Abstract
Thalamic alterations have been reported in autism but the relationships between these abnormalities and clinical symptoms, specifically sensory features, have not been elucidated. The goal of this investigation is to combine two neuroimaging methods to examine further the pathophysiology of thalamic anomalies in autism and to identify any association with sensory deficits. Structural MRI and multi-voxel, short echo-time proton magnetic resonance spectroscopy (1H MRS) measurements were collected from 18 male children with autism and 16 healthy children. Anatomical measurements of thalamic nuclei and absolute concentration levels of key 1H MRS metabolites were obtained. Sensory abnormalities were assessed using a sensory profile questionnaire. Lower levels of N-Acetylaspartate (NAA), phosphocreatine and creatine, and choline-containing metabolites were observed on the left side in the autism group when compared to controls. No differences in thalamic volumes were observed between the two groups. Relationships, although limited, were observed between measures of sensory abnormalities and 1H MRS metabolites. Findings from this study support the role of the thalamus in the pathophysiology of autism and more specifically in the sensory abnormalities observed in this disorder. Further investigations of this structure are warranted, since it plays an important role in information processing as part of the cortico-thalamo-cortical pathways.
Keywords: Sensory profile questionnaire (SPQ), Sensory abnormalities, N-Acetylaspartate (NAA)
1. Introduction
Autism is a pervasive developmental disorder characterized by impairment in the development of reciprocal social interactions and communication abilities and the presence of stereotyped/repetitive behaviors (APA, 2000). In addition to these core deficits, abnormalities in the motor and sensory domains have also been reported. While not necessary to make the diagnosis, these features are clinically important because of their impact on function and quality of life. This is particularly true for the sensory disturbances, where recent evidence pointed to their usefulness in distinguishing autism from mental retardation in very young children with pervasive developmental disorders (Gillberg et al., 1990). However, a limited number of studies have focused on the neurobiological basis of these deficits, and further investigations of their pathophysiology are needed if more effective therapeutic strategies are to be developed.
Sensory abnormalities in autism were initially reported by Kanner (1947) in seven of his eleven original cases, when he described children who were experiencing fascination with visual stimuli and hypersensitivity to noise. Recent work has focused on examining the characteristics of these alterations by assessing their frequency and course in patient populations with different age ranges and variable level of illness severity. These features appear to be common with a reported prevalence rate ranging from 30% to 88 % and even 100% (Kientz and Dunn, 1997; Dawson and Watling, 2000). Sensory abnormalities are also quite diverse with 39% of individuals with autism exhibiting a hypo-responsive pattern, 19% experiencing a hyper-responsive pattern and 36% displaying a mixed response (Greenspan and Wieder, 1997). Interestingly, the consistency of these patterns over time in the same individuals has been questioned with evidence of variability with age (Cesaroni and Garber, 1991). However, the most critical and informative characteristics appear to be related to the relationship between these sensory abnormalities and the core symptoms. Some investigators have reported on links between sensory features and different aspects of social development, such as joint attention (Dahlgren and Gillberg, 1989; Baranek, 1999). Others have suggested a relationship between auditory abnormalities and the development of language and communication (Dawson et al, 1998, Rapin, 1997). In contrast, other studies have associated sensory anomalies and rigidity/repetitive behaviors, and suggested that both types of symptoms might share common pathophysiologic pathways driven by chronic hypo- or hyper- arousal (Rogers and Ozonoff, 2005).
The neural basis of sensory processing has been investigated in animal models and more recently in humans with the advent of non-invasive magnetic resonance technologies. Sensory-specific areas have been described with, for example, specialized brain regions in the occipital cortex, the superior temporal gyrus, and the post-central gyrus responsible for processing visual, auditory and tactile information, respectively. However, this regional specialization becomes more difficult to delineate with the processing of multisensory information such as examining visual stimuli when it is accompanied with a tactile or auditory stimulus. Such complex events are initially processed in sensory specific areas and later on by common multisensory representations in association cortices such as the ventral intraparietal areas (Duhamel et al., 1998) and posterior temporal cortex (both tactile and visual stimulation) (Macaluso and Driver, 2001). While several brain regions are variably involved in simple and complex sensory information, virtually all sensory systems pass through the thalamus which is reciprocally connected through projections to the cerebral cortex. An additional crucial attribute of the thalamus is that it appears to be much more than a key-link in this process since it actively filters the flow of information to the cortex. These characteristics have prompted several investigators in the field of autism, using different methodologies including imaging techniques, to link sensory abnormalities observed in this disorder to the thalamus, but no direct evidence supporting this association has, to date, been reported (Baranek, 2002; Tsatsanis et al., 2003; Hardan et al., 2006).
The focus on the thalamus in the neuroimaging literature has recently increased. Studies investigating the structural and functional integrity of the thalamus in autism have been reported. Two recent morphometric studies in individuals with autism have observed an abnormal relationship between total brain size and thalamic volumes in patients, in the absence of volumetric differences between autistics and controls (Tsatsanis et al., 2003; Hardan et al., 2006). An MRI and 1H spectroscopy study of the thalamus examined a sample of 32 children with autism and 15 controls (Perich et al. 2002). No structural differences were found between the two groups but N-Acetylaspartate(NAA)/phosphocreatine plus creatine (PCr+Cr) ratios, a marker of functioning neuroaxonal tissue (Birken and Oldendorf, 1989; Pouwels et al., 1999), were found to be decreased in the oldest patient subgroup (8–13 years). Additional evidence implicating the thalamus in the pathophysiology of autism comes from a positron emission tomography (PET) study examining a sample of 17 adolescents and adults with autism and 17 healthy matched controls while subjects were performing a serial of verbal learning task. No volumetric alterations in the thalamus were observed between the two groups, but lower relative glucose metabolic rates were found in the thalamus and other brain regions in patients with autism spectrum disorders when compared to controls (Haznedar et al., 2006).
While the above investigations provide evidence of abnormalities in the thalamus in autism, they offer little support to the link between this structure and the clinical manifestations of autism, particularly the sensory abnormalities. Therefore, we conducted this investigation to assess for structural and functional imaging abnormalities in the thalamus as measured by structural MRI and 1H spectroscopy and to examine the association between any neurobiologic alterations and sensory features as measured by the sensory profile questionnaire (SPQ) (Dunn, 1994). Based on previous report, we hypothesized that no structural abnormalities would be observed between the two groups, but decreased NAA levels would be found in the patient group when compared to controls. Additionally, we predicted that neurobiologic measurements would be associated with sensory abnormalities taking in consideration the key role that the thalamus play in information processing.
2. Methods
2.1 Participants
Subjects were 18 boys with autism and 16 healthy male controls between eight and 15 years of age. The autistic subjects represented all consecutive referrals to a research clinic who were eligible to participate in the study and able to complete the imaging procedures. The diagnosis of autism was established through the administration of the Autism Diagnostic Interview-Revised (ADI-R) and Autism Diagnostic Observation Schedule (ADOS) (Lord et al., 1989; Lord et al., 1994) in addition to expert clinical evaluation. Children with secondary autism related to a specific etiology such as tuberous sclerosis or Fragile X were excluded, as were potential subjects with evidence of genetic, metabolic, seizure, or infectious disorders. All subjects had a full-scale IQ (FSIQ) > 70.
Controls were children recruited from the community through advertisements in areas socio-economically comparable to those of the families of origin of the autistic subjects. Healthy subjects were screened by face-to-face evaluations, questionnaires, telephone interviews, and observation during psychometric tests. Individuals with a family history of any neuropsychiatric disorder, such as autism, learning disability, affective disorders, and schizophrenia, were not included. Potential subjects with a history of birth asphyxia, head injury, or a seizure disorder were also excluded. All control subjects had a FSIQ > 70 and no learning disability as assessed by the Wide Range Achievement Test-R. Exclusions for control subjects and individuals with autism were based on history and physical examination as well as laboratory testing when indicated.
The behavioral phenotyping of participants included several cognitive and clinical measures. The Wechsler Intelligence Scale for Children-III was administered to measure cognitive functioning including FSIQ for all participants. The socioeconomic status of the family of origin was assessed using the Hollingshead method (Hollingshead, 1975). The Sensory Profile Questionnaire (SPQ) was obtained from participants’ parents to assess sensory abnormalities (Dunn, 1994). The SPQ is a 125-item parent report questionnaire that evaluates sensory abnormalities and compared to available normative data. The items are written such that low scores reflect undesirable and abnormal behaviors. The SPQ includes a summary section with 9 factors and sensory processing section with 6 subscales. Methodology of the study was approved by the Institutional Review Board. Written informed consent was obtained from parents and assent was obtained from all children.
2.2 MRI Scans and Structural Measurements
MR scans were obtained on a GE 1.5 Tesla Signa whole-body MRI system (GE Medical Systems, Milwaukee, WI). A 3D, T1-weighted spoiled gradient recalled (SPGR) acquisition was performed in the coronal plane to obtain 124 images covering the entire brain (slice thickness=1.5mm, TE = 5ms; TR = 25ms; flip angle = 40 degrees, NEX = 1; FOV = 24cm; matrix = 256 × 192). A double spin-echo sequence was also used to obtain T2 and proton density images in the coronal plane (slice thickness= 5mm; TEs= 17ms and 102ms; TR = 2,500ms; NEX = 1; FOV = 24cm; matrix = 256 × 192; total slices= 24). MRI data were identified by scan number to retain blindness and analyzed using Brain Research: Analysis of Images, Networks and Systems software (BRAINS) while applying previously published methodologies of total brain volume (TBV) measurements (Magnotta et al., 2002). The image processing was performed on a SGI workstation (Silicon Graphics Inc., Mountain View, CA) using the BRAINS2 (University of Iowa, Iowa City, IA, USA) software package. The image data was normalized to standard Talairach stereotactic three-dimensional space (Talairach and Tournoux, 1988) by identifying six brain-limiting points (anterior, posterior, superior, inferior, left, and right); the anterior-posterior commissure line specified the x-axis, a vertical line rising from the x-axis through the interhemispheric fissure specified the y-axis, and a transverse orthogonal line with respect to x and y coordinates specified the z-axis. Registration was performed by aligning the T2 and PD images with a resampled T1 image and then resampling the T2 and PD images themselves (Magnotta et al., 2002). After normalization to a standard three-dimensional space, the pixels representing grey matter, white matter, and cerebrospinal fluid were identified using a segmentation algorithm applied to the T1, T2, and PD image sequences as described elsewhere (White et al., 2003).
Measurements of TBV were performed using the BRAINS2 masks as generated by a neural network and corrected by manual tracing (ICC > 0.95). TBV was defined as the whole supra- and infra-tentorial brain tissue superior to the foramen magnum while excluding cerebrospinal fluid. Similarly, thalamic measurements were generated by a neural network followed by a manual editing of the tracings. Reliability was satisfactory (> 0.95) with one tracer measuring 10 scans independently twice.
2.3 1H Spectroscopy
In vivo proton magnetic resonance spectroscopy (1H MRS) is a non-invasive neuroimaging approach capable of assessing, in localized brain regions, the brain biochemistry of NAA (a marker of functioning neuroaxonal tissue), PCr+Cr (high-energy phosphate metabolites), glycerophosphocholine plus phosphocholine (GPC+PC; catabolic and anabolic metabolite of membrane phospholipids), myo-inositol and the neurotransmitter glutamate. A 2D, multi-voxel 1H spectroscopy acquisition method was applied here combining the stimulated echo acquisition mode (STEAM) sequence (Frahm et al., 1989) with the phase encoding steps of chemical shift imaging (CSI), which is termed STEAMCSI on the GE system. The STEAM sequence localized a 3D region of interest (ROI) with an axial thickness of 20 mm and right/left and anterior posterior dimensions of approximately 100 mm × 80 mm to ensure the CSI signal was within the cerebrum. . The ROI in the axial direction was parallel to the anterior commissure-posterior commissure (AC-PC) line and its infereior limit was at the superior edge of the orbital bone resulting in an ROI encompassing the righ and left thalamus. Acquisition parameters included a repetition time TR of 1.6 s, TE of 20ms, TM of 13.7ms, FOV of 24 cm, 16 ×16 phase encoding steps or 16 × 16 voxels with a nominal voxel size of 15 × 15 × 20mm3, 1,024 complex data points, 2,500 Hz spectral bandwidth, and 4 averages. A water-unsuppressed measurement also was collected with 8 × 8 phase encoding steps.
As part of the post-processing, the CSI was repositioned, in each subject, by shifting the 16 × 16 voxel grid in the right/left and anterior/posterior directions, prior to the 2D inverse Fourier transformation (IFT) to ensure two adjascent voxels were centered in the left and right thalamus (Figure 1). In conjunction with the grid shift, a mild spatial apodization (i.e., Fermi window with 90% diameter and 5% transition width) was applied followed by the 2D IFT. Based on empirical calculations of the point spread of a 16 × 16 CSI matrix with a mild apodization, the effective voxel size was 28% greater than the nominal size (i.e. 1.7 × 1.7 × 2.0 cm3 or 5.8 cm3). For each right and left voxels, no apodization was applied to the signal and the quantification of the spectral metabolites NAA, glutamate, glutamine, myo-inositol (mI), PCr+Cr, GPC+PC, taurine, alanine, aspartate, gamma amino-butyric acid (GABA), glucose, and NAAG, as well as lipid resonances and macromolecule resonances (Seeger et al., 2003), was done using the Linear Combination (LC) Model software version 6.1–4 (Provencher, 1993). Only results of the more reliable metabolites (NAA, PCr+Cr, GPC+PC, myo-inositol and glutamate) were used in the analysis. Quantification of glutamate but not glutamine was possible. The water-unsuppressed signal was utilized to obtain absolute quantification values with units of mmol/kg wet weight as described by Stanley et al. (1995). Grey and white matter tissue fractions were assumed to be constant for all subjects.
Figure 1.
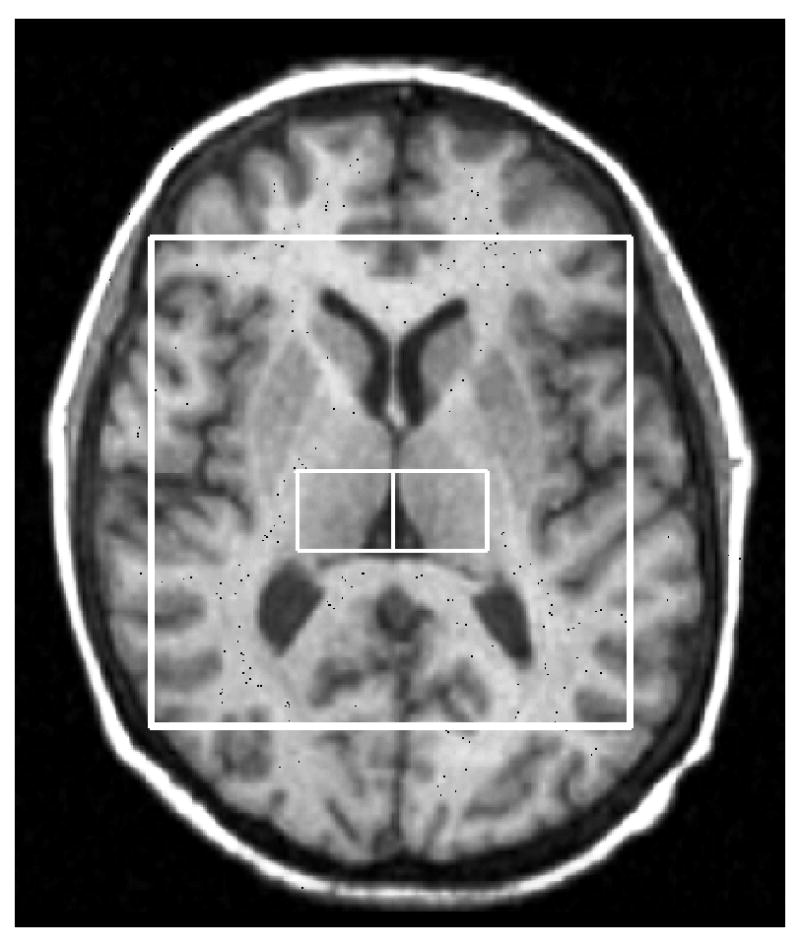
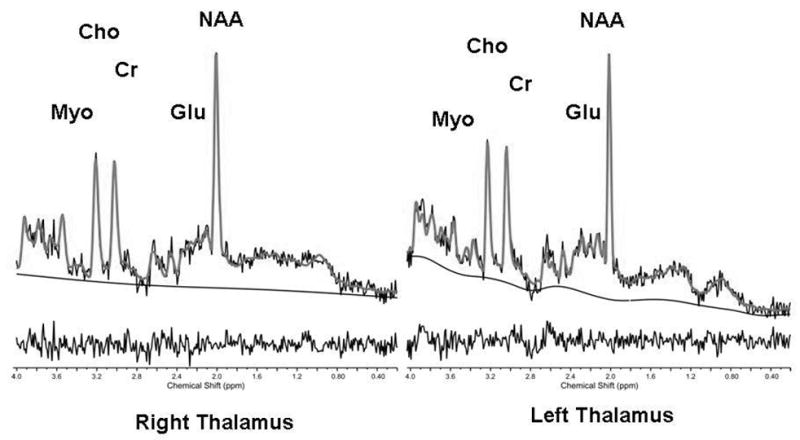
Thalamic voxels localization 1H MRS (1a) and representative STEAM spectra from the right and left thalamus (1b). NAA: N-Acetylaspartate; Cr: creatine + phosphocreatine; Chol: Choline containing compounds: glycerophosphocholine + phosphocholine; Glu: Glutamate.
Between-group differences were analyzed with two-tailed Student t-tests and were reported as means and standard deviations (M ± SD). Furthermore, despite the unclear effect of IQ on brain size in individuals with autism, analyses were conducted for measurements to account for this potential confounding variable such as FSIQ (Hazlett et al., 2005). Analysis of covariance (ANCOVA) was applied to compare the two subject groups on neurobiologic measurements while controlling for confounding factors when indicated. Pearson’s correlation coefficients were used to examine the association between thalamic measurements and confounding factors. Regression analyses were conducted to examine the relationships between the two groups and SPQ. Probability figures were considered significant if they achieved significance at conventional levels (i.e., P < 0.05).
3. Results
No differences were observed between the demographic characteristics of patients and controls, except for FSIQ (Table 1). No differences between the two groups were observed in right or left thalamic volumes (Table 2) before and after controlling for TBV and FSIQ. Bilateral decrease in NAA levels were found in the autistic group when compared to controls but reached statistical differences only on the left side (Table 2). Differences were also observed in PCr+Cr and GPC+PC measures (Table 2). A non-significant reduction in all left-sided metabolites was also observed in individuals with autism without specificity to NAA. The level of significance of all these comparisons remained unchanged when controlling for FSIQ. There was no relationship between FSIQ and structural or spectroscopy measurements. Bilateral positive correlations were observed between TBV and thalamic volumes in patients and controls. SPQ was available from 17 children with autism and 15 controls.
Table 1.
Demographic information
| Autistics | Control | t-test | ||||
|---|---|---|---|---|---|---|
| n = 18 | n = 16 | df = 32 | ||||
| Mean | S.D. | Mean | S.D. | t | P | |
| Age | 11.9 | 2.2 | 11.6 | 1.6 | 0.537 | 0.595 |
| Full-scale IQ | 93.8 | 17.6 | 116.8 | 12.7 | −4.318 | 0.000 |
| SES | 4.4 | 0.6 | 4.4 | 0.5 | 0.358 | 0.723 |
| ADI-R | 51.6 | 7.5 | --- | --- | --- | --- |
| ADOS | 15.6 | 2.7 | --- | --- | --- | --- |
SES: socio-economic status; ADI-R: Autism Diagnostic Interview-Revised; ADOS: Autism Observation Schedule
Table 2.
Volumetric and proton spectroscopy measurements in autistic and normal control subjects
| Thalamic Measurements | Autistics | Control | t-test | |||
|---|---|---|---|---|---|---|
| n = 18 | n = 16 | df = 32 | ||||
| Mean | S.D. | Mean | S.D. | t | P | |
| Volume (cc) | ||||||
| Left Thalamus | 3.9 | 0.5 | 3.9 | 0.3 | 0.506 | 0.616 |
| Right Thalamus | 4.1 | 0.6 | 4.1 | 0.4 | −0.099 | 0.922 |
| Total Brain Volume | 1352.2 | 129.8 | 1341.7 | 90.3 | 0.271 | 0.788 |
| 1H MRS | ||||||
| Left NAA | 4.4 | 1.4 | 5.6 | 1.0 | −2.923 | 0.006 |
| Left PCr+Cr | 2.7 | 0.9 | 3.6 | 1.0 | −2.400 | 0.022 |
| Left GPC+PC | 0.9 | 0.3 | 1.2 | 0.2 | −3.088 | 0.004 |
| Left Myo-Inositol | 2.5 | 0.9 | 3.0 | 0.6 | −1.682 | 0.102 |
| Left Glutamate + Glutamine | 7.21 | 2.93 | 8.75 | 1.91 | −1.795 | 0.082 |
| Right NAA | 4.9 | 1.84 | 5.6 | 1.1 | −1.224 | 0.230 |
| Right PCr+Cr | 3.3 | 1.3 | 3.6 | 0.9 | −0.813 | 0.422 |
| Right GPC+PC | 1.1 | 0.4 | 1.2 | 0.3 | −1.449 | 0.157 |
| Right Myo-Inositol | 2.8 | 1.2 | 2.8 | 0.9 | 0.061 | 0.951 |
| Right Glutmate+ Glutamine | 7.60 | 2.96 | 7.67 | 1.76 | −0.084 | 0.934 |
Metabolite concentration expressed in absolute values; NAA: N-Acetylaspartate; PCr+Cr: creatine + phosphocreatine; GPC+PC: glycerophosphocholine + phosphocholine
Due to the small sample size, a limited number of variables were introduced in the regressions analyses. Only, levels of NAA and glu + gln on the left side were included since they were both clinically relevant. Relationships, although limited, were observed between levels of these compounds and several sensory measures. When controlling for FSIQ and participant groups, trends toward an association was observed between NAA levels on the left side and the sensory seeking factor (B = 0.322, t = 1.738, P = 0.094) and the behavioral outcome of sensory processing (B = 0.293, t = 2.043, P = 0.051). Similarly, trends toward relationships were found between glu + gln levels on the left side and the sensory sensitivity factor (B = −0.376, t = −1.746, P = 0.092), as well as modulation related to body position and movement (B = −0.316, t = −1.866, P = 0.073), and modulation of movement affecting activity level (B = −0.268, t = −1.810, P = 0.081).
4. Discussion
The present study provides further evidence of neurobiological abnormalities involving the thalamus in autism. They were most apparent in the metabolic indices and are particularly interesting because of the role of the thalamus in sensory gating and the prominence and prevalence of sensory complaints in autism and their associated disability.
In the present study, no volumetric differences in the thalamus were observed between autistic subjects and controls. The absence of structural anomalies is consistent with several other morphometric MRI studies reported in this disorder suggesting that obvious structural alterations are not present in the thalamus in autism (Perich et al. 2002; Tsatsanis et al., 2003; Haznedar et al., 2006; Hardan et al., 2006). However, a correlation between thalamic volume and TBV was observed in the autism group. This observation differs from two prior studies (Tsatsanis et al., 2003; Hardan et al., 2006). Tsatsanis et al., (2003) examined the size of the thalamus in twelve high functioning individuals with autism (age range: 10.7–29.5 years) and twelve normal controls and reported a significant positive correlation in controls but not in the autism group (Tsatsanis et al., 2003). A more recent study examining a much larger sample size (40 individuals with autism and 41 controls) with a wider age range (8.8 to 45.7 years) observed similar findings (Hardan et al., 2006). The difference between the current study and the previous ones might be related to the difference in age ranges of the subjects in the three studies. In fact, the other two studies included adult subjects whereas the present investigation involved only children and adolescents (8 to 15 years). Additionally, the application of different morphometric methodologies and software might have also contributed to the discrepant findings.
Decreased levels of several metabolites, including NAA, were observed in the patients group when compared to controls. These findings are consistent with several 1H MRS studies reporting a decrease in the level of several metabolites in cortical and subcortical structures (DeVito et al., 2007; Friedman et al., 2003; Levitt et al., 2003; Perich et al. 2002). Our results are also concordant with some but not all spectroscopy investigations of the thalamus in autism (Perich et al. 2002, Levitt et al., 2003). Our observations are comparable to a previous 1H MRS study that found similar results in 8–13 year old children with autism (Perich et al. 2002). However, they are discordant with a recent investigation reporting a decrease in NAA levels in the body of the caudate but not in the thalamus (Levitt et al., 2003). Nonetheless, all of these observations of decrease NAA levels in different brain structures are consistent with 1H MRS study of a large sample (N=45) of very young children with autism spectrum disorder (mean age and SD: 47.4 ± 4.2 months) examining cortical and subcortical structures (Friedman et al., 2003). Decreased NAA levels were observed in the autism group when compared to typically developing and developmentally disabled children (Friedman et al., 2003). This pattern of reduced NAA levels in subcortical brain regions, such as the thalamus, points to the possibility of decreased neuronal number, reduction in neuronal function or both. These metabolic abnormalities could represent, at least partially, the neurobiologic underpinnings of the aberrant connectivity recently reported in autism (Just et al., 2004). Finally, the reduction in NAA and glutamate levels might be related (Clark et al., 2006) pointing to possible impairment in neuronal oxidative metabolism (DeVito et al., 2007) and consequently providing additional evidence for an imbalance in oxidative stress in autism (Kern, 2003; James et al., 2004).
Despite the prevalence and pervasiveness of sensory abnormalities in autism, little progress has been made in elucidating its neurobiologic underpinnings (O’Neill et al., 1997). The current study provides some evidence, although not strong, linking these features to specific neurobiologic abnormalities. Available research has indirectly implicated the neurotransmitter serotonin and several brain regions including the thalamus. Two positron emission tomography studies have observed disturbances of serotonin synthesis affecting the dentato-thalamo-cortical pathways and have suggested that serotoninergic alterations in this brain network might lead to the sensory integration deficits observed in autism (Chugani et al.,1999; Mueller et al., 1998). Serotonin plays an important role in the modulation of synaptogenesis and consequently, in the development of several brain regions including the primary sensory and somatosensory cortices (Chugani, 2002). Alterations of synaptic modulation in autism, possibly related to the polymorphism of the serotonin transporter, has been suggested to affect thalamo-cortical connectivity and consequently leading to the development of sensory abnormalities (Chugani, 2002). Finally, the most compelling evidence linking the serotoninergic system and the thalamus to sensory abnormalities in autism comes from the involvement of serotonin in obsessive compulsive behaviors (Gilbert et al., 2000) and the recently reported association between these symptoms and sensory abnormalities in autism (Baranek et al., 1997). However, in spite of this mounting evidence, it remains unclear if the nature of this relationship is associative or causative.
This study has a number of limitations. Findings should be interpreted with caution in light of the small sample size, the lack of matching for IQ, and the inclusion of only males. These characteristics limit the generalizability of the results and warrant additional studies with larger sample sizes of individuals with a wide range of intellectual functioning while using objective and laboratory methods to assess sensory deficits. Adjustment of the p-value might be needed in light of the number of comparisons conducted. However, while p-value adjustments diminish the chance of making type I errors, they raise the chance of making type II errors, or necessitate the increase of the sample size (Feise, 2002). The thalamus tracings are limited by the lack of tissue contrast on the images which does not allow the measurement of the different thalamic nuclei when applying the available morphomotric software. Finally, there are several limitations related to the spectroscopy acquisition sequence and data processing. The use of a STEAM CSI sequence might limit the acquired signal when compared with the point resolved spectroscopy (PRESS). Furthermore, in short TR acquisition, the metabolites levels are subject to T1 saturation which might account for group differences observed in this study. Additionally, the overall reduction in metabolites in the left thalamus might suggest that NAA decrease is not very specific. However, this reduction cannot be due to processing effect since the control group shows similar values on both sides. Finally, findings of the present investigation could be due to differences in tissue composition, which was not accounted for in this study including a cerebral spinal fluid correction; nonetheless, the right and left volumes of the thalamus, albeit not exact to the spectroscopy localization, were not significantly different between groups.
In summary, results from this study point to the involvement of the thalamus in the pathophysiology of autism and possibly to the sensory abnormalities observed in this disorder. Future studies should apply multimodal imaging techniques including functional MRI to probe these deficits which will help in shedding light on the circuitry involved. Sensory abnormalities are very disabling and have received little attention. Insight into their neurobiologic basis may open the way to effective treatments.
Acknowledgments
This work was supported in part by NIMH grant MH 64027 (AYH) and NICHD grant HD 35469 (NJM). This study was also supported by an NICHD Collaborative Program of Excellence in Autism (CPEA). The efforts and commitment of the participants and their families in this study are gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adrien JL, Faure M, Perrot A, Hameury L, Garreau B, Barthelemy C, Sauvage D. Autism and family home movies: preliminary findings. Journal of Autism and Developmental Disorders. 1991;21:43–49. doi: 10.1007/BF02206996. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. TR. Washington, DC: American Psychiatric Press; 2000. [Google Scholar]
- Baranek GT. Autism during infancy: a retrospective video analysis of sensory-motor and social behaviors at 9–12 months of age. Journal of Autism and Developmental Disorders. 1999;29:213–224. doi: 10.1023/a:1023080005650. [DOI] [PubMed] [Google Scholar]
- Baranek GT, Foster LG, Berkson G. Tactile defensiveness and stereotyped behaviors. American Journal of Occupational Therapy. 1997;51:91–95. doi: 10.5014/ajot.51.2.91. [DOI] [PubMed] [Google Scholar]
- Baranek GT. Efficacy of sensory and motor interventions for children with autism. Journal of Autism and Developmental Disorders. 2002;32:397–422. doi: 10.1023/a:1020541906063. [DOI] [PubMed] [Google Scholar]
- Birken DL, Oldendorf WH. N-acetyl-L-aspartic acid: a literature review of a compound prominent in 1H-NMR spectroscopic studies of brain. Neuroscience and Biobehavioral Reviews. 1989;13:23–31. doi: 10.1016/s0149-7634(89)80048-x. [DOI] [PubMed] [Google Scholar]
- Cesaroni L, Garber M. Exploring the experience of autism through firsthand accounts. Journal of Autism and Developmental Disorders. 1991;21:303–313. doi: 10.1007/BF02207327. [DOI] [PubMed] [Google Scholar]
- Chugani DC, Muzik O, Behen M, Rothermel R, Janisse JJ, Lee J, Chugani HT. Developmental changes in brain serotonin synthesis capacity in autistic and nonautistic children. Annals of Neurology. 1999;45:287–295. doi: 10.1002/1531-8249(199903)45:3<287::aid-ana3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Chugani DC. Role of altered brain serotonin mechanisms in autism. Molecular Psychiatry. 2002;7:S16–7. doi: 10.1038/sj.mp.4001167. [DOI] [PubMed] [Google Scholar]
- Clark JF, Doepke A, Filosa JA, Wardle RL, Lu A, Meeker TJ, Pyne-Geithman GJ. N-acetylaspartate as a reservoir for glutamate. Medical Hypotheses. 2006;67:506–512. doi: 10.1016/j.mehy.2006.02.047. [DOI] [PubMed] [Google Scholar]
- Dawson G, Finley C, Phillips S, Lewy A. A comparison of hemispheric asymmetries in speech-related brain potentials of autistic and dysphasic children. Brain and Language. 1989;37:26–41. doi: 10.1016/0093-934x(89)90099-0. [DOI] [PubMed] [Google Scholar]
- Dawson G, Watling R. Interventions to facilitate auditory, visual, and motor integration in autism: a review of the evidence. Journal of Autism and Developmental Disorders. 2000;30:415–421. doi: 10.1023/a:1005547422749. [DOI] [PubMed] [Google Scholar]
- Dawson G, Toth K, Abbott R, Osterling J, Munson J, Estes A, Liaw J. Early social attention impairments in autism: social orienting, joint attention, and attention to distress. Developmental Psychology. 2004;40:271–283. doi: 10.1037/0012-1649.40.2.271. [DOI] [PubMed] [Google Scholar]
- Dahlgren SO, Gillberg C. Symptoms in the first two years of life. A preliminary population study of infantile autism. European Archives of Psychiatry and Neurological sciences. 1989;238:169–174. doi: 10.1007/BF00451006. [DOI] [PubMed] [Google Scholar]
- Devito TJ, Drost DJ, Neufeld RW, Rajakumar N, Pavlosky W, Williamson P, Nicolson R. Evidence for cortical dysfunction in autism: a proton magnetic resonance spectroscopic imaging study. Biological Psychiatry. 2007;61:465–73. doi: 10.1016/j.biopsych.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Dunn W. Performance of typical children on the Sensory Profile: an item analysis. American Journal of Occupational Therapy. 1994;48:967–974. doi: 10.5014/ajot.48.11.967. [DOI] [PubMed] [Google Scholar]
- Duhamel JR, Colby CL, Goldberg ME. Ventral intraparietal area of the macaque: congruent visual and somatic response properties. Journal of Neurophysiology. 1998;79:126–136. doi: 10.1152/jn.1998.79.1.126. [DOI] [PubMed] [Google Scholar]
- Feise RJ. Do multiple outcome measures require p-value adjustment? BMC Medical Research Methodology. 2002;2:8. doi: 10.1186/1471-2288-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frahm J, Bruhn H, Gyngell ML, Merboldt KD, Hanicke W, Sauter R. Localized proton NMR spectroscopy in different regions of the human brain in vivo. Relaxation times and concentrations of cerebral metabolites. Magnetic Resonance in Medicine. 1989;11:47–63. doi: 10.1002/mrm.1910110105. [DOI] [PubMed] [Google Scholar]
- Friedman SD, Shaw DW, Artru AA, Richards TL, Gardner J, Dawson G, Posse S, Dager SR. Regional brain chemical alterations in young children with autism spectrum disorder. Neurology. 2003;60:100–107. doi: 10.1212/wnl.60.1.100. [DOI] [PubMed] [Google Scholar]
- Greenspan S, Wieder S. Developmental patterns and outcomes in infants and children with disorders in relating and communicating: A chart review of 200 cases of children with autism spectrum disorders. Journal of Developmental and Learning Disorders. 1997;1:87–141. [Google Scholar]
- Gilbert AR, Moore GJ, Keshavan MS, Paulson LA, Narula V, Mac Master FP, Stewart CM, Rosenberg DR. Decrease in thalamic volumes of pediatric patients with obsessive-compulsive disorder who are taking paroxetine. Archives of General Psychiatry. 2000;57:449–56. doi: 10.1001/archpsyc.57.5.449. [DOI] [PubMed] [Google Scholar]
- Gillberg C, Ehlers S, Schaumann H, Jakobsson G, Dahlgren SO, Lindblom R, Bagenholm A, Tjuus T, Blidner E. Autism under age 3 years: a clinical study of 28 cases referred for autistic symptoms in infancy. Journal of Child Psychology and Psychiatry. 1990;31:921–934. doi: 10.1111/j.1469-7610.1990.tb00834.x. [DOI] [PubMed] [Google Scholar]
- Hardan AY, Girgis RR, Adams J, Gilbert AR, Keshavan MS, Minshew NJ. Abnormal brain size effect on the thalamus in autism. Psychiatry Research. 2006;147:145–151. doi: 10.1016/j.pscychresns.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Poe M, Gerig G, Smith RG, Provenzale J, Ross A, Gilmore J, Piven J. Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Archives of General Psychiatry. 2005;62:1366–1376. doi: 10.1001/archpsyc.62.12.1366. [DOI] [PubMed] [Google Scholar]
- Haznedar MM, Buchsbaum MS, Hazlett EA, LiCalzi EM, Cartwright C, Hollander E. Volumetric analysis and three-dimensional glucose metabolic mapping of the striatum and thalamus in patients with autism spectrum disorders. American Journal of Psychiatry. 2006;163:1252–1263. doi: 10.1176/ajp.2006.163.7.1252. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Four Factor Index of Social Status. Yale University Department of Sociology; New Haven, CT: 1975. [Google Scholar]
- James SJ, Cutler P, Melnyk S, Jernigan S, Janak L, Gaylor DW, Neubrander JA. Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. The American Journal of Clinical Nutrition. 2004;80:1611–1617. doi: 10.1093/ajcn/80.6.1611. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127:1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. The Nervous Child. 1943;2:217–250. [Google Scholar]
- Kern JK. Purkinje cell vulnerability and autism: a possible etiological connection. Brain & Development. 2003;25:377–382. doi: 10.1016/s0387-7604(03)00056-1. [DOI] [PubMed] [Google Scholar]
- Kientz MA, Dunn W. A comparison of the performance of children with and without autism on the Sensory Profile. American Journal of Occupational Therapy. 1997;51:530–537. doi: 10.5014/ajot.51.7.530. [DOI] [PubMed] [Google Scholar]
- Levitt JG, O’Neill J, Blanton RE, Smalley S, Fadale D, McCracken JT, Guthrie D, Toga AW, Alger JR. Proton magnetic resonance spectroscopic imaging of the brain in childhood autism. Biological Psychiatry. 2003;54:1355–66. doi: 10.1016/s0006-3223(03)00688-7. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Goode S, Heemsbergen J, Jordan H, Mawhood L, Schopler E. Autism diagnostic observation schedule: a standardized observation of communicative and social behavior. Journal of Autism and Developmental Disorders. 1989;19:185–212. doi: 10.1007/BF02211841. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, LeCouteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Macaluso E, Driver J. Spatial attention and crossmodal interactions between vision and touch. Neuropsychologia. 2001;39:1304–1316. doi: 10.1016/s0028-3932(01)00119-1. [DOI] [PubMed] [Google Scholar]
- Magnotta VA, Harris G, Andreasen NC, O’Leary DS, Yuh WT, Heckel D. Structural MR image processing using the BRAINS2 toolbox. Computerized Medical Imaging and Graphics. 2002;26:251–264. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- Muller RA, Chugani DC, Behen ME, Rothermel RD, Muzik O, Chakraborty PK, Chugani HT. Impairment of dentato-thalamo-cortical pathway in autistic men: language activation data from positron emission tomography. Neuroscience Letters. 1998;245:1–4. doi: 10.1016/s0304-3940(98)00151-7. [DOI] [PubMed] [Google Scholar]
- O’Neill M, Jones RS. Sensory-perceptual abnormalities in autism: a case for more research? Journal of Autism and Developmental Disorders. 1997;27:283–293. doi: 10.1023/a:1025850431170. [DOI] [PubMed] [Google Scholar]
- Perich-Alsina J, Aduna de Paz M, Valls A, Munoz-Yunta JA. Thalamic spectroscopy using magnetic resonance in autism. Revista de Neurologia. 2002;1:S68–71. [PubMed] [Google Scholar]
- Pouwels PJ, Brockmann K, Kruse B, Wilken B, Wick M, Hanefeld F, Frahm J. Regional age dependence of human brain metabolites from infancy to adulthood as detected by quantitative localized proton MRS. Pediatric Research. 1999;46:474–85. doi: 10.1203/00006450-199910000-00019. [DOI] [PubMed] [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magnetic Resonance in Medicine. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Rapin I. Autism. New England Journal of Medicine. 1997;337:97–104. doi: 10.1056/NEJM199707103370206. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Ozonoff S. Annotation: what do we know about sensory dysfunction in autism? A critical review of the empirical evidence. Journal of Child Psychology and Psychiatry. 2005;46:1255–68. doi: 10.1111/j.1469-7610.2005.01431.x. [DOI] [PubMed] [Google Scholar]
- Seeger U, Klose U, Mader I, Grodd W, Nagele T. Parameterized evaluation of macromolecules and lipids in proton MR spectroscopy of brain diseases. Magnetic Resonance in Medicine. 2003;49:19–28. doi: 10.1002/mrm.10332. [DOI] [PubMed] [Google Scholar]
- Stanley JA, Drost DJ, Williamson PC, Thompson RT. The use of a priori knowledge to quantify short echo in vivo 1H MR spectra. Magnetic Resonance in Medicine. 1995;34:17–24. doi: 10.1002/mrm.1910340105. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. Thieme; New York: 1988. [Google Scholar]
- Tsatsanis KD, Rourke BP, Klin A, Volkmar FR, Cicchetti D, Schultz RT. Reduced thalamic volume in high-functioning individuals with autism. Biological Psychiatry. 2003;53:121–129. doi: 10.1016/s0006-3223(02)01530-5. [DOI] [PubMed] [Google Scholar]
- White T, Andreasen NC, Nopoulos P, Magnotta V. Gyrification abnormalities in childhood- and adolescent-onset schizophrenia. Biological Psychiatry. 2003;54:418–426. doi: 10.1016/s0006-3223(03)00065-9. [DOI] [PubMed] [Google Scholar]


