Abstract
Cyclic nucleotide-gated (CNG) ion channels play a central role in vision and olfaction, generating the electrical responses to light in photoreceptors and to odorants in olfactory receptors. These channels have been detected in many other tissues where their functions are largely unclear. The use of gene knockouts and other methods have yielded some information, but there is a pressing need for potent and specific pharmacological agents directed at CNG channels. To date there has been very little systematic effort in this direction - most of what can be termed CNG channel pharmacology arose from testing reagents known to target protein kinases or other ion channels, or by accident when researchers were investigating other intracellular pathways that may regulate the activity of CNG channels. Predictably, these studies have not produced selective agents. However, taking advantage of emerging structural information and the increasing knowledge of the biophysical properties of these channels, some promising compounds and strategies have begun to emerge. In this review we discuss progress on two fronts, cyclic nucleotide analogs as both activators and competitive inhibitors, and inhibitors that target the pore or gating machinery of the channel. We also discuss the potential of these compounds for treating certain forms of retinal degeneration.
INTRODUCTION
CNG channels play a key role in visual and olfactory signal transduction in retinal photoreceptor cells and olfactory receptor neurons. In these sensory neurons, CNG channels generate an electrical signal by responding to light- and odorant-induced changes in intracellular levels of cyclic nucleotides [1-3]. In retinal rod photoreceptors, the level of cGMP is relatively high in the dark, and the sustained entry of Na+ and Ca2+ ions through CNG channels maintains the cell in a partially depolarized state. When the visual pigment rhodopsin absorbs a photon, it becomes enzymatically active and catalyzes the exchange of GTP for bound GDP on many molecules of the G-protein transducin (for reviews of phototransduction see [4-16]). The GTP-bound form of transducin in turn activates a cGMP phosphodiesterase that catalyzes the hydrolysis of cGMP. As a result, CNG channels in the plasma membrane close, causing a membrane hyperpolarization that decreases the release of transmitter onto second order cells of the retina [17, 18]. Recovery of the dark state requires both shut-off of the excitation pathway and synthesis of cGMP to reopen channels. The interruption of Ca2+ influx through the channels is critical for the timing of recovery. Ca2+ continues to be extruded by a light-independent Na+/Ca2+-K+ exchanger, which causes a decrease in intracellular Ca2+. This stimulates the activation of guanylyl cyclase to resynthesize cGMP and the deactivation of rhodopsin by rhodopsin kinase. A similar pathway operates in cones, but each molecular constituent differs somewhat from its rod counterpart. Cones are much less sensitive to light, give briefer light responses, and adapt over a wider range of light intensities (see reviews cited earlier).
In contrast, the signaling cascade in olfactory sensory neurons generates responses with polarity opposite to those found in rods and cones. Stimulation of a diverse array of odorant receptors triggers an increase in intracellular cAMP via activation of Golf and adenylyl cyclase type III (reviewed in [19-23]). This rise in cAMP directly activates CNG channels leading to a depolarizing influx of Na+ and Ca2+ ions. The olfactory response is further shaped by activation of calcium-activated chloride channels. The olfactory response is terminated by receptor phosphorylation, GTP hydrolysis by Golf, and reduction of CNG channel activity by calcium-calmodulin feedback.
Significant activation of CNG channels requires the binding of three molecules of cGMP [24-30]. Thus, these channels behave as molecular amplifiers, with large changes in activity resulting from small changes in cyclic nucleotide concentration. The gating kinetics of the channel are very rapid, and do not limit the response [31, 32]. CNG channels have been embraced by biophysicists as a paradigm for the study of ligand gating and protein allostery [33-35]. They are well-suited for this purpose because they can be studied at the level of a single protein molecule, but, unlike many other ion channels, they do not inactivate or desensitize.
Native CNG channels are composed of different combinations and splice variants of six pore-forming subunits, including both α (CNGA1-4) and β (CNGB1 & 3) subunits. Although many of the α subunits can be functionally expressed as homomultimers, co-expression of the β subunits is known to confer distinct functional properties, in terms of ion permeation, ligand sensitivity, gating mechanisms, and regulation. Cloning, functional expression, and biochemical experiments have demonstrated that rod channels are composed of two types of subunits: CNGA1 and CNGB1 [36-45]. Recent evidence suggests that the stoichiometry of the native rod photoreceptor channel is 3 CNGA1 to 1 CNGB1 [46-48]. The CNG channel in cone photoreceptors is composed of CNGA3 and CNGB3; in an intriguing twist compared to rod channels, functional evidence suggests that the stoichiometry of the cone channel is 2:2 [49]. The native olfactory channel contains three subunit types, including CNGA2, CNGA4, and CNGB1.3, in a ratio of 2:1:1 [50-54]. Although they are relatively insensitive to membrane potential, CNG channels belong to the superfamily of voltage-gated channels, and both A and B subunits contain six transmembrane helices (S1-S6) and a pore (P) region between S5 and S6 [55, 56]. Each subunit contains a single cGMP-binding site near its cytoplasmic C-terminus [57, 58]. Like voltage-gated potassium channels, evidence indicates that CNG channels function as tetramers [27, 28, 30, 59-62].
CNG channels are permeable to monovalent and divalent cations, including Na+, K+, Ca2+, and Mg2+ [33]. Ca2+ and, to a lesser degree, Mg2+ occupy the pore a high percentage of the time under physiological conditions, which significantly lowers the single channel conductance [24, 25]. The relatively tight binding of Ca2+ to the pore results in the channel showing some selectivity for Ca2+ over other cations [63], and the permeation mechanism bears a qualitative resemblance to that of voltage-gated Ca2+ channels. Homomeric CNG channels are the most similar to Ca2+ channels, because they also have a ring of glutamates that coordinate Ca2+ at the equivalent pore position [64, 65]. At somewhat higher than normal extracellular Ca2+ levels, CNG channels become “pure” Ca2+ channels, albeit with lower throughput rates for Ca2+ than typical voltage-gated Ca2+ channels [63, 66]. These observations illustrate two quantitative differences: first, the affinity and selectivity of CNG channels for Ca2+ is not as high [65, 67], and second, the charge repulsion that creates high Ca2+ flux in voltage-gated Ca2+ channels is much less prominent (or perhaps nonexistent) in CNG channels. Native, heteromeric channels bind Ca2+ less well than homomeric channels [67] because the B subunits contain a glycine at the equivalent position. Although they carry a more mixed cationic current, native channels are still selective for Ca2+: 15% of the dark current in rods is carried by Ca2+ [68], even though the concentration of extracellular Ca2+ is about 1% of the Na+ concentration.
As is true for most eukaryotic ion channels, the three-dimensional structure of CNG channels is not yet known. The pore is thought to be similar in structure to that of other P-loop-containing channels. The seminal work of MacKinnon and colleagues in determining the structure of the bacterial K+ channel KcsA [69-71] has given ion channel researchers a template for understanding pores from this large family. On the other hand, there are substantial differences in permeation and gating between CNG channels and K+ channels, and thus the analogies go only so far. In addition to the different cations that pass through CNG channels, there is evidence that the main gate for ions is in the outer selectivity filter [72-76], rather than at the intracellular entrance to the pore where the gate in K+ channels is found [77-80]. (This region does widen during opening of CNG channels, but the orifice is too large to limit the flow of ions even in the closed channel [76].) Some guidance to the structure of the cyclic nucleotide binding region comes from the recent x-ray crystal structure of the C-terminal fragment of HCN2 [62], a eukaryotic hyperpolarization-activated, cyclic nucleotide-modulated channel. HCN channels are responsible for neuronal and cardiac pacemaker activity. They are primarily voltage-gated, but gating is modulated by the binding of cyclic nucleotides. The C-terminal fragment contains the cytoplasmic cyclic nucleotide binding domain (CNBD) and the C-linker that connects the binding region to the pore. The fragments assemble as tetramers with a four-fold axis of rotational symmetry. The CNBD consists of an eight-stranded antiparallel β roll flanked by α helices. Cyclic nucleotides interact with the β-roll and the C-terminal α-helix. Based on sequence similarity, CNG channels are likely to have the same basic topology in the binding region. However, much remains to be learned, not only about static structure, but also about the sequence of events that underlie channel gating.
CNG channels may be regulated by several physiologically-relevant signaling pathways. Olfactory desensitization is mediated in part by direct binding of calcium-calmodulin to the olfactory CNG channel, which causes a dramatic reduction in the channel’s apparent sensitivity to cAMP. Both the rod and cone CNG channel isoforms are also regulated by calcium-calmodulin, although the effect is less dramatic. The effects of calcium-calmodulin on olfactory and retinal CNG channels are reviewed in [81]. Several other modulators have dramatic effects on channel activation, but their relevance in vivo has yet to be definitively established. Transition metal divalent cations, such as Zn2+ and Ni2+, are known to enhance activation of the rod CNG channel, and inhibit activation of the olfactory isoform [72, 82-84]. Phosphorylation at tyrosine and/or serine residues is known to alter the apparent cyclic nucleotide affinity of the rod and olfactory CNG channels [85-87]. In addition, experiments using the tyrosine-kinase inhibitor genistein, suggest that the rod channel may also be regulated by direct interaction with a tyrosine kinase [88]. Recently, lipid modulators have been shown to regulate the activity of CNG channels. Activation of rod CNG channels is dramatically reduced by application of diacylglycerol analogs and all-trans-retinal [89, 90], while activation of olfactory CNG channels is inhibited by cholesterol depletion [91] and application of phosphatidylinositol-3,4,5-trisphosphate (PIP3) [92]. Finally, modification of C460 by nitric oxide has been reported to activate homomeric channels composed of CNGA2, independently of cyclic nucleotides [93]. Despite the plethora of natural compounds that modulate the activity of CNG channels, this review will focus on modulation of CNG channel function by non-natural pharmacological agents; for consideration of physiological regulation of CNG channel function, the reader is directed to several recent reviews on this topic [33, 81, 86, 94].
CNG channels may have functions in the retina in addition to their key role in phototransduction. CNG channels have been found to modulate transmitter release at the cone-bipolar cell synapse [95]. Perhaps Ca2+ entry through these channels can regulate transmitter release in a mostly voltage-independent fashion at other synapses as well. In this vein, CNG channels may be involved in mediating the presynaptic effects of nitric oxide [96]. Elsewhere in the retina, CNG channels have been reported in a subset of bipolar cells and ganglion cells [97-102]. However, the function, even the presence, of CNG channels in these cells has been difficult to firmly establish and remains controversial [103].
Although we know something about the molecular functions of CNG channels, how they participate in the physiology of non-sensory tissues remains almost purely speculative. In the brain, there is evidence that CNG channels are responsible for the prolonged depolarization of hippocampal CA1 neurons following muscarinic receptor activation [104], and the induction of hippocampal LTP following low-frequency, theta-burst stimulation was reduced in mice in which CNGA2 was genetically ablated [105]. CNG channels have been detected in many other tissues, including heart, kidney, testis, liver, lung, skeletal muscle, adrenal gland, pancreas, and colon [33, 35]. There is intriguing evidence that CNG channels play a part in resistance to colon cancer mediated by bacterial enterotoxins, suggesting possible treatments that would target CNG channels [106].
Until recently there has been very little systematic effort to develop potent and specific pharmacological agents directed at CNG channels.Most of what can loosely be termed CNG channel pharmacology came from testing reagents known to target protein kinases or other ion channels, or by accident (retrospectively termed serendipity) when researchers were investigating other intracellular pathways that might regulate CNG channels. Predictably, these studies have not produced selective agents. The use of gene knockout technology in mice has helped to define some of the purposes of CNG channels, but the foregoing discussion clearly points to a need for specific pharmacological agents.
CYCLIC NUCLEOTIDE DERIVATIVES – ACTIVATORS AND COMPETITIVE INHIBITORS
Modifications of the cyclic nucleotide can alter affinity, gating efficacy, and membrane permeability. However, achieving selectivity for CNG channels is a major challenge as cyclic nucleotide-dependent protein kinases are sensitive to much lower concentrations of the natural cyclic nucleotides. Moreover, the effects of cyclic nucleotide analogues on other targets such as cyclic nucleotide phosphodiesterases (PDEs), Epac, a cAMP-sensitive guanine nucleotide exchange factor, and hyperpolarization-activated, cyclic nucleotide-modulated (HCN) channels need to be evaluated. The latter two classes are relatively recent discoveries and thus have received considerably less attention as cyclic nucleotide binding proteins. While hundreds of cyclic nucleotide analogues have been synthesized over the past four decades (see [107], where many of the synthetic methods were worked out), few systematic studies of their effects on CNG channels have been carried out; the majority of studies have focused on cAMP- and cGMP-dependent protein kinases and cyclic nucleotide phosphodiesterases (reviewed in [108]). Nonetheless, several analogues have been used in studies of CNG channel function and structure, and new strategies for generating CNG-channel-selective cyclic nucleotide analogues are emerging.
Different classes of CNG channels have distinct preferences for cGMP and cAMP (Fig. 1). As CNG channels were discovered and cloned from various tissues it became clear that different combinations of the pore-forming A and B subunits respond to cAMP and cGMP differently. For instance, cAMP and cGMP are full activators of native olfactory CNG channels with similar potencies, while native rod photoreceptor CNG channels require ~10-fold higher cGMP and ~500-fold higher cAMP concentrations for half maximal activation [3, 109, 110]. Furthermore, cAMP is only a partial activator of the rod channel, eliciting 10-40% of the maximal cGMP-activated current at saturating concentrations of cAMP (depending on the species). This nucleotide preference highlights the importance of evaluating more than one class of CNG channel and suggests the additional challenge of producing cyclic nucleotide analogues that are selective for a single type of CNG channel.
Fig. (1).
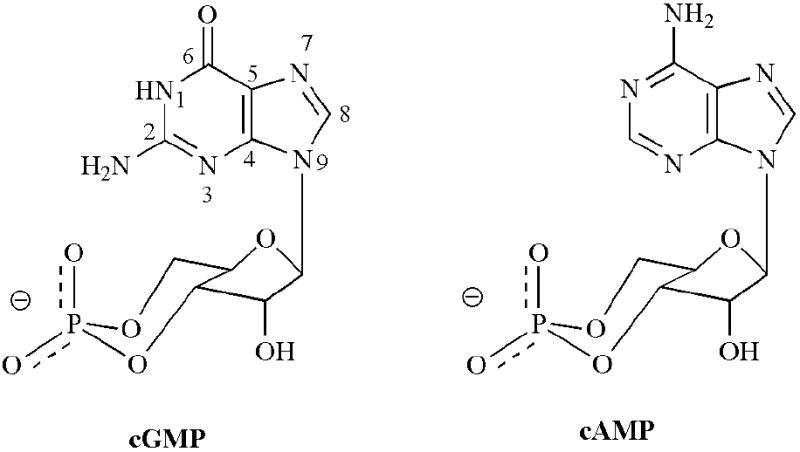
Structures of cGMP and cAMP both drawn in the syn conformation with respect to the N-glycosidic bond. Numbering of the purine ring is shown for cGMP.
An understanding of how cyclic nucleotides bind to and activate CNG channels, as well as the structural features that underlie nucleotide selectivity will be important guides in the development of CNG channel-selective analogues. Although the three-dimensional structures of vertebrate CNG channels are not yet known, the essential structural blueprint for a cyclic nucleotide-binding domain (CNBD) is conserved from bacteria to mammals, as revealed by atomic resolution structures of CNBDs from diverse proteins: a transcription factor, protein kinases, a guanine nucleotide exchange factor, a voltage-sensitive potassium channel and a bacterial potassium channel [62, 111-117]. The CNBD consists of an eight-stranded antiparallel β roll with one α helix (A) at the N-terminus of the domain and two (B and C) at the C-terminus. The cyclic nucleotide binds to a cleft on the β roll with the cyclic phosphate making an electrostatic interaction with a conserved arginine residue (R558 in bovine CNGA1). Mutation of this residue in a chimeric CNG channel results in reduced cyclic nucleotide affinity but allows full activation at saturating cyclic nucleotide concentrations [118]. The ribose binds to the β roll via hydrogen bonding interactions, while contacts between the purine ring and the β roll and are largely hydrophobic. Additional contacts with the purine ring are made by the C-helix, especially in the open state. Of particular note is aspartate 604 in bovine CNGA1, which is thought to be largely responsible for nucleotide preference by contributing two hydrogen bonds to cGMP (N1 and N2) while presenting an unfavorable interaction with the N1 position of cAMP [119]. CNGA2 and CNGA3 contain glutamate and aspartate at this position, and all three subunits show strong selectivity for cGMP over cAMP when they are expressed on their own. In contrast, CNGA4, CNGB1 and CNGB3 do not contain acidic residues here. This is thought to be the primary reason why these subunits, which do not express on their own, make native heteromeric channels more sensitive to cAMP than homomultimers formed from CNGA1-3 alone. In general, though, how cyclic nucleotide binding triggers channel opening is largely unknown. One indicator of the complexity of this process is that CNGA2 exhibits a higher probability of opening than CNGA1 with either cGMP or cAMP bound, and evidence suggests that regions spread throughout the CNGA2 subunit contribute to this [120]. Finally, there is the surprising observation that the CNBD from HCN2 binds cAMP in the anti conformation (about the N-glycosidic bond) and cGMP in the syn conformation even though cAMP and cGMP modulate activation of the channel to the same extent [62].
Ribose and Cyclic Phosphate Modifications
CNG channels are very sensitive to alterations of the ribose and the cyclic phosphate: 2’-deoxy-cGMP, 2’,3’-cGMP and 5’-GMP are unable to activate rod CNG channels [1, 109]. Furthermore, while phosphorothioate derivatives of cGMP, SP-cGMPS and RP-cGMPS (Fig. 2), are able to activate the rod CNG channel, 12- and 70-fold higher concentrations are required relative to cGMP [121]. In studies of the native olfactory CNG channel SP-cAMPS and RP-cAMPS were 4- and 75-fold less potent than cAMP, and RP-cAMPS was only a partial activator, eliciting ~35% of the maximal current at saturation [122]. This sensitivity to modification at the cyclic phosphate has been utilized to generate “caged” cyclic nucleotides, analogues with modifications to the cyclic phosphate that prevent channel activation but can be cleaved by photolysis to rapidly release cGMP [123, 124]. For example, the 4,5-dimethoxy-2-nitrobenzyl ester of cGMP was used in flash photolysis experiments to measure the gating kinetics of the CNG channel from retinal rods [32].
Fig. (2).
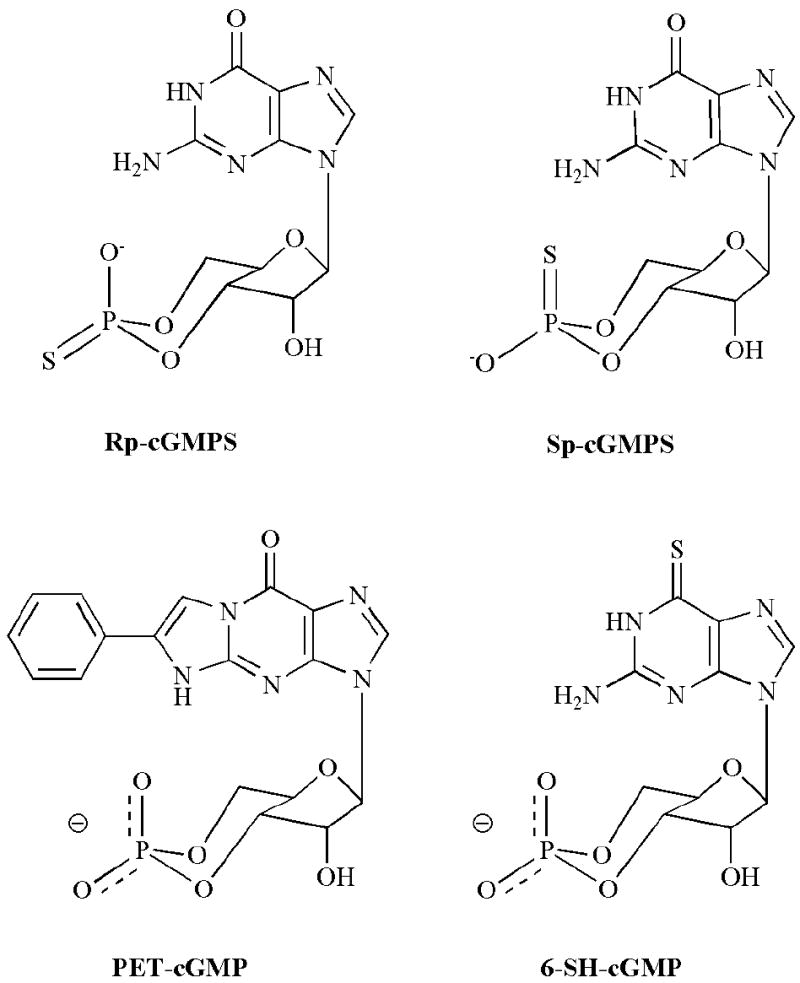
Structures of selected analogues of cGMP modified at the cyclic phosphate and purine ring.
Purine Ring Modifications
Other than at the C8 position, modifications to the purine ring system have resulted in compounds with decreased apparent affinity for CNG channels (Fig. 2). For example, 2-aminopurine 3’,5’-cyclic monophosphate that lacks the C6 oxygen of cGMP is unable to activate CNG channels from retinal rods. Substitution of the 6-oxy group with sulfur is tolerated, albeit with a 3-fold reduction in potency [109]. Modification of both the 2-amino and the nitrogen at position 1 was achieved with the synthesis of β-phenyl-1, N2-etheno-cGMP (PET-cGMP) [125]. This modification introduces significant nonpolar bulk to the purine ring, and PET-cGMP is unable to activate homomeric rat CNGA1 channels when present at 2.5 mM. However, in conjunction with modifications at other positions that rescue binding affinity, the PET modification may turn out to be useful in creating competitive antagonists (see below). PET-cGMP can potentiate channel activation by subsaturating concentrations of cGMP, an effect that is maximal at ~100 μM PET-cGMP, indicating that this analogue can bind to the channel [126].
A number of cGMP analogues with substitutions at the C8 position have been tested on CNG channels (Fig. 3). In addition to exhibiting an increased apparent affinity, these analogues have the advantage of being generally resistant to degradation by PDE [127-130], and hydrophobic substitutions can increase membrane permeability. Early studies of 8-Br-cGMP demonstrated that it is about 10-fold more potent than cGMP in activating retinal rod CNG channels [121, 131, 132]. In addition, 8-N3-cGMP, 8-(p-azidophenacylthio)-cGMP, 8-benzylthio-cGMP, and 8-Fl-cGMP [8-(5-thioacet-amidofluorescein)-cGMP)] were also reported to be potent activators, the latter two more potent than 8-Br-cGMP [57, 109, 132]. To systematically examine the effects of charge and hydrophobicity at this position on channel activation, Brown et al. synthesized a series of 8-amino- and 8-thio-substituted cGMP analogues and tested them on native retinal rod channels [133]. Results from these experiments suggest that amine (n-propylamino, carboxyethylamino, and aminoethylamino) substitutions at the C8 position alter the electronic structure of the guanine ring system, leading to lower potency. Positively charged groups several bond lengths from the guanine ring (aminoethylthio and trimethy-laminoethylthio) are also detrimental. Conversely, nonpolar (n-propylthio) or negatively charged groups at the same position (sulfoethylthio and carboxyethylthio) yield activators that exhibit similar or slightly greater potency than 8-Br-cGMP. The photoaffinity analogue 8-(p-azidophenacylthio)-[32P]cGMP labels a stretch of hydrophobic amino acids within the CNBD. This region may be responsible for the greater potency of cGMP analogues with hydrophobic substituents at the C8 position [58]. One such compound, 8-(p-chlorophenylthio)-cGMP (CPT-cGMP), has found use in several studies as a membrane-permeant activator of CNG channels that is highly resistant to hydrolysis by PDEs [91, 134]. Finally, it appears that the cyclic nucleotide-binding pocket of CNG channels is capable of accommodating very large substituents at the C8 position. This is illustrated by the ability of 8-Fl-cGMP, more than twice the mass of cGMP, to fully activate the rod photoreceptor channel, with potency greater than 8-Br-cGMP [109, 132]. Despite the potency of different C8 substituted derivatives, many of these compounds are also very potent activators of protein kinase G (PKG) [125, 135]. Thus, other modifications will likely be required to produce channel-specific agents.
Fig. (3).
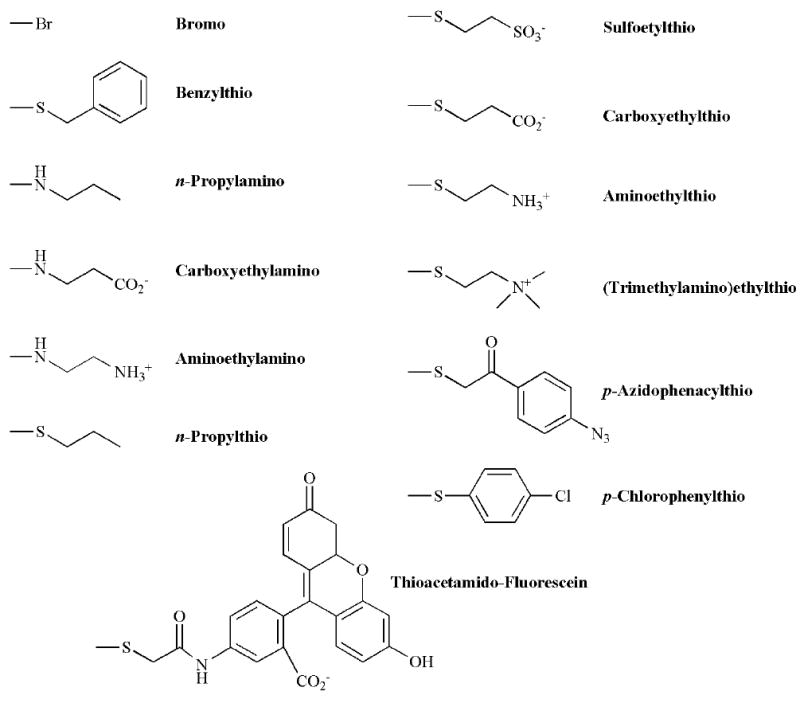
Modification of cGMP at the C8 position. Structures of selected C8 substituents.
Competitive Antagonists
As protein kinases display greater affinity for cAMP and cGMP relative to CNG channels, it seems unlikely that alterations at a single position will yield a CNG channel-selective modulator. Greater success may be achieved by modifying several positions on the nucleotide. Some progress has been made by combining phosphorothioate modification of the cyclic phosphate (either RP or SP isomers) with phenyletheno (PET) derivatization of the 2-amino and N1 positions of the guanine ring in cGMP. Specifically, SP-8-Br-PET-cGMPS was reported to be a potent activator of PKG, but a competitive inhibitor of the rod CNG channel [126]. Conversely, RP-8-Br-cGMPS activates the homomeric rod CNG channel but antagonizes activation of PKG [134]. However, these agents bind quite poorly to CNG channels, and their specificities against different isoforms and other cyclic nucleotide binding proteins remain at issue. Also, it would be desirable to have analogs that act on only one protein at a given concentration. In another study RP-8-CPT-cGMPS was found to be an effective competitive inhibitor of homomeric CNGA2 channels [122]. Further work will be required to determine if it acts similarly on native olfactory channels.
Multivalent Ligands
A novel approach for producing ligands that bind selectively and with high affinity to proteins containing multiple ligand binding sites has been employed in the synthesis of highly potent CNG channel activators. The concept is to connect two ligands with flexible polymer chains of defined average length, until a compound is found that optimally spans two binding sites on the protein of interest (Fig. 4) [136]. The largest enhancement in affinity occurs when the average length of the polymer chain matches the average distance between binding sites. These multivalent compounds are referred to as polymer-linked ligand dimers (PLDs). The enhancement in apparent affinity is due to a dramatic slowing of the off-rate. When one ligand moiety of the pair dissociates, its tendency to rebind is very high because it has a high effective concentration in a hemisphere swept out by the polymer chain (Fig. 4b). Thus, the effective off-rate of the PLD is given by the normal off-rate at a single binding site multiplied by the probability that the second site is not occupied (which is very low).
Fig. (4).
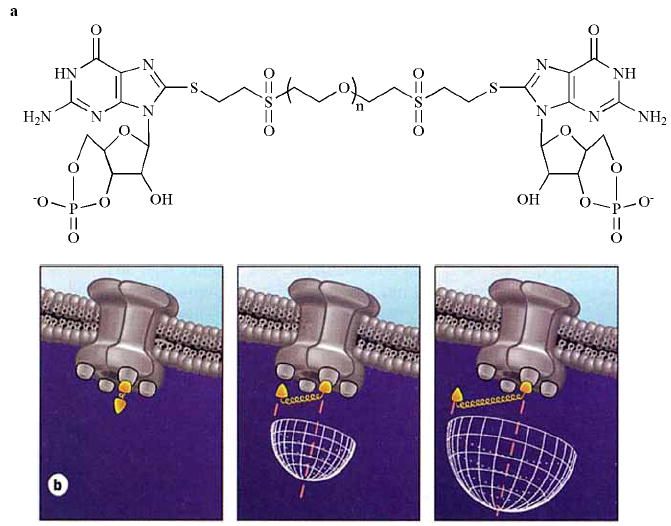
Polymer-linked ligand dimers (PLDs). a, Structure of a PLD containing two cGMP moieties and a polyethylene glycol (PEG) linker, with n ethylene glycol units. b, Diagrams of PLDs binding to a channel with four ligand-binding sites: PLDs are shown with polymers whose average length is too short (left), just right (center), or longer (right) than necessary, to allow the ligands to span two binding sites on the channel. Reproduced from ref. [136] by copyright permission of Nature Publishing Group (http://www.nature.com/).
A series of polymer-linked cGMP dimers of varying length was generated by reacting 8-SH-cGMP with vinyl sulfone-derivatized polyethylene glycol (Fig. 4a) [136]. The compounds were tested on homomeric rod and olfactory CNG channels expressed in oocytes, and on cGMP-dependent protein kinase (PKG). Fig. (5a) shows that for both channels, there was a gradual increase in apparent affinity with increasing polymer length until an optimal average length was reached. Flexible polymers like PEGs have average lengths that are proportional to the square root of the number of monomers [137]. At the optimal length (30 Å for retinal channels and 39 Å for olfactory channels) the enhancement of apparent affinity (cf. cGMP) was 250-fold for the rod channel and 800-fold for the olfactory channel. Kinetic experiments with the olfactory channel showed that the off-rate for the optimal PLD was slowed by a factor of several thousand. The PLDs were less effective in activating PKG (30-fold enhancement – Fig. 5b), most likely because one of the two cGMP-binding sites in each regulatory subunit does not bind C8-substituted cGMP derivatives as well as cGMP itself [135]. The optimal PLD was different for each target protein, showing that the PLD strategy can reveal agents with a selectivity for specific proteins, even if their binding sites are conserved. This was best illustrated by the 39 Å cGMP PLD, which was the first cyclic nucleotide derivative to activate a CNG channel (olfactory CNGA2) more potently than PKG, by a factor of about 20 (Fig. 5). There is significant promise for the development of even more potent and selective CNG channel modulators via changes to the number of ligands (cGMP trimers and tetramers), refinements to the optimal polymer length, alterations of polymer stiffness, or the incorporation of additional modifications to the cyclic nucleotide.
Fig. (5).
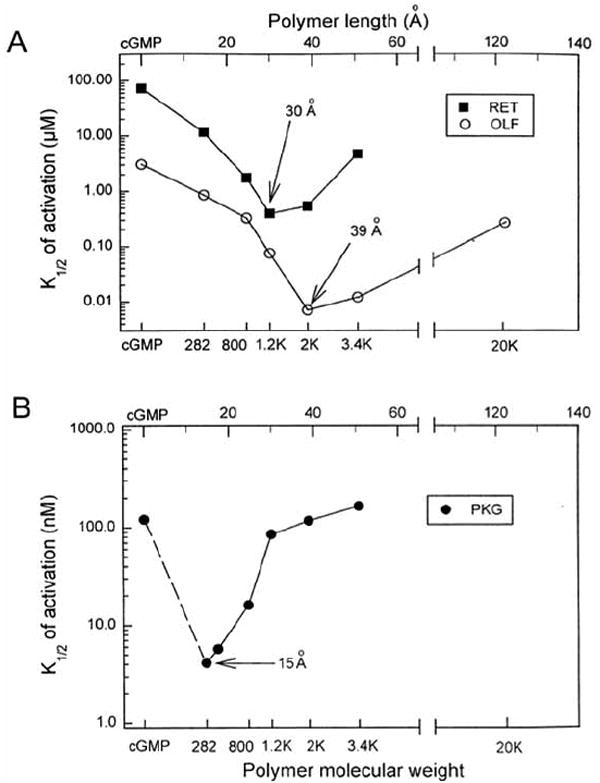
Identification of optimal polymer-linked dimers (PLDs) for cGMP-binding proteins. a, Activation of CNG channels (RET and OLF, CNGA1 and CNGA2). b, Activation of cGMP-dependent protein kinase (PKG). Arrows indicate PLDs optimal for activating each target protein. Polymer lengths were estimated from previous determinations, assuming an increase with the square root of the Mr. Reproduced from ref. [136] by copyright permission of Nature Publishing Group (http://www.nature.com/).
PORE BLOCKERS AND GATING MODIFIERS
The two CNG channel blockers that are most commonly used are l-cis-diltiazem, an optical isomer of the clinically-relevant Cardizem (an antihypertensive drug), and dichloro-benzamil, a derivative of amiloride, the well-known blocker of epithelial sodium channels (Fig. 6). l-cis-diltiazem (LCD) was first reported to block CNG channels in 1985 by Koch and Kaupp, who demonstrated inhibition of cGMP-dependent ion flux from bovine rod outer segment vesicles [131]. This compound was subsequently tested on membrane patches excised from the rod photoreceptors of the salamander, Ambystoma tigrinum. In these studies, LCD was found to inhibit the rod photoreceptor CNG channel with a K1/2 of ~ 1 μM at +30 mV, whereas the more common isomer, d-cis-diltiazem was at least an order of magnitude less effective [138]. LCD has also been shown to block the CNG channels from olfactory receptor neurons and cone photoreceptors, although the affinity for these channels is lower (~ 50 μM) [139-141]. Inhibition of the rod channel by LCD is noncompetitive and voltage-dependent, with the K1/2 increasing about 5 – 10-fold at negative voltages [141-143]. High affinity block of the rod CNG channel by LCD requires the presence of the CNGB1 subunit, as channels formed by the CNGA1 subunit are less sensitive by nearly two orders of magnitude [41]. A similar finding has also been reported for the CNG channel from cone photoreceptors [144]. Thus, LCD can be used as a diagnostic tool to assess the presence of CNG channel β subunits. Because it is relatively membrane-permeant, LCD is also effective when applied from the extracellular surface in the whole-cell recording mode [143]. Although the actual binding site for LCD remains unknown, it is thought to be within the transmembrane domain (zδ ~ 0.5 [141, 145]), and it has been suggested to inhibit channel current through effects on gating, rather than direct blockage of the pore [141]. Although commonly used as a diagnostic tool to demonstrate the presence of CNG channels, these results must be interpreted with caution, as LCD can by no means be considered a specific blocker. Although the affinity of LCD for L-type calcium channels is ~ 10-fold lower than that of d-cis-diltiazem, it is still an effective blocker at low or sub μM concentrations, comparable to those required to inhibit the rod CNG channel, and much lower than the concentrations required to inhibit the olfactory and cone isoforms [146, 147]. More recently, LCD has also been shown to have cardioprotective effects that are not related to block of calcium channels [148, 149], and it has been shown to block voltage-dependent sodium channels at μM concentrations [150]. Despite these limitations, however, LCD has been proposed for use in clinical treatment of retinal degeneration due to altered cGMP metabolism, which causes calcium overload and apoptosis, and it has been shown to be partially neuroprotective in rodent models of retinal degeneration [151].
Fig. (6).
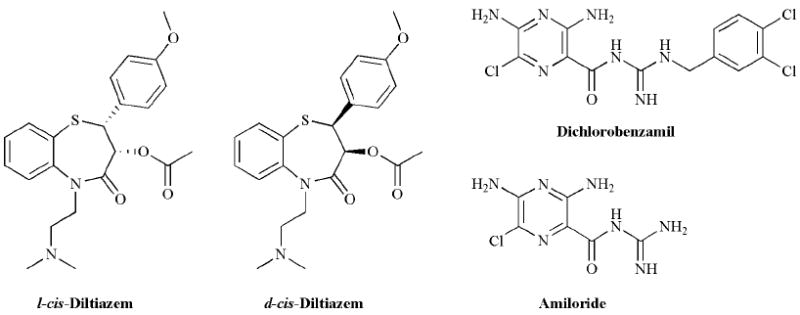
Structures of l-cis- and d-cis- diltiazem, dichlorobenzamil and amiloride.
Dichlorobenzamil (DCB), an amiloride derivative, was shown to inhibit CNG channel currents in ion-flux experiments and when applied to the cytoplasmic face of inside-out patches excised from frog rod outer segments [152] (Fig. 6). Block was half-maximal at low μM concentrations and was relatively independent of voltage. This compound is equally effective on CNG channels found in the sensory neurons of rat olfactory epithelium [139, 153], but, to our knowledge, its effectiveness on the channel isoform found in cone photoreceptors has not been reported. DCB has been used to demonstrate the presence of CNG channels in hippocampal neurons, airway epithelium, and intestine [104, 154, 155]. Once again, however, DCB cannot be considered a selective antagonist of CNG channels. Although originally developed as an inhibitor of the sodium-calcium exchanger at low μM concentrations [156], it has also been shown to inhibit voltage-gated sodium, calcium, and potassium channels in a similar concentration range [157-159].
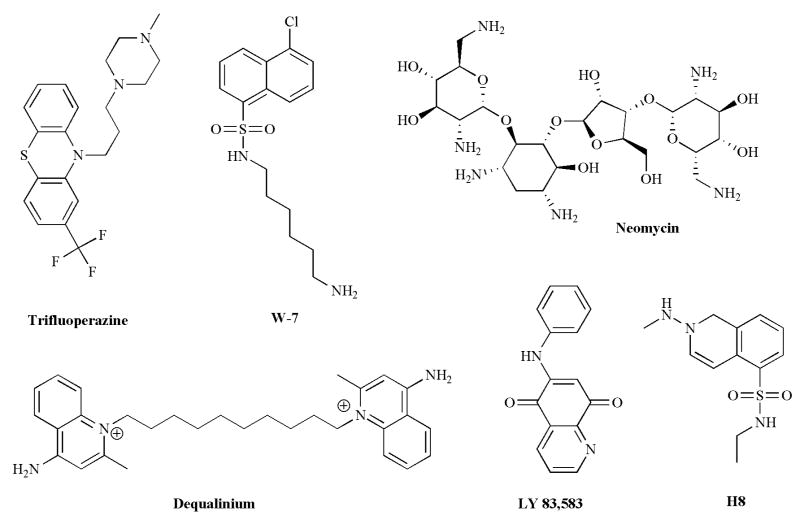
Several additional pharmacological agents known to target other ion channels have also been shown to block CNG channels (Fig. 7). Pimozide, a calcium channel blocker used as an antipsychotic drug [160] has been reported to block the CNG channel in frog rod photoreceptors when applied to inside-out patches at low μM concentrations [161], and the related compound, clopimozide, was reported to block the channel from bovine rods in ion flux experiments when applied at low μM concentrations [131]. However, attempts to reproduce the effect of pimozide in ion flux experiments and on excised inside-out patches containing the heterologously-expressed bovine rod channels (CNGA1 + CNGB1) have not been successful ([131] and Fig. 8). Amiloride, typically used at very low μM concentrations to block the epithelial sodium channel [162], has been shown to block rod and olfactory CNG channels [140, 143]. The block by amiloride is strongly voltage-dependent, with the K1/2 for the olfactory channel increasing from 17 μM at +100 mV to 400 μM at -100 mV [140]. D-600, a derivative of the L-type calcium channel blocker verapamil, has also been shown to block the olfactory CNG channel at relatively high concentrations (Ki = 200 μM) at -20 mV, but was more effective at depolarized potentials (Ki = 12 μM at +100 mV) [140]. However, other calcium channel blockers, such as nimodipine, nifedipine, and verapamil are ineffective on the rod CNG channel [131]. Nifedipine, however, has been reported to block the frog olfactory CNG channel [163]. More recently, dequalinium, a blocker of small-conductance calcium-activated potassium channels, has been reported to be a high-affinity and voltage-dependent blocker of both homomeric and heteromeric rod CNG channels [164, 165].
Fig. (7).
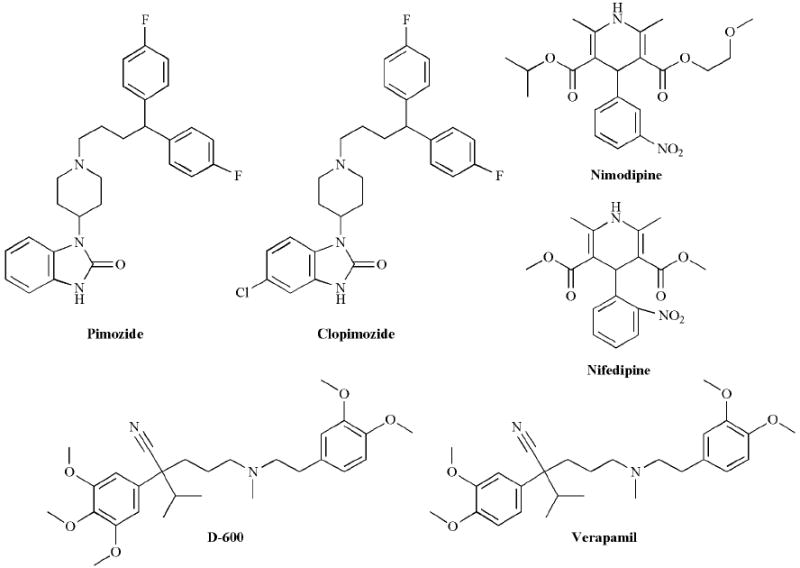
Structures of Ca2+ channel blockers that have been reported to block CNG channels.
Fig. (8).
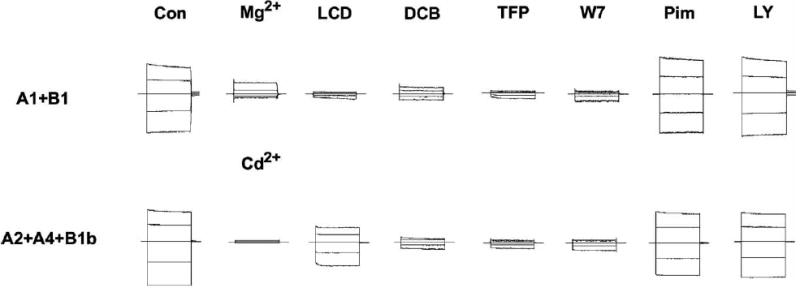
Extent of inhibition of heteromeric retinal rod and olfactory CNG channels by various pharmacological agents. Conventional whole-cell currents were recorded from HEK-293 cells expressing the rod (CNGA1 + CNGB1) or olfactory (CNGA2 + CNGA4 + CNGB1b) channels. Expression of all subunits was confirmed by measuring cyclic nucleotide dose-response relations and LCD affinity on excised inside-out patches. Currents were elicited by 250 ms voltage steps from a holding potential of 0 mV to potentials ranging from -50 to +50 mV in 25 mV increments. Control (Con) currents in the absence of inhibitor for both heteromeric channels ranged from 1-5 nA at -50 mV. Because the effects of many of the agents were only partially reversible, each set of traces was obtained from a different cell and normalized to a control trace taken at -50 mV on the same cell. Abbreviations for inhibitors not defined in the text are: TFP, trifluoperazine; Pim, Pimozide; and LY, LY83,583. External Mg2+ and Cd2+ were applied at 10 mM and 3 mM respectively; all other pharmacological agents were present at 50 μM. The currents shown in the presence of inhibitors were obtained after steady-state block was achieved, usually several minutes after extracellular application. Pimozide and LY83,583 are known to be membrane-permeant on the timescale of this experiment (~10 min), yet no channel block was detected (see text). The extracellular solution contained 140 mM NaCl, 5 mM KCl, 0.5 mM EDTA, and 10 mM Hepes, pH 7.4; the internal solution contained 140 mM KCl, 5 mM NaCl, 0.5 mM EDTA, 1 mM MgCl2, 10 mM Hepes, pH 7.4, and 1 mM cGMP.
Finally, tetracaine (compound 1, Table 1), a local anesthetic that blocks both sodium and calcium channels, has been shown to block rod CNG channels at micromolar concentrations [142, 166, 167]. (Mutagenesis studies of sodium channels have suggested a bimodal interaction of local anesthetics with the pore; an electrostatic interaction with negatively charged residues in the selectivity filter and a hydrophobic interaction with aromatic residues on the pore-lining helix [168-172].) Further work on homomeric retinal (CNGA1) and olfactory (CNGA2) channels established that block by tetracaine is voltage- and state-dependent. The apparent KD for block of open channels is 170 μM in contrast to 220 nM for closed channels [173]. Evidence was also presented that tetracaine interacts with the pore glutamate residues [73]. The very strong preference of tetracaine for closed channels may have to do with the precise arrangement of glutamate residues in the closed configuration. For a closed state blocker the efficiency of block increases with the fraction of closed channels. This can be a desirable property in a therapeutic setting for channels like CNG channels that are thought to spend most of their time in the closed state. More recently, a multiply charged tetracaine derivative that blocks CNG channels at subnanomolar concentrations was reported (compound 4, Table 1) [174]. Like tetracaine this compound binds preferentially to closed channels. The extra charges increase the apparent affinity for the pore, and also confer a steep voltage dependence of block. Compound 4 is selective for CNG channels over brain Na+ channels. The highly charged moiety likely interacts more strongly with the selectivity filter of rod CNG channels, which contain three (heteromeric channel) to four (homomeric channel) glutamates, than the selectivity filter of Na+ channels, which contains aspartate, glutamate, and lysine [175]. Several additional tetracaine derivatives were synthesized to examine the effects of increased positive charge and alterations to the hydrophobic character of tetracaine for blockade of homomeric CNGA1 (Table 1) [176]. The results show that the addition of one or two positively charged groups to the tertiary amine end of tetracaine results in dramatic increases in potency and voltage-dependence of block. In contrast, addition of a positively charged amine to the hydrophobic butyl tail of tetracaine is deleterious to block. A derivative with a polar N-acetyl group at the butyl tail or a derivative lacking the butyl tail are both very poor blockers, further reinforcing the importance of hydrophobic character at this end. Among this series of tetracaine derivatives the apparent KD for block varies over nearly eight orders of magnitude, which provides a fair amount of guidance for how to produce blockers that may be more specific for CNG channels.
Table 1.
Tetracaine Analogue Structures and Block of CNGA1 Channels
| Compound | KD(40) (μM) | zδ | |
|---|---|---|---|
| 1 |
|
6.8 | 0.47 |
| 2 |
|
3.1 | 0.45 |
| 3 |
|
0.074 | 1.8 |
| 4 |
|
0.00003 | 2.6 |
| 5 |
|
210 | 1.1 |
| 6 |
|
2500 | 0.47 |
| 7 |
|
2300 | 0.91 |
KD(40): The apparent dissociation constant at +40 mV. zδ: the charge carried by a blocker (z) multiplied by the fraction of the electric field (δ) traversed to reach its binding site. Each structure shown is the predominant form at neutral pH.
A second category of CNG channel blockers includes a number of calmodulin antagonists (Fig. 9). Undoubtedly, many of the CNG channel blockers in this category were discovered during experiments designed to elucidate the mechanism and physiological role of calcium-calmodulin (Ca-CaM) regulation in olfactory transduction. Binding of the Ca-CaM complex is known to reduce the cyclic nucleotide affinity of all three isoforms of CNG channels [177-179]. Although the physiological significance of this regulation is unclear for rod and cone channels [180, 181], binding of Ca-CaM to the native olfactory channel causes a twentyfold increase in the K1/2 for cAMP, which is thought to underlie olfactory desensitization [81, 178]. Kleene [182] reported that application of the calmodulin antagonists, trifluoperazine and W-7 (N-(6-aminohexyl)-5-chloro-1-naphthalene-sulfonamide hydrochloride), reduced the cAMP-dependent current when applied at low μM concentrations to the cytoplasmic face of membrane patches excised from frog olfactory neurons. Thus, although they can discriminate between the cAMP-activated current and the Ca2+-activated chloride current in olfactory neurons, these reagents will be of little use for investigating the Ca-CaM regulation of olfactory transduction. Furthermore, trifluoperazine and W-7 have been reported to inhibit other ion channels, including calcium channels [183], sodium channels [184], potassium channels [185], and chloride channels [186], at similar concentrations, so they certainly cannot be regarded as specific blockers of CNG channels.
Fig. (9).
Structures of compounds with diverse activities: Ca2+/CaM antagonists, trifluoperazine and W-7; an antibiotic, neomycin; an SK K+ channel blocker, dequalinium; a guanylyl cyclase inhibitor, LY83,583; a PKA inhibitor, H-8. All of these compounds have been reported to block CNG channels.
Pharmacological agents targeting other signaling pathways have also been shown to block CNG channels (Fig. 9). An inhibitor of soluble guanylyl cyclase, LY83,583, has been reported to block olfactory CNG channels in salamander [187], but this compound is ineffective on rat ([188] and Fig. 8) and zebrafish channels [189]. LY83,583 is also ineffective at blocking heteromeric rod channels (Fig. 8), suggesting caution in extrapolating blocking efficacy across both species and channel isoforms. Reagents targeting Ca2+-signaling pathways have also been reported to block CNG channels. Ruthenium red, a well-known blocker of the ryanodine receptor, has been shown to be a high-affinity, but voltage-dependent, blocker of the zebrafish olfactory CNG channel [189]. At -60 mV, ruthenium red was shown to block cAMP-activated currents at sub-μM concentrations, but the efficacy was reduced by over two orders of magnitude at +60 mV. Neomycin, an aminoglycoside antibiotic known to inhibit the activity of phospholipase C, blocks the zebrafish olfactory CNG channel at 100 – 200 μM [189]. Finally, H-8, an inhibitor of the cAMP-dependent protein kinase, has also been shown to directly inhibit the rod CNG channel at mM concentrations [190].
The information above has important consequences for all of ion channel pharmacology. When a blocker that is widely used on a well-known channel is found to block a lesser-known class of channels such as CNG channels, its utility plummets. Thus, many cherished pharmacological agents will have to be reevaluated.
In an attempt to discover more specific, high-affinity blockers for CNG channels, Brown and colleagues screened a variety of snake, spider, and scorpions venoms for peptide toxins that would inhibit CNG channels when applied to the extracellular face of the channel. In 1999, these efforts resulted in the purification of pseudechetoxin (PsTx) from the venom of the Australian King Brown snake (Pseudechis australis) [191]. This 25 kDa toxin is a member of the family of cysteine-rich secretory proteins (CRISP), which are widespread in the venoms of elapid snakes [192]. Other members of this family are thought to inhibit L-type calcium channels [193] and large conductance calcium-activated potassium channels [194]. When applied to the extracellular face of HEK-293 cells expressing rat CNGA2, PsTx blocked currents at low nM concentrations. It was slightly less effective on CNGA1 channels, and almost totally ineffective on the cone channel isoform, CNGA3. PsTx is also largely ineffective on heteromeric CNG channels containing CNGB1 or CNGB3 subunits [195], reducing its utility in native preparations. PsTx has been shown to inhibit channel current by forming high-affinity contacts with the extracellular pore turret, thereby occluding the entrance to the transmembrane pore [195]. A comparision of PsTx with the homolog, pseudecin, which is 97% identical but blocks CNGA2 with a 20-fold lower affinity, led to the suggestion that the cysteine-rich carboxy terminus might be the site of channel interaction [192]. Recently, the crystal structure has been solved for stecrisp, triflin, and natrin, other members of the CRISP family [194, 196, 197]. The structure reveals that the cysteine-rich carboxy terminus forms a separate domain, which is similar in structure to sea anemone toxins, BgK and ShK, that target potassium channels. If this domain from PsTx can be expressed as a functional inhibitor, it will facilitate further development of PsTx as a tool to study the structure and function of CNG channels.
TARGETING CNG CHANNELS IN RETINAL DISEASE
The involvement of CNG channels in retinal disease can be divided into two categories. In the first category mutations in rod and cone CNG channel subunits impair vision by causing improper functioning or trafficking of the channels. In the second category mutations cause defects in cGMP metabolism, and CNG channels are important in the pathophysiology of the diseases. Regarding the first category, mutations in either the CNGA1 [198-200] or CNGB1 [201] subunits of rods cause autosomal recessive forms of retinitis pigmentosa, a heterogeneous group of diseases that cause progressive degeneration of rod and cone photoreceptors, and ultimately blindness (see also [202]). The known mutants either lack major channel domains, or are functional but mostly fail to reach the cell surface [198, 203, 204]. Mutations in either the CNGA3 [205-207] or CNGB3 [208-210] subunits of cones cause complete achromatopsia, also known as total colorblindness or rod monochromacy. This is an autosomal recessive disorder characterized by a complete inability to discriminate between colors and a severe loss of visual acuity. Regarding the second category, a number of forms of retinitis pigmentosa affect phototransduction and cGMP metabolism [211, 212]. Mutations that result in increased cGMP levels, such as mutations in the cGMP phosphodiesterase of rods or in guanylyl cyclase activating protein 1, cause a massive influx of Na+ and Ca2+ through CNG channels [211, 213, 214]. This leads to metabolic overload, as well as direct toxicity and apparent activation of the apoptotic pathway [215]. (Interestingly, some of the disease-associated mutations in the cone channel subunits CNGB3 [216, 217] and CNGA3 [218] have been shown recently to cause a gain of channel function, which would result in the same problems as a rise in cGMP.) An important experimental model for the phosphodiesterase defect has been the rd mutation in mice [219, 220]. Six years ago it was reported that the Ca2+ channel blocker d-cis-diltiazem can rescue photoreceptors and preserve visual function in the rd mouse [221]. Unlike its optical isomer, l-cis-diltiazem, this compound exhibits little or no interaction with rod CNG channels [138]. The hypothesis for why it was able to rescue photoreceptors is that the high cGMP levels in rd rods and consequent depolarization caused by CNG channels leads to the activation of voltage-gated Ca2+ channels and excessive Ca2+ entry. However, this conclusion has been called into question in two more recent studies [222, 223], where no beneficial effects of d-cis-diltiazem were observed. Even if protective effects are seen under some circumstances, Ca2+ channel blockers will have many other effects on retinal function. It has been noted in a number of reviews that an attractive treatment for the disorders involving high cGMP levels would be a specific and slowly reversible blocker of CNG channels [224]. Although l-cis-diltiazem was found to be partially neuroprotective in mouse studies [151], CNG channel blockers that are more potent, specific, and membrane-permeant would likely be extremely useful for treating these blinding diseases.
HYPERPOLARIZATION-ACTIVATED, CYCLIC NUCLEOTIDE-MODULATED CHANNELS
Another physiologically important class of ion channels that is modulated by the direct binding of cyclic nucleotides is the hyperpolarization-activated, cyclic nucleotide-modulated (HCN) channels [225]. These channels are formed from the products of four known genes termed HCN1-HCN4. Two of these members (HCN2 and HCN4) are exquisitely sensitive to intracellular levels of cyclic nucleotides, which enhance their activity. Structurally and functionally, HCN channels can be described as a chimera between a voltage-activated potassium channel (albeit with a reversed polarity and reduced potassium selectivity) and a cyclic nucleotide-gated ion channel. Each subunit of an HCN channel contains six putative transmembrane helices and a reentrant loop forming a cation-selective pore that permits the flow of both potassium and sodium ions. The carboxy-terminal region of the channel contains a cyclic nucleotide-binding domain, which regulates the voltage-dependence of channel activation. The four isoforms of HCN channels vary in terms of voltage-dependence, gating kinetics, sensitivity to cAMP, and tissue localization.
HCN channels are activated by membrane hyperpolarization, giving rise to the depolarizing Ih current, which plays a key role in the generation of rhythmic activity in a variety of excitable tissues, including the heart and brain. This current is best known for its role as the “pacemaker” channel in the sinoatrial (SA) node of the heart. In cells of the SA node, HCN channels are activated by the hyperpolarization following the cardiac action potential. The inward mixed-cation current carried by these channels slowly depolarizes the cell until threshold is reached, and another action potential is initiated. These channels are also thought to play a role in generation of rhythmic activity in the brain, taste transduction, and synaptic plasticity. Finally, HCN channels have been implicated in the pathophysiology of neuropathic pain [226].
Further efforts to characterize the functions of HCN channels in general, and individual isoforms in particular, have been hampered by a striking lack of selective pharmacological tools. Ih is often distinguished by substantial block by extracellular Cs+ in the low mM range, and insensitivity to 2 mM intracellular Ba2+. However, several other K+ channels are also blocked by Cs+ in this concentration range. The best-known blocker of HCN channels is the Zeneca compound, ZD7288 [227]. A Ki of 41 μM has been reported for block of mouse HCN1, although this may be an underestimate of the true affinity due to a slow phase of block that did not reach steady state [228]. Block of HCN channels by ZD7288 has a complex dependence on voltage and exhibits an unexpectedly high zδ between 3 and 5. Although block of Ih by ZD7288 was originally reported to not be state-dependent, block is now thought to require prior activation of the channel, and ZD7288 can be trapped in the pore by channel closure [228]. It seems likely that some of the discrepancies in the literature arose from differences in the experimental protocols. Although this blocker is relatively effective, it does not select between HCN isoforms, and it has also been shown to inhibit currents through AMPA and NMDA receptors [229], as well as T-type calcium channels [230], calling its specificity into question. Two other organic blockers, zatebradine (UL-FS49) [231, 232] and ivabradine (S-16257) [233] have also been used to block HCN channels.
Acknowledgments
The work in the authors’ laboratories was supported by grants from the National Eye Institute (EY009275 to J.W.K. and EY012837 to R.L.B.).
References
References 234-236 are related articles recently published in Current Pharmaceutical Design.
- 1.Fesenko EE, Kolesnikov SS, Lyubarsky AL. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 1985;313:310–3. doi: 10.1038/313310a0. [DOI] [PubMed] [Google Scholar]
- 2.Yau KW, Baylor DA. Cyclic GMP-activated conductance of retinal photoreceptor cells. Annu Rev Neurosci. 1989;12:289–327. doi: 10.1146/annurev.ne.12.030189.001445. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura T, Gold GH. A cyclic nucleotide-gated conductance in olfactory receptor cilia. Nature. 1987;325:442–4. doi: 10.1038/325442a0. [DOI] [PubMed] [Google Scholar]
- 4.Molday RS. Photoreceptor membrane proteins, phototransduction, and retinal degenerative diseases. The Friedenwald Lecture. Invest Ophthalmol Vis Sci. 1998;39:2491–513. [PubMed] [Google Scholar]
- 5.Burns ME, Baylor DA. Activation, deactivation, and adaptation in vertebrate photoreceptor cells. Annu Rev Neurosci. 2001;24:779–805. doi: 10.1146/annurev.neuro.24.1.779. [DOI] [PubMed] [Google Scholar]
- 6.Ebrey T, Koutalos Y. Vertebrate photoreceptors. Prog Retin Eye Res. 2001;20:49–94. doi: 10.1016/s1350-9462(00)00014-8. [DOI] [PubMed] [Google Scholar]
- 7.Fain GL, Matthews HR, Cornwall MC, Koutalos Y. Adaptation in vertebrate photoreceptors. Physiol Rev. 2001;81:117–51. doi: 10.1152/physrev.2001.81.1.117. [DOI] [PubMed] [Google Scholar]
- 8.Hurley JB, Chen J. Evaluation of the contributions of recoverin and GCAPs to rod photoreceptor light adaptation and recovery to the dark state. Prog Brain Res. 2001;131:395–405. doi: 10.1016/s0079-6123(01)31032-4. [DOI] [PubMed] [Google Scholar]
- 9.Arshavsky VY, Lamb TD, Pugh EN., Jr G proteins and phototransduction. Annu Rev Physiol. 2002;64:153–87. doi: 10.1146/annurev.physiol.64.082701.102229. [DOI] [PubMed] [Google Scholar]
- 10.Calvert PD, Makino CL. The time course of light adaptation in vertebrate retinal rods. Adv Exp Med Biol. 2002;514:37–60. doi: 10.1007/978-1-4615-0121-3_3. [DOI] [PubMed] [Google Scholar]
- 11.Koch KW, Duda T, Sharma RK. Photoreceptor specific guanylate cyclases in vertebrate phototransduction. Mol Cell Biochem. 2002;230:97–106. [PubMed] [Google Scholar]
- 12.Korenbrot JI, Rebrik TI. Tuning outer segment Ca2+ homeostasis to phototransduction in rods and cones. Adv Exp Med Biol. 2002;514:179–203. doi: 10.1007/978-1-4615-0121-3_11. [DOI] [PubMed] [Google Scholar]
- 13.Nakatani K, Chen C, Yau KW, Koutalos Y. Calcium and phototransduction. Adv Exp Med Biol. 2002;514:1–20. doi: 10.1007/978-1-4615-0121-3_1. [DOI] [PubMed] [Google Scholar]
- 14.Senin II, Koch KW, Akhtar M, Philippov PP. Ca2+-dependent control of rhodopsin phosphorylation: recoverin and rhodopsin kinase. Adv Exp Med Biol. 2002;514:69–99. doi: 10.1007/978-1-4615-0121-3_5. [DOI] [PubMed] [Google Scholar]
- 15.Maeda T, Imanishi Y, Palczewski K. Rhodopsin phosphorylation: 30 years later. Prog Retin Eye Res. 2003;22:417–34. doi: 10.1016/s1350-9462(03)00017-x. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, Cote RH. cGMP signaling in vertebrate retinal photoreceptor cells. Front Biosci. 2005;10:1191–204. doi: 10.2741/1612. [DOI] [PubMed] [Google Scholar]
- 17.Dowling JE, Ripps H. Effect of magnesium on horizontal cell activity in the skate retina. Nature. 1973;242:101–3. doi: 10.1038/242101a0. [DOI] [PubMed] [Google Scholar]
- 18.Baylor DA, Fettiplace R. Transmission from photoreceptors to ganglion cells in turtle retina. J Physiol. 1977;271:391–424. doi: 10.1113/jphysiol.1977.sp012006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Firestein S. A nobel nose: the 2004 Nobel Prize in Physiology and Medicine. Neuron. 2005;45:333–8. doi: 10.1016/j.neuron.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 20.Mombaerts P. Genes and ligands for odorant, vomeronasal and taste receptors. Nat Rev Neurosci. 2004;5:263–78. doi: 10.1038/nrn1365. [DOI] [PubMed] [Google Scholar]
- 21.Gaillard I, Rouquier S, Giorgi D. Olfactory receptors. Cell Mol Life Sci. 2004;61:456–69. doi: 10.1007/s00018-003-3273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matthews HR, Reisert J. Calcium, the two-faced messenger of olfactory transduction and adaptation. Curr Opin Neurobiol. 2003;13:469–75. doi: 10.1016/s0959-4388(03)00097-7. [DOI] [PubMed] [Google Scholar]
- 23.Breer H. Olfactory receptors: molecular basis for recognition and discrimination of odors. Anal Bioanal Chem. 2003;377:427–33. doi: 10.1007/s00216-003-2113-9. [DOI] [PubMed] [Google Scholar]
- 24.Haynes LW, Kay AR, Yau KW. Single cyclic GMP-activated channel activity in excised patches of rod outer segment membrane. Nature. 1986;321:66–70. doi: 10.1038/321066a0. [DOI] [PubMed] [Google Scholar]
- 25.Zimmerman AL, Baylor DA. Cyclic GMP-sensitive conductance of retinal rods consists of aqueous pores. Nature. 1986;321:70–2. doi: 10.1038/321070a0. [DOI] [PubMed] [Google Scholar]
- 26.Matthews G. In: Ion Channel Pharmacology. Soria B, Cena V, editors. Oxford University Press; New York: 1998. pp. 383–96. [Google Scholar]
- 27.Ruiz M, Karpen JW. Single cyclic nucleotide-gated channels locked in different ligand-bound states. Nature. 1997;389:389–92. doi: 10.1038/38744. [DOI] [PubMed] [Google Scholar]
- 28.Liu DT, Tibbs GR, Paoletti P, Siegelbaum SA. Constraining ligand-binding site stoichiometry suggests that a cyclic nucleotide-gated channel is composed of two functional dimers. Neuron. 1998;21:235–48. doi: 10.1016/s0896-6273(00)80530-9. [DOI] [PubMed] [Google Scholar]
- 29.Ruiz M, Brown RL, He Y, Haley TL, Karpen JW. The single-channel dose-response relation is consistently steep for rod cyclic nucleotide-gated channels: implications for the interpretation of macroscopic dose-response relations. Biochemistry. 1999;38:10642–8. doi: 10.1021/bi990532w. [DOI] [PubMed] [Google Scholar]
- 30.Ruiz M, Karpen JW. Opening mechanism of a cyclic nucleotide-gated channel based on analysis of single channels locked in each liganded state. J Gen Physiol. 1999;113:873–95. doi: 10.1085/jgp.113.6.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cobbs WH, Pugh EN., Jr Kinetics and components of the flash photocurrent of isolated retinal rods of the larval salamander, Ambystoma tigrinum. J Physiol. 1987;394:529–72. doi: 10.1113/jphysiol.1987.sp016884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karpen JW, Zimmerman AL, Stryer L, Baylor DA. Gating kinetics of the cyclic-GMP-activated channel of retinal rods: flash photolysis and voltage-jump studies. Proc Natl Acad Sci USA. 1988;85:1287–91. doi: 10.1073/pnas.85.4.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaupp UB, Seifert R. Cyclic nucleotide-gated ion channels. Physiol Rev. 2002;82:769–824. doi: 10.1152/physrev.00008.2002. [DOI] [PubMed] [Google Scholar]
- 34.Karpen JW, Ruiz M. Ion channels: does each subunit do something on its own? Trends Biochem Sci. 2002;27:402–9. doi: 10.1016/s0968-0004(02)02124-2. [DOI] [PubMed] [Google Scholar]
- 35.Matulef K, Zagotta WN. Cyclic nucleotide-gated ion channels. Annu Rev Cell Dev Biol. 2003;19:23–44. doi: 10.1146/annurev.cellbio.19.110701.154854. [DOI] [PubMed] [Google Scholar]
- 36.Cook NJ, Hanke W, Kaupp UB. Identification, purification, and functional reconstitution of the cyclic GMP-dependent channel from rod photoreceptors. Proc Natl Acad Sci USA. 1987;84:585–9. doi: 10.1073/pnas.84.2.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaupp UB, Niidome T, Tanabe T, Terada S, Bonigk W, Stuhmer W, et al. Primary structure and functional expression from complementary DNA of the rod photoreceptor cyclic GMP-gated channel. Nature. 1989;342:762–6. doi: 10.1038/342762a0. [DOI] [PubMed] [Google Scholar]
- 38.Cook NJ, Molday LL, Reid D, Kaupp UB, Molday RS. The cGMP-gated channel of bovine rod photoreceptors is localized exclusively in the plasma membrane. J Biol Chem. 1989;264:6996–9. [PubMed] [Google Scholar]
- 39.Molday LL, Cook NJ, Kaupp UB, Molday RS. The cGMP-gated cation channel of bovine rod photoreceptor cells is associated with a 240-kDa protein exhibiting immunochemical cross-reactivity with spectrin. J Biol Chem. 1990;265:18690–5. [PubMed] [Google Scholar]
- 40.Molday RS, Molday LL, Dose A, Clark-Lewis I, Illing M, Cook NJ, et al. The cGMP-gated channel of the rod photoreceptor cell characterization and orientation of the amino terminus. J Biol Chem. 1991;266:21917–22. [PubMed] [Google Scholar]
- 41.Chen TY, Peng YW, Dhallan RS, Ahamed B, Reed RR, Yau KW. A new subunit of the cyclic nucleotide-gated cation channel in retinal rods. Nature. 1993;362:764–7. doi: 10.1038/362764a0. [DOI] [PubMed] [Google Scholar]
- 42.Korschen HG, Illing M, Seifert R, Sesti F, Williams A, Gotzes S, et al. A 240 kDa protein represents the complete β subunit of the cyclic nucleotide-gated channel from rod photoreceptor. Neuron. 1995;15:627–36. doi: 10.1016/0896-6273(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 43.Colville CA, Molday RS. Primary structure and expression of the human β-subunit and related proteins of the rod photoreceptor cGMP-gated channel. J Biol Chem. 1996;271:32968–74. doi: 10.1074/jbc.271.51.32968. [DOI] [PubMed] [Google Scholar]
- 44.Ardell MD, Aragon I, Oliveira L, Porche GE, Burke E, Pittler SJ. The β subunit of human rod photoreceptor cGMP-gated cation channel is generated from a complex transcription unit. FEBS Lett. 1996;389:213–8. doi: 10.1016/0014-5793(96)00588-1. [DOI] [PubMed] [Google Scholar]
- 45.Ardell MD, Bedsole DL, Schoborg RV, Pittler SJ. Genomic organization of the human rod photoreceptor cGMP-gated cation channel β-subunit gene. Gene. 2000;245:311–8. doi: 10.1016/s0378-1119(00)00023-8. [DOI] [PubMed] [Google Scholar]
- 46.Weitz D, Ficek N, Kremmer E, Bauer PJ, Kaupp UB. Subunit stoichiometry of the CNG channel of rod photoreceptors. Neuron. 2002;36:881–9. doi: 10.1016/s0896-6273(02)01098-x. [DOI] [PubMed] [Google Scholar]
- 47.Zheng J, Trudeau MC, Zagotta WN. Rod cyclic nucleotide-gated channels have a stoichiometry of three CNGA1 subunits and one CNGB1 subunit. Neuron. 2002;36:891–6. doi: 10.1016/s0896-6273(02)01099-1. [DOI] [PubMed] [Google Scholar]
- 48.Zhong H, Molday LL, Molday RS, Yau KW. The heteromeric cyclic nucleotide-gated channel adopts a 3A:1B stoichiometry. Nature. 2002;420:193–8. doi: 10.1038/nature01201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peng C, Rich ED, Varnum MD. Subunit configuration of heteromeric cone cyclic nucleotide-gated channels. Neuron. 2004;42:401–10. doi: 10.1016/s0896-6273(04)00225-9. [DOI] [PubMed] [Google Scholar]
- 50.Bradley J, Li J, Davidson N, Lester HA, Zinn K. Heteromeric olfactory cyclic nucleotide-gated channels: a subunit that confers increased sensitivity to cAMP. Proc Natl Acad Sci USA. 1994;91:8890–4. doi: 10.1073/pnas.91.19.8890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liman ER, Buck LB. A second subunit of the olfactory cyclic nucleotide-gated channel confers high sensitivity to cAMP. Neuron. 1994;13:611–21. doi: 10.1016/0896-6273(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 52.Sautter A, Zong X, Hofmann F, Biel M. An isoform of the rod photoreceptor cyclic nucleotide-gated channel beta subunit expressed in olfactory neurons. Proc Natl Acad Sci USA. 1998;95:4696–701. doi: 10.1073/pnas.95.8.4696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bonigk W, Bradley J, Muller F, Sesti F, Boekhoff I, Ronnett GV, et al. The native rat olfactory cyclic nucleotide-gated channel is composed of three distinct subunits. J Neurosci. 1999;19:5332–47. doi: 10.1523/JNEUROSCI.19-13-05332.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng J, Zagotta WN. Stoichiometry and assembly of olfactory cyclic nucleotide-gated channels. Neuron. 2004;42:411–21. doi: 10.1016/s0896-6273(04)00253-3. [DOI] [PubMed] [Google Scholar]
- 55.Heginbotham L, Abramson T, MacKinnon R. A functional connection between the pores of distantly related ion channels as revealed by mutant K+ channels. Science. 1992;258:1152–5. doi: 10.1126/science.1279807. [DOI] [PubMed] [Google Scholar]
- 56.Henn DK, Baumann A, Kaupp UB. Probing the transmembrane topology of cyclic nucleotide-gated ion channels with a gene fusion approach. Proc Natl Acad Sci USA. 1995;92:7425–9. doi: 10.1073/pnas.92.16.7425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brown RL, Gerber WV, Karpen JW. Specific labeling and permanent activation of the retinal rod cGMP-activated channel by the photoaffinity analog 8-p-azidophenacylthio-cGMP. Proc Natl Acad Sci USA. 1993;90:5369–73. doi: 10.1073/pnas.90.11.5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brown RL, Gramling R, Bert RJ, Karpen JW. Cyclic GMP contact points within the 63-kDa subunit and a 240-kDa associated protein of retinal rod cGMP-activated channels. Biochemistry. 1995;34:8365–70. doi: 10.1021/bi00026a018. [DOI] [PubMed] [Google Scholar]
- 59.Gordon SE, Zagotta WN. Subunit interactions in coordination of Ni2+ in cyclic nucleotide-gated channels. Proc Natl Acad Sci USA. 1995;92:10222–6. doi: 10.1073/pnas.92.22.10222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu DT, Tibbs GR, Siegelbaum SA. Subunit stoichiometry of cyclic nucleotide-gated channels and effects of subunit order on channel function. Neuron. 1996;16:983–90. doi: 10.1016/s0896-6273(00)80121-x. [DOI] [PubMed] [Google Scholar]
- 61.Varnum MD, Zagotta WN. Subunit interactions in the activation of cyclic nucleotide-gated ion channels. Biophys J. 1996;70:2667–79. doi: 10.1016/S0006-3495(96)79836-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zagotta WN, Olivier NB, Black KD, Young EC, Olson R, Gouaux E. Structural basis for modulation and agonist specificity of HCN pacemaker channels. Nature. 2003;425:200–5. doi: 10.1038/nature01922. [DOI] [PubMed] [Google Scholar]
- 63.Dzeja C, Hagen V, Kaupp UB, Frings S. Ca2+ permeation in cyclic nucleotide-gated channels. EMBO J. 1999;18:131–44. doi: 10.1093/emboj/18.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Root MJ, MacKinnon R. Identification of an external divalent cation-binding site in the pore of a cGMP-activated channel. Neuron. 1993;11:459–66. doi: 10.1016/0896-6273(93)90150-p. [DOI] [PubMed] [Google Scholar]
- 65.Eismann E, Muller F, Heinemann SH, Kaupp UB. A single negative charge within the pore region of a cGMP-gated channel controls rectification, Ca2+ blockage, and ionic selectivity. Proc Natl Acad Sci USA. 1994;91:1109–13. doi: 10.1073/pnas.91.3.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Frings S, Seifert R, Godde M, Kaupp UB. Profoundly different calcium permeation and blockage determine the specific function of distinct cyclic nucleotide-gated channels. Neuron. 1995;15:169–79. doi: 10.1016/0896-6273(95)90074-8. [DOI] [PubMed] [Google Scholar]
- 67.Seifert R, Eismann E, Ludwig J, Baumann A, Kaupp UB. Molecular determinants of a Ca2+-binding site in the pore of cyclic nucleotide-gated channels: S5/S6 segments control affinity of intrapore glutamates. EMBO J. 1999;18:119–30. doi: 10.1093/emboj/18.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakatani K, Yau KW. Calcium and magnesium fluxes across the plasma membrane of the toad rod outer segment. J Physiol. 1988;395:695–729. doi: 10.1113/jphysiol.1988.sp016942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, et al. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 70.Morais-Cabral JH, Zhou Y, MacKinnon R. Energetic optimization of ion conduction rate by the K+ selectivity filter. Nature. 2001;414:37–42. doi: 10.1038/35102000. [DOI] [PubMed] [Google Scholar]
- 71.Zhou Y, Morais-Cabral JH, Kaufman A, MacKinnon R. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 A resolution. Nature. 2001;414:43–8. doi: 10.1038/35102009. [DOI] [PubMed] [Google Scholar]
- 72.Karpen JW, Brown RL, Stryer L, Baylor DA. Interactions between divalent cations and the gating machinery of cyclic GMP-activated channels in salamander retinal rods. J Gen Physiol. 1993;101:1–25. doi: 10.1085/jgp.101.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fodor AA, Black KD, Zagotta WN. Tetracaine reports a conformational change in the pore of cyclic nucleotide-gated channels. J Gen Physiol. 1997;110:591–600. doi: 10.1085/jgp.110.5.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Becchetti A, Gamel K, Torre V. Cyclic nucleotide-gated channels. Pore topology studied through the accessibility of reporter cysteines. J Gen Physiol. 1999;114:377–92. doi: 10.1085/jgp.114.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu J, Siegelbaum SA. Change of pore helix conformational state upon opening of cyclic nucleotide-gated channels. Neuron. 2000;28:899–909. doi: 10.1016/s0896-6273(00)00162-8. [DOI] [PubMed] [Google Scholar]
- 76.Flynn GE, Zagotta WN. Conformational changes in S6 coupled to the opening of cyclic nucleotide-gated channels. Neuron. 2001;30:689–98. doi: 10.1016/s0896-6273(01)00324-5. [DOI] [PubMed] [Google Scholar]
- 77.Liu Y, Holmgren M, Jurman ME, Yellen G. Gated access to the pore of a voltage-dependent K+ channel. Neuron. 1997;19:175–84. doi: 10.1016/s0896-6273(00)80357-8. [DOI] [PubMed] [Google Scholar]
- 78.Holmgren M, Shin KS, Yellen G. The activation gate of a voltage-gated K+ channel can be trapped in the open state by an intersubunit metal bridge. Neuron. 1998;21:617–21. doi: 10.1016/s0896-6273(00)80571-1. [DOI] [PubMed] [Google Scholar]
- 79.Jiang Y, Lee A, Chen J, Cadene M, Chait BT, MacKinnon R. Crystal structure and mechanism of a calcium-gated potassium channel. Nature. 2002;417:515–22. doi: 10.1038/417515a. [DOI] [PubMed] [Google Scholar]
- 80.Jiang Y, Lee A, Chen J, Cadene M, Chait BT, MacKinnon R. The open pore conformation of potassium channels. Nature. 2002;417:523–6. doi: 10.1038/417523a. [DOI] [PubMed] [Google Scholar]
- 81.Bradley J, Reisert J, Frings S. Regulation of cyclic nucleotide-gated channels. Curr Opin Neurobiol. 2005;15:343–9. doi: 10.1016/j.conb.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 82.Ildefonse M, Crouzy S, Bennett N. Gating of retinal rod cation channel by different nucleotides: comparative study of unitary currents. J Membr Biol. 1992;130:91–104. doi: 10.1007/BF00233741. [DOI] [PubMed] [Google Scholar]
- 83.Gordon SE, Zagotta WN. A histidine residue associated with the gate of the cyclic nucleotide-activated channels in rod photoreceptors. Neuron. 1995;14:177–83. doi: 10.1016/0896-6273(95)90252-x. [DOI] [PubMed] [Google Scholar]
- 84.Gordon SE, Zagotta WN. Localization of regions affecting an allosteric transition in cyclic nucleotide-activated channels. Neuron. 1995;14:857–64. doi: 10.1016/0896-6273(95)90229-5. [DOI] [PubMed] [Google Scholar]
- 85.Gordon SE, Brautigan DL, Zimmerman AL. Protein phosphatases modulate the apparent agonist affinity of the light-regulated ion channel in retinal rods. Neuron. 1992;9:739–48. doi: 10.1016/0896-6273(92)90036-d. [DOI] [PubMed] [Google Scholar]
- 86.Kramer RH, Molokanova E. Modulation of cyclic-nucleotide-gated channels and regulation of vertebrate phototransduction. J Exp Biol. 2001;204:2921–31. doi: 10.1242/jeb.204.17.2921. [DOI] [PubMed] [Google Scholar]
- 87.Muller F, Bonigk W, Sesti F, Frings S. Phosphorylation of mammalian olfactory cyclic nucleotide-gated channels increases ligand sensitivity. J Neurosci. 1998;18:164–73. doi: 10.1523/JNEUROSCI.18-01-00164.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Molokanova E, Savchenko A, Kramer RH. Noncatalytic inhibition of cyclic nucleotide-gated channels by tyrosine kinase induced by genistein. J Gen Physiol. 1999;113:45–56. doi: 10.1085/jgp.113.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gordon SE, Downing-Park J, Tam B, Zimmerman AL. Diacylglycerol analogs inhibit the rod cGMP-gated channel by a phosphorylation-independent mechanism. Biophys J. 1995;69:409–17. doi: 10.1016/S0006-3495(95)79913-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dean DM, Nguitragool W, Miri A, McCabe SL, Zimmerman AL. All-trans-retinal shuts down rod cyclic nucleotide-gated ion channels: a novel role for photoreceptor retinoids in the response to bright light? Proc Natl Acad Sci USA. 2002;99:8372–7. doi: 10.1073/pnas.122681899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brady JD, Rich TC, Le X, Stafford K, Fowler CJ, Lynch L, et al. Functional role of lipid raft microdomains in cyclic nucleotide-gated channel activation. Mol Pharmacol. 2004;65:503–11. doi: 10.1124/mol.65.3.503. [DOI] [PubMed] [Google Scholar]
- 92.Zhainazarov AB, Spehr M, Wetzel CH, Hatt H, Ache BW. Modulation of the olfactory CNG channel by Ptdlns(3,4,5)P3. J Membr Biol. 2004;201:51–7. doi: 10.1007/s00232-004-0707-4. [DOI] [PubMed] [Google Scholar]
- 93.Broillet M-C. A single intracellular cysteine residue is responsible for the activation of the olfactory cyclic nucleotide-gated channel by NO. J Biol Chem. 2000;275:15135–41. doi: 10.1074/jbc.275.20.15135. [DOI] [PubMed] [Google Scholar]
- 94.Warren R, Molday RS. Regulation of the rod photoreceptor cyclic nucleotide-gated channel. Adv Exp Med Biol. 2002;514:205–23. doi: 10.1007/978-1-4615-0121-3_12. [DOI] [PubMed] [Google Scholar]
- 95.Rieke F, Schwartz EA. A cGMP-gated current can control exocytosis at cone synapses. Neuron. 1994;13:863–73. doi: 10.1016/0896-6273(94)90252-6. [DOI] [PubMed] [Google Scholar]
- 96.Savchenko A, Barnes S, Kramer RH. Cyclic-nucleotide-gated channels mediate synaptic feedback by nitric oxide. Nature. 1997;390:694–8. doi: 10.1038/37803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nawy S, Jahr CE. cGMP-gated conductance in retinal bipolar cells is suppressed by the photoreceptor transmitter. Neuron. 1991;7:677–83. doi: 10.1016/0896-6273(91)90380-i. [DOI] [PubMed] [Google Scholar]
- 98.Shiells RA, Falk G. Properties of the cGMP-activated channel of retinal on-bipolar cells. Proc Roy Soc Lond B. 1992;247:21–5. doi: 10.1098/rspb.1992.0004. [DOI] [PubMed] [Google Scholar]
- 99.Ahmad I, Leinders-Zufall T, Kocsis JD, Shepherd GM, Zufall F, Barnstable CJ. Retinal ganglion cells express a cGMP-gated cation conductance activatable by nitric oxide donors. Neuron. 1994;12:155–65. doi: 10.1016/0896-6273(94)90160-0. [DOI] [PubMed] [Google Scholar]
- 100.Kawai F, Sterling P. AMPA receptor activates a G-protein that suppresses a cGMP-gated current. J Neurosci. 1999;19:2954–9. doi: 10.1523/JNEUROSCI.19-08-02954.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kawa F, Sterling P. cGMP modulates spike responses of retinal ganglion cells via a cGMP-gated current. Vis Neurosci. 2002;19:373–80. [PubMed] [Google Scholar]
- 102.Henry D, Burke S, Shishido E, Matthews G. Retinal bipolar neurons express the cyclic nucleotide-gated channel of cone photoreceptors. J Neurophysiol. 2003;89:754–61. doi: 10.1152/jn.00771.2002. [DOI] [PubMed] [Google Scholar]
- 103.Snellman J, Nawy S. cGMP-dependent kinase regulates response sensitivity of the mouse on bipolar cell. J Neurosci. 2004;24:6621–8. doi: 10.1523/JNEUROSCI.1474-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kuzmiski JB, MacVicar BA. Cyclic nucleotide-gated channels contribute to the cholinergic plateau potential in hippocampal CA1 pyramidal neurons. J Neurosci. 2001;21:8707–14. doi: 10.1523/JNEUROSCI.21-22-08707.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Parent A, Schrader K, Munger SD, Reed RR, Linden DJ, Ronnett GV. Synaptic transmission and hippocampal long-term potentiation in olfactory cyclic nucleotide-gated channel type 1 null mouse. J Neurophysiol. 1998;79:3295–301. doi: 10.1152/jn.1998.79.6.3295. [DOI] [PubMed] [Google Scholar]
- 106.Pitari GM, Zingman LV, Hodgson DM, Alekseev AE, Kazerounian S, Bienengraeber M, et al. Bacterial enterotoxins are associated with resistance to colon cancer. Proc Natl Acad Sci USA. 2003;100:2695–9. doi: 10.1073/pnas.0434905100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Miller JP, Boswell KH, Muneyama K, Simon LN, Robins RK, Shuman DA. Synthesis and biochemical studies of various 8-substituted derivatives of guanosine 3’,5’-cyclic phosphate, inosine 3’,5’-cyclic phosphate, and xanthosine 3’,5’-cyclic phosphate. Biochemistry. 1973;12:5310–9. doi: 10.1021/bi00750a014. [DOI] [PubMed] [Google Scholar]
- 108.Schwede F, Maronde E, Genieser H, Jastorff B. Cyclic nucleotide analogs as biochemical tools and prospective drugs. Pharmacol Ther. 2000;87:199–226. doi: 10.1016/s0163-7258(00)00051-6. [DOI] [PubMed] [Google Scholar]
- 109.Tanaka JC, Eccleston JF, Furman RE. Photoreceptor channel activation by nucleotide derivatives. Biochemistry. 1989;28:2776–84. doi: 10.1021/bi00433a006. [DOI] [PubMed] [Google Scholar]
- 110.Gavazzo P, Picco C, Maxia L, Menini A. In: Ionic Channels, Neurons, and the Brain. Torre V, Conti F, editors. Plenum Press; New York: 1996. pp. 75–83. [Google Scholar]
- 111.Weber IT, Steitz TA. Structure of a complex of catabolite gene activator protein and cyclic AMP refined at 2.5 A resolution. J Mol Biol. 1987;198:311–26. doi: 10.1016/0022-2836(87)90315-9. [DOI] [PubMed] [Google Scholar]
- 112.Su Y, Dostmann WR, Herberg FW, Durick K, Xuong NH, Ten Eyck L, et al. Regulatory subunit of protein kinase A: structure of deletion mutant with cAMP binding domains. Science. 1995;269:807–13. doi: 10.1126/science.7638597. [DOI] [PubMed] [Google Scholar]
- 113.Diller TC, Madhusudan, Xuong NH, Taylor SS. Molecular basis for regulatory subunit diversity in cAMP-dependent protein kinase: crystal structure of the type II beta regulatory subunit. Structure (Camb) 2001;9:73–82. doi: 10.1016/s0969-2126(00)00556-6. [DOI] [PubMed] [Google Scholar]
- 114.Wu J, Brown S, Xuong NH, Taylor SS. RIalpha subunit of PKA: a cAMP-free structure reveals a hydrophobic capping mechanism for docking cAMP into site B. Structure (Camb) 2004;12:1057–65. doi: 10.1016/j.str.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 115.Kim C, Xuong NH, Taylor SS. Crystal structure of a complex between the catalytic and regulatory (RIalpha) subunits of PKA. Science. 2005;307:690–6. doi: 10.1126/science.1104607. [DOI] [PubMed] [Google Scholar]
- 116.Rehmann H, Prakash B, Wolf E, Rueppel A, De Rooij J, Bos JL, et al. Structure and regulation of the cAMP-binding domains of Epac2. Nat Struct Biol. 2003;10:26–32. doi: 10.1038/nsb878. [DOI] [PubMed] [Google Scholar]
- 117.Clayton GM, Silverman WR, Heginbotham L, Morais-Cabral JH. Structural basis of ligand activation in a cyclic nucleotide regulated potassium channel. Cell. 2004;119:615–27. doi: 10.1016/j.cell.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 118.Tibbs GR, Liu DT, Leypold BG, Siegelbaum SA. A state-independent interaction between ligand and a conserved arginine residue in cyclic nucleotide-gated channels reveals a functional polarity of the cyclic nucleotide binding site. J Biol Chem. 1998;273:4497–505. doi: 10.1074/jbc.273.8.4497. [DOI] [PubMed] [Google Scholar]
- 119.Varnum MD, Black KD, Zagotta WN. Molecular mechanism for ligand discrimination of cyclic nucleotide-gated channels. Neuron. 1995;15:619–25. doi: 10.1016/0896-6273(95)90150-7. [DOI] [PubMed] [Google Scholar]
- 120.Zagotta WN, Siegelbaum SA. Structure and function of cyclic nucleotide-gated channels. Ann Rev Neurosci. 1996;19:235–63. doi: 10.1146/annurev.ne.19.030196.001315. [DOI] [PubMed] [Google Scholar]
- 121.Zimmerman AL, Yamanaka G, Eckstein F, Baylor DA, Stryer L. Interaction of hydrolysis-resistant analogs of cyclic GMP with the phosphodiesterase and light-sensitive channel of retinal rod outer segments. Proc Natl Acad Sci USA. 1985;82:8813–7. doi: 10.1073/pnas.82.24.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kramer RH, Tibbs GR. Antagonists of cyclic nucleotide-gated channels and molecular mapping of their site of action. J Neurosci. 1996;16:1285–93. doi: 10.1523/JNEUROSCI.16-04-01285.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nerbonne JM, Richard S, Nargeot J, Lester HA. New photoactivatable cyclic nucleotides produce intracellular jumps in cyclic AMP and cyclic GMP concentrations. Nature. 1984;310:74–6. doi: 10.1038/310074a0. [DOI] [PubMed] [Google Scholar]
- 124.Hagen V, Bendig J, Frings S, Eckardt T, Helm S, Reuter D, et al. Highly efficient and ultrafast phototriggers for cAMP and cGMP by using long-wavelength UV/Vis-activation. Angew Chem Int Ed Engl. 2001;40:1045–8. doi: 10.1002/1521-3773(20010316)40:6<1045::aid-anie10450>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 125.Sekhar KR, Hatchett RJ, Shabb JB, Wolfe L, Francis SH, Wells JN, et al. Relaxation of pig coronary arteries by new and potent cGMP analogs that selectively activate type I alpha, compared with type I beta, cGMP-dependent protein kinase. Mol Pharmacol. 1992;42:103–8. [PubMed] [Google Scholar]
- 126.Wei JY, Cohen ED, Yan YY, Genieser HG, Barnstable CJ. Identification of competitive antagonists of the rod photoreceptor cGMP-gated cation channel: beta-phenyl-1,N2-etheno-substituted cGMP analogues as probes of the cGMP-binding site. Biochemistry. 1996;35:16815–23. doi: 10.1021/bi961763v. [DOI] [PubMed] [Google Scholar]
- 127.Francis SH, Noblett BD, Todd BW, Wells JN, Corbin JD. Relaxation of vascular and tracheal smooth muscle by cyclic nucleotide analogs that preferentially activate purified cGMP-dependent protein kinase. Mol Pharmacol. 1988;34:506–17. [PubMed] [Google Scholar]
- 128.Butt E, Nolte C, Schulz S, Beltman J, Beavo JA, Jastorff B, et al. Analysis of the functional role of cGMP-dependent protein kinase in intact human platelets using a specific activator 8-para-chlorophenylthio-cGMP. Biochem Pharmacol. 1992;43:2591–600. doi: 10.1016/0006-2952(92)90148-c. [DOI] [PubMed] [Google Scholar]
- 129.Butt E, Pohler D, Genieser HG, Huggins JP, Bucher B. Inhibition of cyclic GMP-dependent protein kinase-mediated effects by (Rp)-8-bromo-PET-cyclic GMPS. Br J Pharmacol. 1995;116:3110–6. doi: 10.1111/j.1476-5381.1995.tb15112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Thomas MK, Francis SH, Beebe SJ, Gettys TW, Corbin JD. Partial mapping of cyclic nucleotide sites and studies of regulatory mechanisms of phosphodiesterases using cyclic nucleotide analogues. Adv Second Messenger Phosphoprotein Res. 1992;25:45–53. [PubMed] [Google Scholar]
- 131.Koch K-W, Kaupp UB. Cyclic GMP directly regulates a cation conductance in membranes of bovine rods by a cooperative mechanism. J Biol Chem. 1985;260:6788–800. [PubMed] [Google Scholar]
- 132.Caretta A, Cavaggioni A, Sorbi RT. Binding stoichiometry of a fluorescent cGMP analogue to membranes of retinal rod outer segments. Eur J Biochem. 1985;153:49–53. doi: 10.1111/j.1432-1033.1985.tb09265.x. [DOI] [PubMed] [Google Scholar]
- 133.Brown RL, Bert RJ, Evans FE, Karpen JW. Activation of retinal rod cGMP-gated channels: what makes for an effective 8-substituted derivative of cGMP? Biochemistry. 1993;32:10089–95. doi: 10.1021/bi00089a026. [DOI] [PubMed] [Google Scholar]
- 134.Wei JY, Cohen ED, Genieser HG, Barnstable CJ. Substituted cGMP analogs can act as selective agonists of the rod photoreceptor cGMP-gated cation channel. J Mol Neurosci. 1998;10:53–64. doi: 10.1007/BF02737085. [DOI] [PubMed] [Google Scholar]
- 135.Corbin JD, Ogreid D, Miller JP, Suva RH, Jastorff B, Doskeland SO. Studies of cGMP analog specificity and function of the two intrasubunit binding sites of cGMP-dependent protein kinase. J Biol Chem. 1986;261:1208–14. [PubMed] [Google Scholar]
- 136.Kramer RH, Karpen JW. Spanning binding sites on allosteric proteins with polymer-linked ligand dimers. Nature. 1998;395:710–3. doi: 10.1038/27227. [DOI] [PubMed] [Google Scholar]
- 137.Knoll D, Hermans J. Polymer-protein interactions. Comparison of experiment and excluded volume theory. J Biol Chem. 1983;258:5710–5. [PubMed] [Google Scholar]
- 138.Stern JH, Kaupp UB, MacLeish PR. Control of the light-regulated current in rod photoreceptors by cyclic GMP, calcium, and l-cis-diltiazem. Proc Natl Acad Sci USA. 1986;83:1163–7. doi: 10.1073/pnas.83.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kolesnikov SS, Zhainazarov AB, Kosolapov AV. Cyclic nucleotide-activated channels in the frog olfactory receptor plasma membrane. FEBS Lett. 1990;266:96–8. doi: 10.1016/0014-5793(90)81515-p. [DOI] [PubMed] [Google Scholar]
- 140.Frings S, Lynch JW, Lindemann B. Properties of cyclic nucleotide-gated channels mediating olfactory transduction. Activation, selectivity, and blockage. J Gen Physiol. 1992;100:45–67. doi: 10.1085/jgp.100.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Haynes LW. Block of the cyclic GMP-gated channel of vertebrate rod and cone photoreceptors by l-cis-diltiazem. J Gen Physiol. 1992;100:783–801. doi: 10.1085/jgp.100.5.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Quandt FN, Nicol GD, Schnetkamp PPM. Voltage-dependent gating and block of the cyclic-GMP-dependent current in bovine rod outer segments. Neuroscience. 1991;42:629–38. doi: 10.1016/0306-4522(91)90032-j. [DOI] [PubMed] [Google Scholar]
- 143.McLatchie LM, Matthews HR. Voltage-dependent block by L-cis-diltiazem of the cyclic GMP-activated conductance of salamander rods. Proc Biol Sci. 1992;247:113–9. doi: 10.1098/rspb.1992.0016. [DOI] [PubMed] [Google Scholar]
- 144.Gerstner A, Zong X, Hofmann F, Biel M. Molecular cloning and functional characterization of a new modulatory cyclic nucleotide-gated channel subunit from mouse retina. J Neurosci. 2000;20:1324–32. doi: 10.1523/JNEUROSCI.20-04-01324.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.McLatchie LM, Matthews HR. The effect of pH on the block by L-cis-diltiazem and amiloride of the cyclic GMP-activated conductance of salamander rods. Proc Biol Sci. 1994;255:231–6. doi: 10.1098/rspb.1994.0033. [DOI] [PubMed] [Google Scholar]
- 146.Galizzi JP, Borsotto M, Barhanin J, Fosset M, Lazdunski M. Characterization and photoaffinity labeling of receptor sites for the Ca2+ channel inhibitors d-cis-diltiazem, (+/-)-bepridil, desmethoxyverapamil, and (+)-PN 200-110 in skeletal muscle transverse tubule membranes. J Biol Chem. 1986;261:1393–7. [PubMed] [Google Scholar]
- 147.Ikeda S, Oka J, Nagao T. Effects of four diltiazem stereoisomers on binding of d-cis-[3H]diltiazem and (+)-[3H]PN200-110 to rabbit T-tubule calcium channels. Eur J Pharmacol. 1991;208:199–205. doi: 10.1016/0922-4106(91)90096-z. [DOI] [PubMed] [Google Scholar]
- 148.Kaburaki M, Inoue H, Doi H, Yasuhara M, Narita H. Cardiovascular effects of 1,5-benzothiazepine derivatives having a l-cis and d-cis configuration in anesthetized dogs. Biol Pharm Bull. 1998;21:50–5. doi: 10.1248/bpb.21.50. [DOI] [PubMed] [Google Scholar]
- 149.Sakamoto K, Ishikawa M, Koga K, Urushidani T, Nagao T. Energy preserving effect of l-cis diltiazem in isolated ischemic and reperfused guinea pig hearts: a 31P-NMR study. Jpn J Pharmacol. 2000;83:225–32. doi: 10.1254/jjp.83.225. [DOI] [PubMed] [Google Scholar]
- 150.Hashimoto Y, Yabana H, Murata S. Electrophysiological effect of l-cis-diltiazem, the stereoisomer of d-cis-diltiazem, on isolated guinea-pig left ventricular myocytes. Eur J Pharmacol. 2000;391:217–23. doi: 10.1016/s0014-2999(00)00077-7. [DOI] [PubMed] [Google Scholar]
- 151.Fox DA, Poblenz AT, He L, Harris JB, Medrano CJ. Pharmacological strategies to block rod photoreceptor apoptosis caused by calcium overload: a mechanistic target-site approach to neuroprotection. Eur J Ophthalmol. 2003;13:S44–S56. doi: 10.1177/112067210301303s08. [DOI] [PubMed] [Google Scholar]
- 152.Nicol GD, Schnetkamp PP, Saimi Y, Cragoe EJ, Jr, Bownds MD. A derivative of amiloride blocks both the light-regulated and cyclic GMP-regulated conductances in rod photoreceptors. J Gen Physiol. 1987;90:651–69. doi: 10.1085/jgp.90.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kolesnikov SS, Kosolapov AV. Cyclic nucleotide-activated channels in carp olfactory receptor cells. Biochim Biophys Acta. 1993;1150:63–72. doi: 10.1016/0005-2736(93)90122-g. [DOI] [PubMed] [Google Scholar]
- 154.Xu W, Leung S, Wright J, Guggino SE. Expression of cyclic nucleotide-gated cation channels in airway epithelial cells. J Membr Biol. 1999;171:117–26. doi: 10.1007/s002329900564. [DOI] [PubMed] [Google Scholar]
- 155.Qiu W, Lee B, Lancaster M, Xu W, Leung S, Guggino SE. Cyclic nucleotide-gated cation channels mediate sodium and calcium influx in rat colon. Am J Physiol Cell Physiol. 2000;278:C336–C43. doi: 10.1152/ajpcell.2000.278.2.C336. [DOI] [PubMed] [Google Scholar]
- 156.Kaczorowski GJ, Barros F, Dethmers JK, Trumble MJ, Cragoe EJ., Jr Inhibition of Na+/Ca2+ exchange in pituitary plasma membrane vesicles by analogues of amiloride. Biochemistry. 1985;24:1394–403. doi: 10.1021/bi00327a017. [DOI] [PubMed] [Google Scholar]
- 157.Suarez-Kurtz G, Kaczorowski GJ. Effects of dichlorobenzamil on calcium currents in clonal GH3 pituitary cells. J Pharmacol Exp Ther. 1988;247:248–53. [PubMed] [Google Scholar]
- 158.Garcia ML, King VF, Shevell JL, Slaughter RS, Suarez-Kurtz G, Winquist RJ, et al. Amiloride analogs inhibit L-type calcium channels and display calcium entry blocker activity. J Biol Chem. 1990;265:3763–71. [PubMed] [Google Scholar]
- 159.Bollensdorff C, Zimmer T, Benndorf K. Amiloride derivatives are potent blockers of KATP channels. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:351–8. doi: 10.1007/s002100100466. [DOI] [PubMed] [Google Scholar]
- 160.Gould RJ, Murphy KM, Reynolds IJ, Snyder SH. Antischizophrenic drugs of the diphenylbutylpiperidine type act as calcium channel antagonists. Proc Natl Acad Sci USA. 1983;80:5122–5. doi: 10.1073/pnas.80.16.5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nicol GD. The calcium channel antagonist, pimozide, blocks the cyclic GMP-activated current in rod photoreceptors. J Pharmacol Exp Ther. 1993;265:626–32. [PubMed] [Google Scholar]
- 162.Benos DJ. Amiloride: a molecular probe of sodium transport in tissues and cells. Am J Physiol. 1982;242:C131–45. doi: 10.1152/ajpcell.1982.242.3.C131. [DOI] [PubMed] [Google Scholar]
- 163.Zufall F, Firestein S. Divalent cations block the cyclic nucleotide-gated channel of olfactory receptor neurons. J Neurophysiol. 1993;69:1758–68. doi: 10.1152/jn.1993.69.5.1758. [DOI] [PubMed] [Google Scholar]
- 164.Rosenbaum T, Islas LD, Carlson AE, Gordon SE. Dequalinium: a novel, high-affinity blocker of CNGA1 channels. J Gen Physiol. 2003;121:37–47. doi: 10.1085/jgp.20028716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rosenbaum T, Gordon-Shaag A, Islas LD, Cooper J, Munari M, Gordon SE. State-dependent block of CNG channels by dequalinium. J Gen Physiol. 2004;123:295–304. doi: 10.1085/jgp.200308925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Schnetkamp PP. Sodium ions selectively eliminate the fast component of guanosine cyclic 3’,5’-phosphate induced Ca2+ release from bovine rod outer segment disks. Biochemistry. 1987;26:3249–53. doi: 10.1021/bi00386a002. [DOI] [PubMed] [Google Scholar]
- 167.Schnetkamp PP. Cation selectivity of and cation binding to the cGMP-dependent channel in bovine rod outer segment membranes. J Gen Physiol. 1990;96:517–34. doi: 10.1085/jgp.96.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sunami A, Dudley SC, Jr, Fozzard HA. Sodium channel selectivity filter regulates antiarrhythmic drug binding. Proc Natl Acad Sci USA. 1997;94:14126–31. doi: 10.1073/pnas.94.25.14126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. Molecular determinants of state-dependent block of Na+ channels by local anesthetics. Science. 1994;265:1724–8. doi: 10.1126/science.8085162. [DOI] [PubMed] [Google Scholar]
- 170.Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. Common molecular determinants of local anesthetic, antiarrhythmic, and anticonvulsant block of voltage-gated Na+ channels. Proc Natl Acad Sci USA. 1996;93:9270–5. doi: 10.1073/pnas.93.17.9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Catterall WA. Molecular mechanisms of gating and drug block of sodium channels. Novartis Found Symp. 2002;241:206–18. [PubMed] [Google Scholar]
- 172.Nau C, Wang GK. Interactions of local anesthetics with voltage-gated Na+ channels. J Membr Biol. 2004;201:1–8. doi: 10.1007/s00232-004-0702-y. [DOI] [PubMed] [Google Scholar]
- 173.Fodor AA, Gordon SE, Zagotta WN. Mechanism of tetracaine block of cyclic nucleotide-gated channels. J Gen Physiol. 1997;109:3–14. doi: 10.1085/jgp.109.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ghatpande AS, Uma R, Karpen JW. A multiply charged tetracaine derivative blocks cyclic nucleotide-gated channels at subnanomolar concentrations. Biochemistry. 2003;42:265–70. doi: 10.1021/bi027031m. [DOI] [PubMed] [Google Scholar]
- 175.Heinemann SH, Terlau H, Stuhmer W, Imoto K, Numa S. Calcium channel characteristics conferred on the sodium channel by single mutations. Nature. 1992;356:441–3. doi: 10.1038/356441a0. [DOI] [PubMed] [Google Scholar]
- 176.Strassmaier T, Uma R, Ghatpande AS, Bandyopadhyay T, Schaffer M, Witte J, et al. Modifications to the tetracaine scaffold produce cyclic nucleotide-gated channel blockers with widely varying efficacies. J Med Chem. 2005;48:5805–12. doi: 10.1021/jm0502485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hsu YT, Molday RS. Modulation of the cGMP-gated channel of rod photoreceptor cells by calmodulin. Nature. 1993;361:76–9. doi: 10.1038/361076a0. [DOI] [PubMed] [Google Scholar]
- 178.Chen TY, Yau KW. Direct modulation by Ca2+-calmodulin of cyclic nucleotide-activated channel of rat olfactory receptor neurons. Nature. 1994;368:545–8. doi: 10.1038/368545a0. [DOI] [PubMed] [Google Scholar]
- 179.Hackos DH, Korenbrot JI. Calcium modulation of ligand affinity in the cyclic GMP-gated ion channels of cone photoreceptors. J Gen Physiol. 1997;110:515–28. doi: 10.1085/jgp.110.5.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Koutalos Y, Yau K-W. Regulation of sensitivity in vertebrate rod photoreceptors by calcium. Trends Neurosci. 1996;19:73–81. doi: 10.1016/0166-2236(96)89624-x. [DOI] [PubMed] [Google Scholar]
- 181.Rebrik TI, Korenbrot JI. In intact mammalian photoreceptors, Ca 2+-dependent modulation of cGMP-gated ion channels is detectable in cones but not in rods. J Gen Physiol. 2004;123:63–75. doi: 10.1085/jgp.200308952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kleene SJ. Inhibition of olfactory cyclic nucleotide-activated current by calmodulin antagonists. Br J Pharmacol. 1994;111:469–72. doi: 10.1111/j.1476-5381.1994.tb14760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Greenberg DA, Carpenter CL, Messing RO. Interaction of calmodulin inhibitors and protein kinase C inhibitors with voltage-dependent calcium channels. Brain Res. 1987;404:401–4. doi: 10.1016/0006-8993(87)91403-x. [DOI] [PubMed] [Google Scholar]
- 184.Ichikawa M, Urayama M, Matsumoto G. Anticalmodulin drugs block the sodium gating current of squid giant axons. J Membr Biol. 1991;120:211–22. doi: 10.1007/BF01868532. [DOI] [PubMed] [Google Scholar]
- 185.Kihira M, Matsuzawa K, Tokuno H, Tomita T. Effects of calmodulin antagonists on calcium-activated potassium channels in pregnant rat myometrium. Br J Pharmacol. 1990;100:353–9. doi: 10.1111/j.1476-5381.1990.tb15808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yang J, Zorumski CF. Trifluoperazine blocks GABA-gated chloride currents in cultured chick spinal cord neurons. J Neurophysiol. 1989;61:363–73. doi: 10.1152/jn.1989.61.2.363. [DOI] [PubMed] [Google Scholar]
- 187.Leinders-Zufall T, Zufall F. Block of cyclic nucleotide-gated channels in salamander olfactory receptor neurons by the guanylyl cyclase inhibitor LY83583. J Neurophysiol. 1995;74:2759–62. doi: 10.1152/jn.1995.74.6.2759. [DOI] [PubMed] [Google Scholar]
- 188.Gold GH. Controversial issues in vertebrate olfactory transduction. Annu Rev Physiol. 1999;61:857–71. doi: 10.1146/annurev.physiol.61.1.857. [DOI] [PubMed] [Google Scholar]
- 189.Ma L, Michel WC. Drugs affecting phospholipase C-mediated signal transduction block the olfactory cyclic nucleotide-gated current of adult zebrafish. J Neurophysiol. 1998;79:1183–92. doi: 10.1152/jn.1998.79.3.1183. [DOI] [PubMed] [Google Scholar]
- 190.Wei JY, Cohen ED, Barnstable CJ. Direct blockade of both cloned rat rod photoreceptor cyclic nucleotide-gated non-selective cation (CNG) channel alpha-subunit and native CNG channels from Xenopus rod outer segments by H-8, a non-specific cyclic nucleotide-dependent protein kinase inhibitor. Neurosci Lett. 1997;233:37–40. doi: 10.1016/s0304-3940(97)00622-8. [DOI] [PubMed] [Google Scholar]
- 191.Brown RL, Haley TL, West KA, Crabb JW. Pseudechetoxin: a peptide blocker of cyclic nucleotide-gated ion channels. Proc Natl Acad Sci USA. 1999;96:754–9. doi: 10.1073/pnas.96.2.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yamazaki Y, Brown RL, Morita T. Purification and cloning of toxins from elapid venoms that target cyclic nucleotide-gated ion channels. Biochemistry. 2002;41:11331–7. doi: 10.1021/bi026132h. [DOI] [PubMed] [Google Scholar]
- 193.Yamazaki Y, Koike H, Sugiyama Y, Motoyoshi K, Wada T, Hishinuma S, et al. Cloning and characterization of novel snake venom proteins that block smooth muscle contraction. Eur J Biochem. 2002;269:2708–15. doi: 10.1046/j.1432-1033.2002.02940.x. [DOI] [PubMed] [Google Scholar]
- 194.Wang J, Shen B, Guo M, Lou X, Duan Y, Cheng XP, et al. Blocking effect and crystal structure of natrin toxin, a cysteine-rich secretory protein from Naja atra venom that targets the BKCa channel. Biochemistry. 2005;44:10145–52. doi: 10.1021/bi050614m. [DOI] [PubMed] [Google Scholar]
- 195.Brown RL, Lynch LL, Haley TL, Arsanjani R. Pseudechetoxin binds to the pore turret of cyclic nucleotide-gated ion channels. J Gen Physiol. 2003;122:749–60. doi: 10.1085/jgp.200308823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Guo M, Teng M, Niu L, Liu Q, Huang Q, Hao Q. Crystal structure of the cysteine-rich secretory protein stecrisp reveals that the cysteine-rich domain has a K + channel inhibitor-like fold. J Biol Chem. 2005;280:12405–12. doi: 10.1074/jbc.M413566200. [DOI] [PubMed] [Google Scholar]
- 197.Shikamoto Y, Suto K, Yamazaki Y, Morita T, Mizuno H. Crystal structure of a CRISP family Ca2+-channel blocker derived from snake venom. J Mol Biol. 2005;350:735–43. doi: 10.1016/j.jmb.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 198.Dryja TP, Finn JT, Peng YW, McGee TL, Berson EL, Yau KW. Mutations in the gene encoding the α subunit of the rod cGMP-gated channel in autosomal recessive retinitis pigmentosa. Proc Natl Acad Sci USA. 1995;92:10177–81. doi: 10.1073/pnas.92.22.10177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Paloma E, Martinez-Mir A, Garcia-Sandoval B, Ayuso C, Vilageliu L, Gonzalez-Duarte R, et al. Novel homozygous mutation in the alpha subunit of the rod cGMP gated channel (CNGA1) in two Spanish sibs affected with autosomal recessive retinitis pigmentosa. J Med Genet. 2002;39:E66. doi: 10.1136/jmg.39.10.e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhang Q, Zulfiqar F, Riazuddin SA, Xiao X, Ahmad Z, Riazuddin S, et al. Autosomal recessive retinitis pigmentosa in a Pakistani family mapped to CNGA1 with identification of a novel mutation. Mol Vis. 2004;10:884–9. [PubMed] [Google Scholar]
- 201.Bareil C, Hamel CP, Delague V, Arnaud B, Demaille J, Claustres M. Segregation of a mutation in CNGB1 encoding the β-subunit of the rod cGMP-gated channel in a family with autosomal recessive retinitis pigmentosa. Hum Genet. 2001;108:328–34. doi: 10.1007/s004390100496. [DOI] [PubMed] [Google Scholar]
- 202.Michaelides M, Aligianis IA, Ainsworth JR, Good P, Mollon JD, Maher ER, et al. Progressive cone dystrophy associated with mutation in CNGB3. Invest Ophthalmol Vis Sci. 2004;45:1975–82. doi: 10.1167/iovs.03-0898. [DOI] [PubMed] [Google Scholar]
- 203.Mallouk N, Ildefonse M, Pages F, Ragno M, Bennett N. Basis for intracellular retention of a human mutant of the retinal rod channel alpha subunit. J Membr Biol. 2002;185:129–36. doi: 10.1007/s00232-001-0119-9. [DOI] [PubMed] [Google Scholar]
- 204.Trudeau MC, Zagotta WN. An intersubunit interaction regulates trafficking of rod cyclic nucleotide-gated channels and is disrupted in an inherited form of blindness. Neuron. 2002;34:197–207. doi: 10.1016/s0896-6273(02)00647-5. [DOI] [PubMed] [Google Scholar]
- 205.Kohl S, Marx T, Giddings I, Jagle H, Jacobson SG, Apfelstedt-Sylla E, et al. Total colourblindness is caused by mutations in the gene encoding the alpha-subunit of the cone photoreceptor cGMP-gated cation channel. Nat Genet. 1998;19:257–9. doi: 10.1038/935. [DOI] [PubMed] [Google Scholar]
- 206.Wissinger B, Gamer D, Jagle H, Giorda R, Marx T, Mayer S, et al. CNGA3 mutations in hereditary cone photoreceptor disorders. Am J Hum Genet. 2001;69:722–37. doi: 10.1086/323613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Patel KA, Bartoli KM, Fandino RA, Ngatchou AN, Woch G, Carey J, et al. Transmembrane S1 mutations in CNGA3 from achromatopsia 2 patients cause loss of function and impaired cellular trafficking of the cone CNG channel. Invest Ophthalmol Vis Sci. 2005;46:2282–90. doi: 10.1167/iovs.05-0179. [DOI] [PubMed] [Google Scholar]
- 208.Kohl S, Baumann B, Broghammer M, Jagle H, Sieving P, Kellner U, et al. Mutations in the CNGB3 gene encoding the beta-subunit of the cone photoreceptor cGMP-gated channel are responsible for achromatopsia (ACHM3) linked to chromosome 8q21. Hum Mol Genet. 2000;9:2107–16. doi: 10.1093/hmg/9.14.2107. [DOI] [PubMed] [Google Scholar]
- 209.Kohl S, Varsanyi B, Antunes GA, Baumann B, Hoyng CB, Jagle H, et al. CNGB3 mutations account for 50% of all cases with autosomal recessive achromatopsia. Eur J Hum Genet. 2005;13:302–8. doi: 10.1038/sj.ejhg.5201269. [DOI] [PubMed] [Google Scholar]
- 210.Nishiguchi KM, Sandberg MA, Gorji N, Berson EL, Dryja TP. Cone cGMP-gated channel mutations and clinical findings in patients with achromatopsia, macular degeneration, and other hereditary cone diseases. Hum Mutat. 2005;25:248–58. doi: 10.1002/humu.20142. [DOI] [PubMed] [Google Scholar]
- 211.Pierce EA. Pathways to photoreceptor cell death in inherited retinal degenerations. Bioessays. 2001;23:605–18. doi: 10.1002/bies.1086. [DOI] [PubMed] [Google Scholar]
- 212.Pacione LR, Szego MJ, Ikeda S, Nishina PM, McInnes RR. Progress toward understanding the genetic and biochemical mechanisms of inherited photoreceptor degenerations. Annu Rev Neurosci. 2003;26:657–700. doi: 10.1146/annurev.neuro.26.041002.131416. [DOI] [PubMed] [Google Scholar]
- 213.Olshevskaya EV, Calvert PD, Woodruff ML, Peshenko IV, Savchenko AB, Makino CL, et al. The Y99C mutation in guanylyl cyclase-activating protein 1 increases intracellular Ca2+ and causes photoreceptor degeneration in transgenic mice. J Neurosci. 2004;24:6078–85. doi: 10.1523/JNEUROSCI.0963-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Nishiguchi KM, Sokal I, Yang L, Roychowdhury N, Palczewski K, Berson EL, et al. A novel mutation (I143NT) in guanylate cyclase-activating protein 1 (GCAP1) associated with autosomal dominant cone degeneration. Invest Ophthalmol Vis Sci. 2004;45:3863–70. doi: 10.1167/iovs.04-0590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Rohrer B, Pinto FR, Hulse KE, Lohr HR, Zhang L, Almeida JS. Multi-destructive pathways triggered in photoreceptor cell death of the RD mouse as determined through gene expression profiling. J Biol Chem. 2004;24:24. doi: 10.1074/jbc.M405085200. [DOI] [PubMed] [Google Scholar]
- 216.Peng C, Rich ED, Varnum MD. Achromatopsia-associated mutation in the human cone photoreceptor cyclic nucleotide-gated channel CNGB3 subunit alters the ligand sensitivity and pore properties of heteromeric channels. J Biol Chem. 2003;278:34533–40. doi: 10.1074/jbc.M305102200. [DOI] [PubMed] [Google Scholar]
- 217.Okada A, Ueyama H, Toyoda F, Oda S, Ding WG, Tanabe S, et al. Functional role of hCNGB3 in regulation of human cone CNG channel: effect of rod monochromacy-associated mutations in hCNGB3 on channel function. Invest Ophthalmol Vis Sci. 2004;45:2324–32. doi: 10.1167/iovs.03-1094. [DOI] [PubMed] [Google Scholar]
- 218.Liu C, Varnum MD. Functional consequences of progressive cone dystrophy-associated mutations in the human cone photoreceptor cyclic nucleotide-gated channel CNGA3 subunit. Am J Physiol Cell Physiol. 2005;289:C187–98. doi: 10.1152/ajpcell.00490.2004. [DOI] [PubMed] [Google Scholar]
- 219.Farber DB, Lolley RN. Light-induced reduction in cyclic GMP of retinal photoreceptor cells in vivo: abnormalities in the degenerative diseases of RCS rats and rd mice. J Neurochem. 1977;28:1089–95. doi: 10.1111/j.1471-4159.1977.tb10673.x. [DOI] [PubMed] [Google Scholar]
- 220.Chader GJ. Animal models in research on retinal degenerations: past progress and future hope. Vision Res. 2002;42:393–9. doi: 10.1016/s0042-6989(01)00212-7. [DOI] [PubMed] [Google Scholar]
- 221.Frasson M, Sahel JA, Fabre M, Simonutti M, Dreyfus H, Picaud S. Retinitis pigmentosa: rod photoreceptor rescue by a calcium-channel blocker in the rd mouse. Nat Med. 1999;5:1183–7. doi: 10.1038/13508. [DOI] [PubMed] [Google Scholar]
- 222.Pearce-Kelling SE, Aleman TS, Nickle A, Laties AM, Aguirre GD, Jacobson SG, et al. Calcium channel blocker D-cis-diltiazem does not slow retinal degeneration in the PDE6B mutant rcd1 canine model of retinitis pigmentosa. Mol Vis. 2001;7:42–7. [PubMed] [Google Scholar]
- 223.Pawlyk BS, Li T, Scimeca MS, Sandberg MA, Berson EL. Absence of photoreceptor rescue with D-cis-diltiazem in the rd mouse. Invest Ophthalmol Vis Sci. 2002;43:1912–5. [PubMed] [Google Scholar]
- 224.Delyfer MN, Leveillard T, Mohand-Said S, Hicks D, Picaud S, Sahel JA. Inherited retinal degenerations: therapeutic prospects. Biol Cell. 2004;96:261–9. doi: 10.1016/j.biolcel.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 225.Robinson RB, Siegelbaum SA. Hyperpolarization-activated cation currents: from molecules to physiological function. Annu Rev Physiol. 2003;65:453–80. doi: 10.1146/annurev.physiol.65.092101.142734. [DOI] [PubMed] [Google Scholar]
- 226.Chaplan SR, Guo HQ, Lee DH, Luo L, Liu C, Kuei C, et al. Neuronal hyperpolarization-activated pacemaker channels drive neuropathic pain. J Neurosci. 2003;23:1169–78. doi: 10.1523/JNEUROSCI.23-04-01169.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.BoSmith RE, Briggs I, Sturgess NC. Inhibitory actions of ZENECA ZD7288 on whole-cell hyperpolarization activated inward current (If) in guinea-pig dissociated sinoatrial node cells. Br J Pharmacol. 1993;110:343–9. doi: 10.1111/j.1476-5381.1993.tb13815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Shin KS, Rothberg BS, Yellen G. Blocker state dependence and trapping in hyperpolarization-activated cation channels: evidence for an intracellular activation gate. J Gen Physiol. 2001;117:91–101. doi: 10.1085/jgp.117.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Chen C. ZD7288 inhibits postsynaptic glutamate receptor-mediated responses at hippocampal perforant path-granule cell synapses. Eur J Neurosci. 2004;19:643–9. doi: 10.1111/j.0953-816x.2003.03174.x. [DOI] [PubMed] [Google Scholar]
- 230.Felix R, Sandoval A, Sanchez D, Gomora JC, De la Vega-Beltran JL, Trevino CL, et al. ZD7288 inhibits low-threshold Ca2+ channel activity and regulates sperm function. Biochem Biophys Res Commun. 2003;311:187–92. doi: 10.1016/j.bbrc.2003.09.197. [DOI] [PubMed] [Google Scholar]
- 231.DiFrancesco D. Some properties of the UL-FS 49 block of the hyperpolarization-activated current (i(f)) in sino-atrial node myocytes. Pflugers Arch. 1994;427:64–70. doi: 10.1007/BF00585943. [DOI] [PubMed] [Google Scholar]
- 232.Van Bogaert PP, Pittoors F. Use-dependent blockade of cardiac pacemaker current (If) by cilobradine and zatebradine. Eur J Pharmacol. 2003;478:161–71. doi: 10.1016/j.ejphar.2003.08.083. [DOI] [PubMed] [Google Scholar]
- 233.Bois P, Bescond J, Renaudon B, Lenfant J. Mode of action of bradycardic agent, S 16257, on ionic currents of rabbit sinoatrial node cells. Br J Pharmacol. 1996;118:1051–7. doi: 10.1111/j.1476-5381.1996.tb15505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Fozzard HA, Lee PJ, Lipkind GM. Mechanism of local anesthetic drug action on voltage-gated sodium channels. Curr Pharm Des. 2005;11(21):2671–86. doi: 10.2174/1381612054546833. [DOI] [PubMed] [Google Scholar]
- 235.Rodrigo GC, Standen NB. ATP-sensitive potassium channels. Curr Pharm Des. 2005;11(15):1915–40. doi: 10.2174/1381612054021015. [DOI] [PubMed] [Google Scholar]
- 236.Schmitz C, Perraud AL. The TRPM cation channels in the immune context. Curr Pharm Des. 2005;11(21):2765–78. doi: 10.2174/1381612054546851. [DOI] [PubMed] [Google Scholar]