morpholino injection.
S. Fig. 2. Protein sequence alignment of zebrafish lysyl oxidase family members with human orthologues. (A) lox, loxl1, loxl5a, and loxl5b; (B) loxl2a and loxl2b; (C) loxl3a and loxl3b with Xenopus laevis clone XL051k10 included for comparison. A number of conserved elements are indicated including predicted signal peptides (bold, underlined), BMP-1 cleavage sequences (bold, italicized), scavenger receptor cysteine-rich domains (gray, underlined), copper binding domains (gray), and lysyl-tyrosyl quinone cofactor residues (bold).
S. Fig. 3. Lysyl oxidase and collagen II splice morpholinos decrease the abundance of wild-type splice forms and cause specific changes in splice site usage. (A) loxl1 (B) loxl2b (C) loxl3b (D) loxl5b and (E) col2a1. Wild-type embryos injected with the indicated doses of morpholino were harvested at 20 hpf and cDNA prepared from equal amounts of RNA. The intensity of the wild-type (WT) product obtained by PCR was quantified and the percentage of wild-type splice form remaining after morpholino knockdown determined, as noted. This measure is independent of processes that may depress levels of the mutant (Mut) splice products, including degradation through nonsense-mediated decay. The primer set used to amplify loxl1 does not amplify the mutant band.
S. Fig. 4. Persistent expression of col2a1 by notochord vacuolar cells after lysyl oxidase inhibition is not due to mechanical distortion of the notochord. Wild-type embryos incubated with (B) or without (A) 2 μM neocuproine were probed for col2a1 expression at 24 hpf. col2a1 expression persists in the notochord vacuolar cells after treatment with 2 μM neocuproine, which affects crosslinking but does not cause mechanical distortion. This suggests that the signal to downregulate col2a1 expression in the vacuolar cells of the notochord originates from the products of the crosslinking reaction.
S. Table 1. Morpholinos to combinations of lysyl oxidase family members other than loxl1 and loxl5b do not result in notochord distortion. Wild-type embryos were injected with the indicated morpholino doses and examined at 24 hpf for notochord distortion. The number of embryos examined includes dead embryos that could not be scored for notochord phenotype. Each result is pooled from three independent experiments.
Acknowledgments
We thank Robert Mecham and Erik Madsen for critical review of the manuscript and Marilyn Levy for electron microscopy. J.M.G and B.A.M. were supported by NIH Medical Scientist Training Program grant T32 GM07200. This work was supported by NIH grants GM56988 (S.L.J.), HD39952 (J.D.G.), DK61763 (J.D.G.), and DK44464 (J.D.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asuncion L, Fogelgren B, Fong KS, Fong SF, Kim Y, Csiszar K. A novel human lysyl oxidase-like gene (LOXL4) on chromosome 10q24 has an altered scavenger receptor cysteine rich domain. Matrix Biol. 2001;20:487–91. doi: 10.1016/s0945-053x(01)00161-5. [DOI] [PubMed] [Google Scholar]
- Barrow MV, Simpson CF, Miller EJ. Lathyrism: a review. Q Rev Biol. 1974;49:101–28. doi: 10.1086/408017. [DOI] [PubMed] [Google Scholar]
- Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol. 2004;340:783–95. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Borel A, Eichenberger D, Farjanel J, Kessler E, Gleyzal C, Hulmes DJ, Sommer P, Font B. Lysyl oxidase-like protein from bovine aorta. Isolation and maturation to an active form by bone morphogenetic protein-1. J Biol Chem. 2001;276:48944–9. doi: 10.1074/jbc.M109499200. [DOI] [PubMed] [Google Scholar]
- Cerda J, Grund C, Franke WW, Brand M. Molecular characterization of Calymmin, a novel notochord sheath-associated extracellular matrix protein in the zebrafish embryo. Dev Dyn. 2002;224:200–9. doi: 10.1002/dvdy.10101. [DOI] [PubMed] [Google Scholar]
- Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ, Higgins DG, Thompson JD. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 2003;31:3497–500. doi: 10.1093/nar/gkg500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver O, Krieg PA. VEGF mediates angioblast migration during development of the dorsal aorta in Xenopus. Development. 1998;125:3905–14. doi: 10.1242/dev.125.19.3905. [DOI] [PubMed] [Google Scholar]
- Coutinho P, Parsons MJ, Thomas KA, Hirst EM, Saude L, Campos I, Williams PH, Stemple DL. Differential requirements for COPI transport during vertebrate early development. Dev Cell. 2004;7:547–58. doi: 10.1016/j.devcel.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Csiszar K. Lysyl oxidases: a novel multifunctional amine oxidase family. Prog Nucleic Acid Res Mol Biol. 2001;70:1–32. doi: 10.1016/s0079-6603(01)70012-8. [DOI] [PubMed] [Google Scholar]
- Epstein CJ. The new dysmorphology: application of insights from basic developmental biology to the understanding of human birth defects. Proc Natl Acad Sci U S A. 1995;92:8566–73. doi: 10.1073/pnas.92.19.8566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson J, Lofberg J. Development of the hypochord and dorsal aorta in the zebrafish embryo (Danio rerio) J Morphol. 2000;244:167–76. doi: 10.1002/(SICI)1097-4687(200006)244:3<167::AID-JMOR2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Erler JT, Bennewith KL, Nicolau M, Dornhofer N, Kong C, Le QT, Chi JT, Jeffrey SS, Giaccia AJ. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–6. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- Felsenstein J PHYLIP (Phylogeny Inference Package) Department of Genome Sciences. University of Washington; Seattle: 2005. Distributed by the author. [Google Scholar]
- Fleming A, Keynes R, Tannahill D. A central role for the notochord in vertebral patterning. Development. 2004;131:873–80. doi: 10.1242/dev.00952. [DOI] [PubMed] [Google Scholar]
- Geach TJ, Dale L. Members of the lysyl oxidase family are expressed during the development of the frog Xenopus laevis. Differentiation. 2005;73:414–24. doi: 10.1111/j.1432-0436.2005.00041.x. [DOI] [PubMed] [Google Scholar]
- Grotmol S, Kryvi H, Keynes R, Krossoy C, Nordvik K, Totland GK. Stepwise enforcement of the notochord and its intersection with the myoseptum: an evolutionary path leading to development of the vertebra? J Anat. 2006;209:339–57. doi: 10.1111/j.1469-7580.2006.00618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornstra IK, Birge S, Starcher B, Bailey AJ, Mecham RP, Shapiro SD. Lysyl oxidase is required for vascular and diaphragmatic development in mice. J Biol Chem. 2003;278:14387–93. doi: 10.1074/jbc.M210144200. [DOI] [PubMed] [Google Scholar]
- Ito H, Akiyama H, Iguchi H, Iyama K, Miyamoto M, Ohsawa K, Nakamura T. Molecular cloning and biological activity of a novel lysyl oxidase-related gene expressed in cartilage. J Biol Chem. 2001;276:24023–9. doi: 10.1074/jbc.M100861200. [DOI] [PubMed] [Google Scholar]
- Jensen PJ, Gitlin JD, Carayannopoulos MO. GLUT1 deficiency links nutrient availability and apoptosis during embryonic development. J Biol Chem. 2006;281:13382–7. doi: 10.1074/jbc.M601881200. [DOI] [PubMed] [Google Scholar]
- Kagan HM, Li W. Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J Cell Biochem. 2003;88:660–72. doi: 10.1002/jcb.10413. [DOI] [PubMed] [Google Scholar]
- Kawakami K, Takeda H, Kawakami N, Kobayashi M, Matsuda N, Mishina M. A transposon-mediated gene trap approach identifies developmentally regulated genes in zebrafish. Dev Cell. 2004;7:133–44. doi: 10.1016/j.devcel.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Lau YK, Gobin AM, West JL. Overexpression of lysyl oxidase to increase matrix crosslinking and improve tissue strength in dermal wound healing. Ann Biomed Eng. 2006;34:1239–46. doi: 10.1007/s10439-006-9130-8. [DOI] [PubMed] [Google Scholar]
- Lee JE, Kim Y. A tissue-specific variant of the human lysyl oxidase-like protein 3 (LOXL3) functions as an amine oxidase with substrate specificity. J Biol Chem. 2006 doi: 10.1074/jbc.M600977200. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhao Y, Gao J, Pawlyk B, Starcher B, Spencer JA, Yanagisawa H, Zuo J, Li T. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat Genet. 2004;36:178–82. doi: 10.1038/ng1297. [DOI] [PubMed] [Google Scholar]
- Lynch M, Force A. The probability of duplicate gene preservation by subfunctionalization. Genetics. 2000;154:459–73. doi: 10.1093/genetics/154.1.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki JM, Rasanen J, Tikkanen H, Sormunen R, Makikallio K, Kivirikko KI, Soininen R. Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation. 2002;106:2503–9. doi: 10.1161/01.cir.0000038109.84500.1e. [DOI] [PubMed] [Google Scholar]
- Maki JM, Sormunen R, Lippo S, Kaarteenaho-Wiik R, Soininen R, Myllyharju J. Lysyl oxidase is essential for normal development and function of the respiratory system and for the integrity of elastic and collagen fibers in various tissues. Am J Pathol. 2005;167:927–36. doi: 10.1016/S0002-9440(10)61183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki JM, Tikkanen H, Kivirikko KI. Cloning and characterization of a fifth human lysyl oxidase isoenzyme: the third member of the lysyl oxidase-related subfamily with four scavenger receptor cysteine-rich domains. Matrix Biol. 2001;20:493–6. doi: 10.1016/s0945-053x(01)00157-3. [DOI] [PubMed] [Google Scholar]
- Mendelsohn BA, Yin C, Johnson SL, Wilm TP, Solnica-Krezel L, Gitlin JD. Atp7a determines a hierarchy of copper metabolism essential for notochord development. Cell Metab. 2006;4:155–62. doi: 10.1016/j.cmet.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Miles HL, Hofman PL, Cutfield WS. Fetal origins of adult disease: a paediatric perspective. Rev Endocr Metab Disord. 2005;6:261–8. doi: 10.1007/s11154-005-6184-0. [DOI] [PubMed] [Google Scholar]
- Molnar J, Fong KS, He QP, Hayashi K, Kim Y, Fong SF, Fogelgren B, Szauter KM, Mink M, Csiszar K. Structural and functional diversity of lysyl oxidase and the LOX-like proteins. Biochim Biophys Acta. 2003;1647:220–4. doi: 10.1016/s1570-9639(03)00053-0. [DOI] [PubMed] [Google Scholar]
- Nasevicius A, Ekker SC. Effective targeted gene ‘knockdown’ in zebrafish. Nat Genet. 2000;26:216–20. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- Odenthal J, Haffter P, Vogelsang E, Brand M, van Eeden FJ, Furutani-Seiki M, Granato M, Hammerschmidt M, Heisenberg CP, Jiang YJ, Kane DA, Kelsh RN, Mullins MC, Warga RM, Allende ML, Weinberg ES, Nusslein-Volhard C. Mutations affecting the formation of the notochord in the zebrafish, Danio rerio. Development. 1996;123:103–15. doi: 10.1242/dev.123.1.103. [DOI] [PubMed] [Google Scholar]
- Pagni M, Ioannidis V, Cerutti L, Zahn-Zabal M, Jongeneel CV, Falquet L. MyHits: a new interactive resource for protein annotation and domain identification. Nucleic Acids Res. 2004;32:W332–5. doi: 10.1093/nar/gkh479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchenko MV, Stetler-Stevenson WG, Trubetskoy OV, Gacheru SN, Kagan HM. Metalloproteinase activity secreted by fibrogenic cells in the processing of prolysyl oxidase. Potential role of procollagen C-proteinase. J Biol Chem. 1996;271:7113–9. doi: 10.1074/jbc.271.12.7113. [DOI] [PubMed] [Google Scholar]
- Parsons MJ, Pollard SM, Saude L, Feldman B, Coutinho P, Hirst EM, Stemple DL. Zebrafish mutants identify an essential role for laminins in notochord formation. Development. 2002;129:3137–46. doi: 10.1242/dev.129.13.3137. [DOI] [PubMed] [Google Scholar]
- Rasband WS. Image J. U.S. National Institutes of Health; Bethesda, Maryland, USA: 1997–2006. [Google Scholar]
- Scott A, Stemple DL. Zebrafish notochordal basement membrane: signaling and structure. Curr Top Dev Biol. 2005;65:229–53. doi: 10.1016/S0070-2153(04)65009-5. [DOI] [PubMed] [Google Scholar]
- Stemple DL. Structure and function of the notochord: an essential organ for chordate development. Development. 2005;132:2503–12. doi: 10.1242/dev.01812. [DOI] [PubMed] [Google Scholar]
- Stemple DL, Solnica-Krezel L, Zwartkruis F, Neuhauss SC, Schier AF, Malicki J, Stainier DY, Abdelilah S, Rangini Z, Mountcastle-Shah E, Driever W. Mutations affecting development of the notochord in zebrafish. Development. 1996;123:117–28. doi: 10.1242/dev.123.1.117. [DOI] [PubMed] [Google Scholar]
- Tang SS, Trackman PC, Kagan HM. Reaction of aortic lysyl oxidase with beta-aminopropionitrile. J Biol Chem. 1983;258:4331–8. [PubMed] [Google Scholar]
- Taylor MR, Hurley JB, Van Epps HA, Brockerhoff SE. A zebrafish model for pyruvate dehydrogenase deficiency: rescue of neurological dysfunction and embryonic lethality using a ketogenic diet. Proc Natl Acad Sci U S A. 2004;101:4584–9. doi: 10.1073/pnas.0307074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teraoka H, Urakawa S, Nanba S, Nagai Y, Dong W, Imagawa T, Tanguay RL, Svoboda K, Handley-Goldstone HM, Stegeman JJ, Hiraga T. Muscular contractions in the zebrafish embryo are necessary to reveal thiuram-induced notochord distortions. Toxicol Appl Pharmacol. 2006;212:24–34. doi: 10.1016/j.taap.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Thisse C, Thisse B, Schilling TF, Postlethwait JH. Structure of the zebrafish snail1 gene and its expression in wild-type, spadetail and no tail mutant embryos. Development. 1993;119:1203–15. doi: 10.1242/dev.119.4.1203. [DOI] [PubMed] [Google Scholar]
- Tiller GE, Polumbo PA, Weis MA, Bogaert R, Lachman RS, Cohn DH, Rimoin DL, Eyre DR. Dominant mutations in the type II collagen gene, COL2A1, produce spondyloepimetaphyseal dysplasia, Strudwick type. Nat Genet. 1995;11:87–9. doi: 10.1038/ng0995-87. [DOI] [PubMed] [Google Scholar]
- Tiller GE, Rimoin DL, Murray LW, Cohn DH. Tandem duplication within a type II collagen gene (COL2A1) exon in an individual with spondyloepiphyseal dysplasia. Proc Natl Acad Sci U S A. 1990;87:3889–93. doi: 10.1073/pnas.87.10.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilton F, La Du JK, Vue M, Alzarban N, Tanguay RL. Dithiocarbamates have a common toxic effect on zebrafish body axis formation. Toxicol Appl Pharmacol. 2006;216:55–68. doi: 10.1016/j.taap.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The zebrafish book: a guide for the laboratory use of zebrafish Danio (Brachydanio) rerio. University of Oregon Press; Eugene, Or: 1993. [Google Scholar]
- Woods IG, Kelly PD, Chu F, Ngo-Hazelett P, Yan YL, Huang H, Postlethwait JH, Talbot WS. A comparative map of the zebrafish genome. Genome Res. 2000;10:1903–14. doi: 10.1101/gr.10.12.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan YL, Hatta K, Riggleman B, Postlethwait JH. Expression of a type II collagen gene in the zebrafish embryonic axis. Dev Dyn. 1995;203:363–76. doi: 10.1002/aja.1002030308. [DOI] [PubMed] [Google Scholar]
- Yeowell HN, Walker LC. Mutations in the lysyl hydroxylase 1 gene that result in enzyme deficiency and the clinical phenotype of Ehlers-Danlos syndrome type VI. Mol Genet Metab. 2000;71:212–24. doi: 10.1006/mgme.2000.3076. [DOI] [PubMed] [Google Scholar]
- Yu Q, Horak K, Larson DF. Role of T lymphocytes in hypertension-induced cardiac extracellular matrix remodeling. Hypertension. 2006;48:98–104. doi: 10.1161/01.HYP.0000227247.27111.b2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
S. Fig. 1. Comparison of the notochord distortion obtained in zebrafish using three distinct methods of lysyl oxidase inhibition at 24 hpf. Wild-type embryos were incubated in 10 μM neocuproine (A), 10 mM β-aminopropionitrile supplemented with 10 μM CuCl2 (B), 100 mM β-aminopropionitrile supplemented with 10 μM CuCl2 (C), or injected with 6 ng each of loxl1 and loxl5b start (ATG) morpholino (D). 10 μM neocuproine (A) causes greater notochord distortion than 10 mM β-aminopropionitrile (B), but an identical phenotype is obtained with 100 mM β-aminopropionitrile (A vs. C). Melanin pigmentation has not yet developed in the embryos pictured, but is not affected by β-aminopropionitrile treatment or by the morpholino injection.
S. Fig. 2. Protein sequence alignment of zebrafish lysyl oxidase family members with human orthologues. (A) lox, loxl1, loxl5a, and loxl5b; (B) loxl2a and loxl2b; (C) loxl3a and loxl3b with Xenopus laevis clone XL051k10 included for comparison. A number of conserved elements are indicated including predicted signal peptides (bold, underlined), BMP-1 cleavage sequences (bold, italicized), scavenger receptor cysteine-rich domains (gray, underlined), copper binding domains (gray), and lysyl-tyrosyl quinone cofactor residues (bold).
S. Fig. 3. Lysyl oxidase and collagen II splice morpholinos decrease the abundance of wild-type splice forms and cause specific changes in splice site usage. (A) loxl1 (B) loxl2b (C) loxl3b (D) loxl5b and (E) col2a1. Wild-type embryos injected with the indicated doses of morpholino were harvested at 20 hpf and cDNA prepared from equal amounts of RNA. The intensity of the wild-type (WT) product obtained by PCR was quantified and the percentage of wild-type splice form remaining after morpholino knockdown determined, as noted. This measure is independent of processes that may depress levels of the mutant (Mut) splice products, including degradation through nonsense-mediated decay. The primer set used to amplify loxl1 does not amplify the mutant band.
S. Fig. 4. Persistent expression of col2a1 by notochord vacuolar cells after lysyl oxidase inhibition is not due to mechanical distortion of the notochord. Wild-type embryos incubated with (B) or without (A) 2 μM neocuproine were probed for col2a1 expression at 24 hpf. col2a1 expression persists in the notochord vacuolar cells after treatment with 2 μM neocuproine, which affects crosslinking but does not cause mechanical distortion. This suggests that the signal to downregulate col2a1 expression in the vacuolar cells of the notochord originates from the products of the crosslinking reaction.
S. Table 1. Morpholinos to combinations of lysyl oxidase family members other than loxl1 and loxl5b do not result in notochord distortion. Wild-type embryos were injected with the indicated morpholino doses and examined at 24 hpf for notochord distortion. The number of embryos examined includes dead embryos that could not be scored for notochord phenotype. Each result is pooled from three independent experiments.